
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Benjamin Peter Carr | -- | 3449 | 2022-10-21 03:13:41 | | | |
| 2 | Amina Yu | -5 word(s) | 3444 | 2022-10-24 03:09:36 | | | | |
| 3 | Amina Yu | Meta information modification | 3444 | 2022-10-24 03:10:22 | | |
Video Upload Options
Collagen is the most prevalent structural protein in the extracellular matrix, resulting in the biopolymer being extensively investigated for biofabrication-based applications. However, its utilisation has been impeded due to a lack of sufficient mechanical toughness and the inability of the scaffolds to mimic complex natural tissues. The anisotropic alignment of collagen has been proven to be an effective method to enhance overall mechanical properties and produce biomimetic scaffolds. The alignment of collagen can be achieved by several methods, namely, gravity and extrusion-based fluidic alignment, static magnetic alignment, magnetic-flow alignment, cell-based stress-induced self-alignment, electrospinning, and electrophoretic-based electro-compaction (EC). Each existing approach of aligning collagen is described and compared with a sharper focus on electro-compaction.
1. Overview of Collagen Alignment Techniques
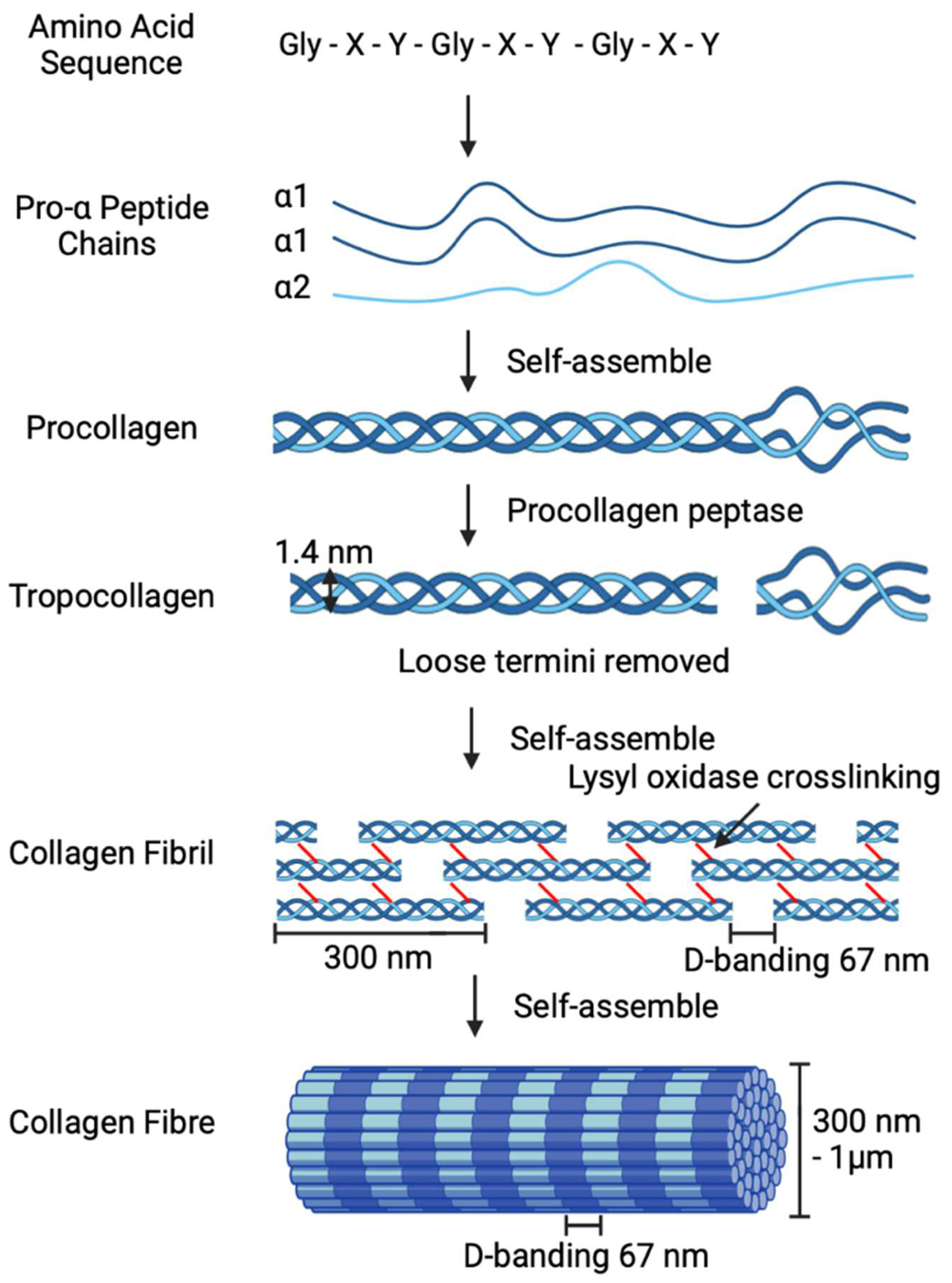
2. Electro-Compaction
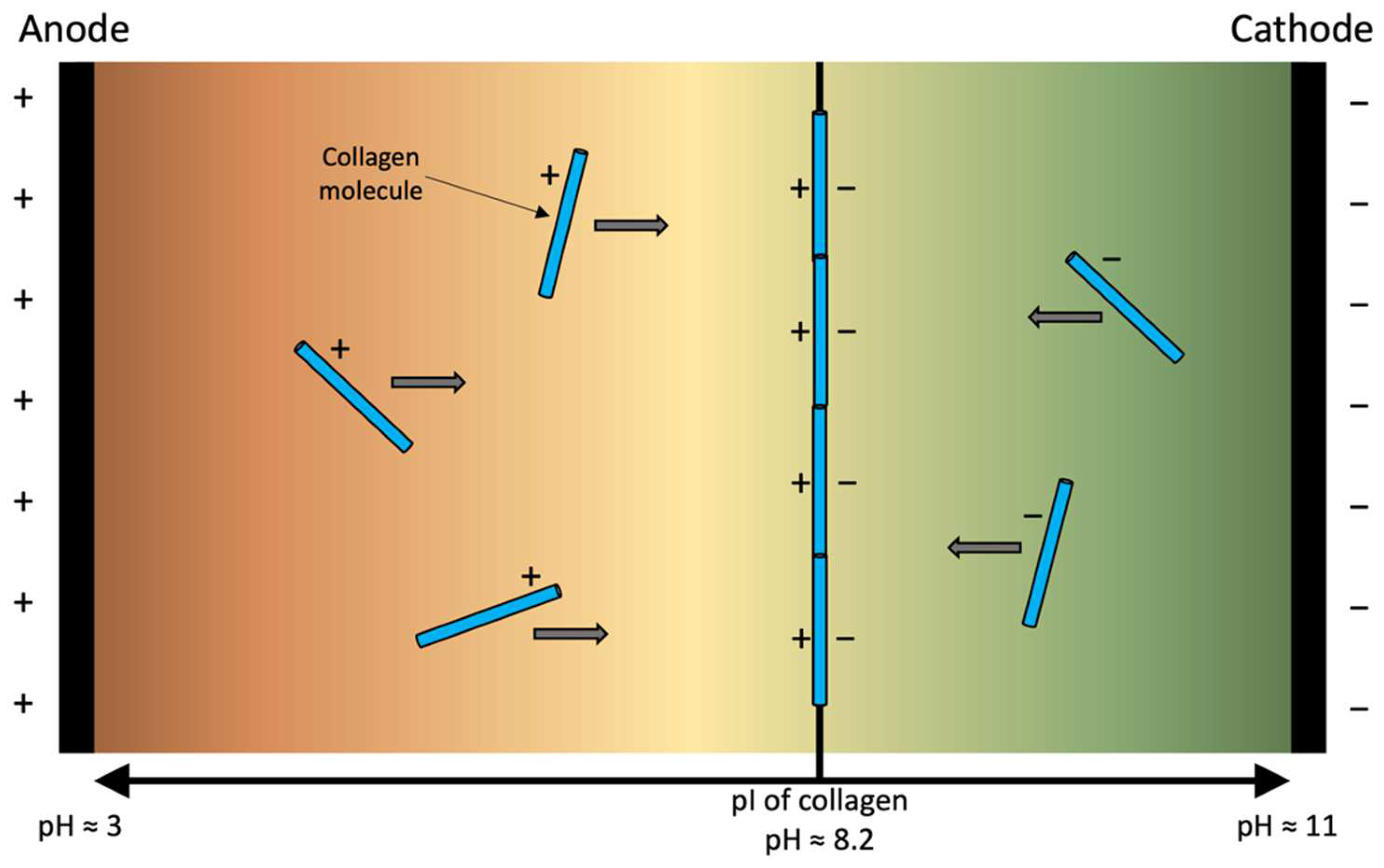
2.1. Sources of Collagen for Electro-Compaction
2.2. Electro-Compacted Scaffold Types
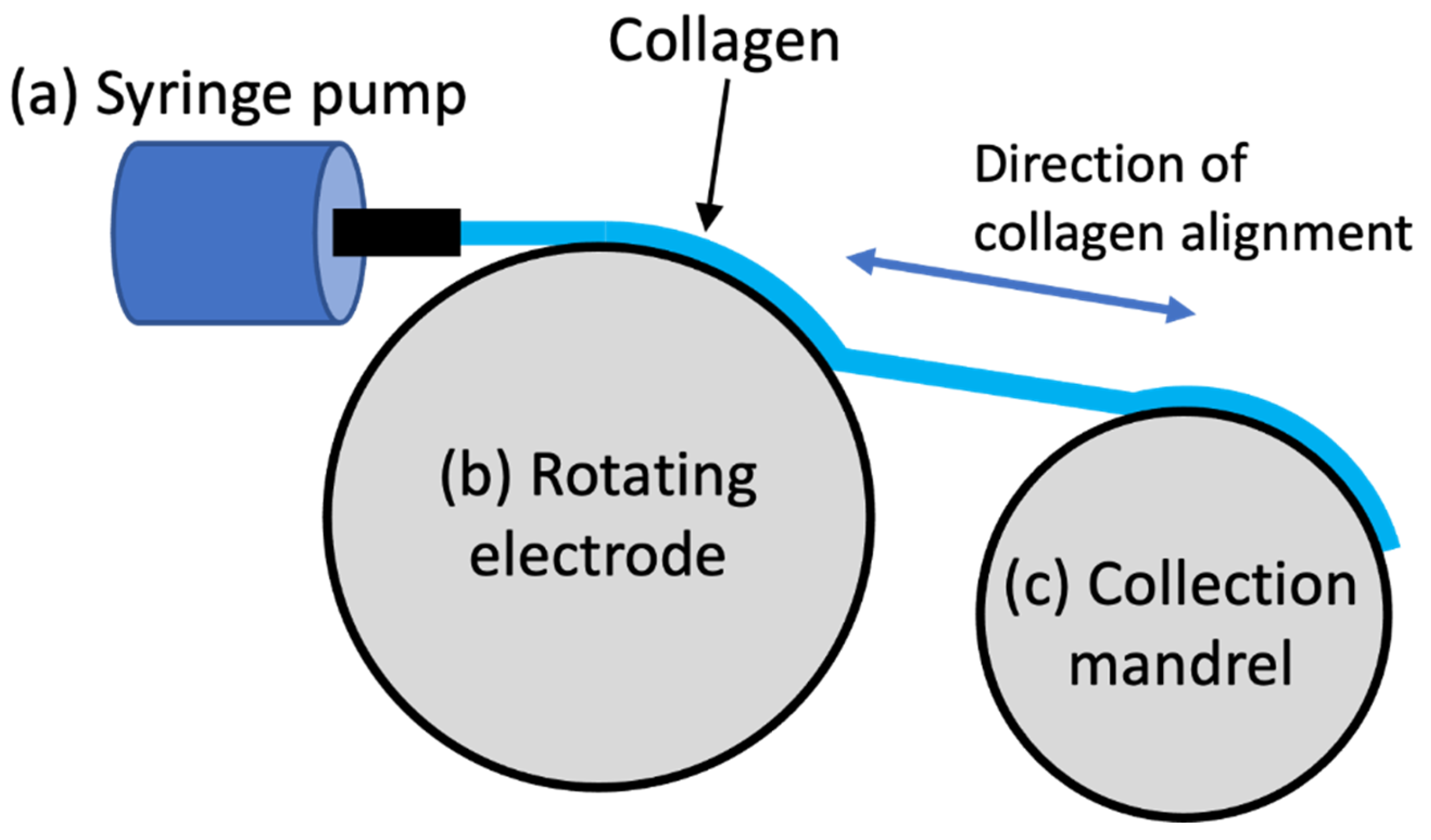
2.3. Enhancing Electro-Compacted Collagen Strength
2.4. Co-Electro-Compaction of Collagen with Fillers
2.5. Post-Alignment Fabrication Methods
2.6. Clinical Applications Using Electro-Compacted Collagen Scaffolds
References
- Dewle, A.; Pathak, N.; Rakshasmare, P.; Srivastava, A. Multifarious fabrication approaches of producing aligned collagen scaffolds for tissue engineering applications. ACS Biomater. Sci. Eng. 2020, 6, 779–797.
- Nguyen, T.U.; Shojaee, M.; Bashur, C.A.; Kishore, V. Electrochemical fabrication of a biomimetic elastin-containing bi-layered scaffold for vascular tissue engineering. Biofabrication 2019, 11, 015007.
- Caliari, S.R.; Harley, B.A.C. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 2011, 32, 5330–5340.
- Chen, Z.; Liu, X.; You, J.; Song, Y.; Tomaskovic-Crook, E.; Sutton, G.; Crook, J.M.; Wallace, G.G. Biomimetic corneal stroma using electro-compacted collagen. Acta Biomater. 2020, 113, 360–371.
- Wang, T.; Chen, P.; Zheng, M.; Wang, A.; Lloyd, D.; Leys, T.; Zheng, Q.; Zheng, M.H. In vitro loading models for tendon mechanobiology. J. Orthop. Res. 2018, 36, 566–575.
- Kishore, V.; Bullock, W.; Sun, X.; Van Dyke, W.S.; Akkus, O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials 2012, 33, 2137–2144.
- Kirkwood, J.E.; Fuller, G.G. Liquid crystalline collagen: A self-assembled morphology for the orientation of mammalian cells. Langmuir 2009, 25, 3200–3206.
- Debons, N.; Matsumoto, K.; Hirota, N.; Coradin, T.; Ikoma, T.; Aimé, C. Magnetic field alignment, a perspective in the engineering of collagen-silica composite biomaterials. Biomolecules 2021, 11, 749.
- Guo, C.; Kaufman, L.J. Flow and magnetic field induced collagen alignment. Biomaterials 2007, 28, 1105–1114.
- Wilks, B.T.; Evans, E.B.; Nakhla, M.N.; Morgan, J.R. Directing fibroblast self-assembly to fabricate highly-aligned, collagen-rich matrices. Acta Biomater. 2018, 81, 70–79.
- Blackstone, B.N.; Gallentine, S.C.; Powell, H.M. Collagen-based electrospun materials for tissue engineering: A systematic review. Bioengineering 2021, 8, 39.
- Cheng, X.; Gurkan, U.A.; Dehen, C.J.; Tate, M.P.; Hillhouse, H.W.; Simpson, G.J.; Akkus, O. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials 2008, 29, 3278–3288.
- Kishore, V.; Iyer, R.; Frandsen, A.; Nguyen, T.U. In vitro characterization of electrochemically compacted collagen matrices for corneal applications. Biomed. Mater. 2016, 11, 055008.
- Younesi, M.; Islam, A.; Kishore, V.; Panit, S.; Akkus, O. Fabrication of compositionally and topographically complex robust tissue forms by 3D-electrochemical compaction of collagen. Biofabrication 2015, 7, 035001.
- Oliveira, V.d.M.; Assis, C.R.D.; Costa, B.d.A.M.; Neri, R.C.d.A.; Monte, F.T.D.; Freitas, H.M.S.d.C.V.; França, R.C.P.; Santos, J.F.; Bezerra, R.d.S.; Porto, A.L.F. Physical, biochemical, densitometric and spectroscopic techniques for characterization collagen from alternative sources: A review based on the sustainable valorization of aquatic by-products. J. Mol. Struct. 2021, 1224, 129023.
- Uquillas, J.A.; Kishore, V.; Akkus, O. Effects of phosphate-buffered saline concentration and incubation time on the mechanical and structural properties of electrochemically aligned collagen threads. Biomed. Mater. 2011, 6, 035008.
- Cross, V.L.; Zheng, Y.; Choi, N.W.; Verbridge, S.S.; Sutermaster, B.A.; Bonassar, L.J.; Fischbach, C.; Stroock, A.D. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 2010, 31, 8596–8607.
- Giraud Guille, M.M.; Helary, C.; Vigier, S.; Nassif, N. Dense fibrillar collagen matrices for tissue repair. Soft Matter 2010, 6, 4963–4967.
- Islam, A.; Chapin, K.; Younesi, M.; Akkus, O. Computer aided biomanufacturing of mechanically robust pure collagen meshes with controlled macroporosity. Biofabrication 2015, 7, 035005.
- Kumar, M.R.; Merschrod, S.E.F.; Poduska, K.M. Correlating mechanical properties with aggregation processes in electrochemically fabricated collagen membranes. Biomacromolecules 2009, 10, 1970–1975.
- Sun, W.; Paulovich, J.; Webster-Wood, V. Tuning the mechanical and geometric properties of electrochemically aligned collagen threads toward applications in biohybrid robotics. J. Biomech. Eng. 2021, 143, 051005.
- Xie, Y.; Chen, J.; Celik, H.; Akkus, O.; King, M.W. Evaluation of an electrochemically aligned collagen yarn for textile scaffold fabrication. Biomed. Mater. 2021, 16, 025001.
- Karami, A.; Tebyanian, H.; Soufdoost, R.S.; Motavallian, E.; Barkhordari, A.; Nourani, M.R. Extraction and characterization of collagen with cost-effective method from human placenta for biomedical applications. World J. Plast. Surg. 2019, 8, 352–358.
- Gao, L.; Wang, Z.; Li, Z.; Zhang, C.; Zhang, D. The characterization of acid and pepsin soluble collagen from ovine bones (Ujumuqin sheep). J. Integr. Agric. 2018, 17, 704–711.
- Salvatore, L.; Gallo, N.; Aiello, D.; Lunetti, P.; Barca, A.; Blasi, L.; Madaghiele, M.; Bettini, S.; Giancane, G.; Hasan, M.; et al. An insight on type I collagen from horse tendon for the manufacture of implantable devices. Int. J. Biol. Macromol. 2020, 154, 291–306.
- Akram, A.N.; Zhang, C. Extraction of collagen-II with pepsin and ultrasound treatment from chicken sternal cartilage; physicochemical and functional properties. Ultrason. Sonochem. 2020, 64, 105053.
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from marine biological sources and medical applications. Chem. Biodivers. 2018, 15, e1700557.
- Liu, S.; Lau, C.-S.; Liang, K.; Wen, F.; Teoh, S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103.
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater 2021, 10, 100098.
- Maher, M.K.; White, J.F.; Glattauer, V.; Yue, Z.; Hughes, T.C.; Ramshaw, J.A.M.; Wallace, G.G. Variation in hydrogel formation and network structure for Telo-, Atelo- and Methacrylated collagens. Polymers 2022, 14, 1775.
- Blidi, O.E.; Omari, N.E.; Balahbib, A.; Ghchime, R.; Menyiy, N.E.; Ibrahimi, A.; Kaddour, K.B.; Bouyahya, A.; Chokairi, O.; Barkiyou, M. Extraction methods, characterization and biomedical applications of collagen: A review. Biointerface Res. Appl. Chem. 2021, 11, 13587–13613.
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed collagen-sources and applications. Molecules 2019, 24, 4031.
- Matinong, A.M.E.; Chisti, Y.; Pickering, K.L.; Haverkamp, R.G. Collagen extraction from animal skin. Biology 2022, 11, 905.
- León-López, A.; Fuentes-Jiménez, L.; Hernández-Fuentes, A.D.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Hydrolysed collagen from sheepskins as a source of functional peptides with antioxidant activity. Int. J. Mol. Sci. 2019, 20, 3931.
- Pan, B.S.; En Chen, H.O.A.; Sung, W.C. Molecular and thermal characteristics of acid-soluble collagen from orbicular batfish: Effects of deep-sea water culturing. Int. J. Food Prop. 2018, 21, 1080–1090.
- Kezwoń, A.; Chromińska, I.; Frączyk, T.; Wojciechowski, K. Effect of enzymatic hydrolysis on surface activity and surface rheology of type I collagen. Colloids Surf. B 2016, 137, 60–69.
- Zhang, G.; Sun, A.; Li, W.; Liu, T.; Su, Z. Mass spectrometric analysis of enzymatic digestion of denatured collagen for identification of collagen type. J. Chromatogr. A 2006, 1114, 274–277.
- Li, Z.; Wang, B.; Chi, C.; Gong, Y.; Luo, H.; Ding, G. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293.
- Baker, H.R.; Merschrod, S.E.F.; Poduska, K.M. Electrochemically controlled growth and positioning of suspended collagen membranes. Langmuir 2008, 24, 2970–2972.
- Webster, V.A.; Hawley, E.L.; Akkus, O.; Chiel, H.J.; Quinn, R.D. Effect of actuating cell source on locomotion of organic living machines with electrocompacted collagen skeleton. Bioinspir. Biomim. 2016, 11, 036012.
- Younesi, M.; Islam, A.; Kishore, V.; Anderson, J.M.; Akkus, O. Tenogenic induction of human MSCs by anisotropically aligned collagen biotextiles. Adv. Funct. Mater. 2014, 24, 5762–5770.
- Zhang, F.; Bambharoliya, T.; Xie, Y.; Liu, L.; Celik, H.; Wang, L.; Akkus, O.; King, M.W. A hybrid vascular graft harnessing the superior mechanical properties of synthetic fibers and the biological performance of collagen filaments. Mater. Sci. Eng. C 2021, 118, 111418.
- Cudjoe, E.; Younesi, M.; Cudjoe, E.; Akkus, O.; Rowan, S.J. Synthesis and fabrication of nanocomposite fibers of collagen-cellulose nanocrystals by coelectrocompaction. Biomacromolecules 2017, 18, 1259–1267.
- Abu-Rub, M.T.; Billiar, K.L.; van Es, M.H.; Knight, A.; Rodriguez, B.J.; Zeugolis, D.I.; McMahon, S.; Windebank, A.J.; Pandit, A. Nano-textured self-assembled aligned collagen hydrogels promote directional neurite guidance and overcome inhibition by myelin associated glycoprotein. Soft Matter 2011, 7, 2770–2781.
- Kang, L.; Liu, X.; Yue, Z.; Chen, Z.; Baker, C.; Winberg, P.C.; Wallace, G.G. Fabrication and in vitro characterization of electrochemically compacted collagen/sulfated xylorhamnoglycuronan matrix for wound healing applications. Polymers 2018, 10, 415.
- Nguyen, T.U.; Bashur, C.A.; Kishore, V. Impact of elastin incorporation into electrochemically aligned collagen fibers on mechanical properties and smooth muscle cell phenotype. Biomed. Mater. 2016, 11, 025008.
- Okamoto, O.; Fujiwara, S. Dermatopontin, a novel player in the biology of the extracellular matrix. Connect Tissue Res. 2006, 47, 177–189.
- Paderi, J.E.; Panitch, A. Design of a synthetic collagen-binding peptidoglycan that modulates collagen fibrillogenesis. Biomacromolecules 2008, 9, 2562–2566.
- Kishore, V.; Paderi, J.E.; Akkus, A.; Smith, K.M.; Balachandran, D.; Beaudoin, S.; Panitch, A.; Akkus, O. Incorporation of a decorin biomimetic enhances the mechanical properties of electrochemically aligned collagen threads. Acta Biomater. 2011, 7, 2428–2436.
- Pergande, M.R.; Cologna, S.M. Isoelectric point separations of peptides and proteins. Proteomes 2017, 5, 4.
- Vindin, H.; Mithieux, S.M.; Weiss, A.S. Elastin architecture. Matrix Biol. 2019, 84, 4–16.
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro- and nanocellulose in polymer composite materials: A Review. Polymers 2021, 13, 231.
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From fundamentals to advanced applications. Front. Chem. 2020, 8, 392.
- Yuan, H.; Chen, L.; Hong, F.F. Homogeneous and efficient production of a bacterial nanocellulose-lactoferrin-collagen composite under an electric field as a matrix to promote wound healing. Biomater. Sci. 2021, 9, 930–941.
- Xu, C.; Zhang Molino, B.; Wang, X.; Cheng, F.; Xu, W.; Molino, P.; Bacher, M.; Su, D.; Rosenau, T.; Willför, S.; et al. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J. Mater. Chem. B 2018, 6, 7066–7075.
- Akbari, M.; Tamayol, A.; Bagherifard, S.; Serex, L.; Mostafalu, P.; Faramarzi, N.; Mohammadi, M.H.; Khademhosseini, A. Textile technologies and tissue engineering: A path toward organ weaving. Adv. Healthc. Mater. 2016, 5, 751–766.
- Kishore, V.; Uquillas, J.A.; Dubikovsky, A.; Alshehabat, M.A.; Snyder, P.W.; Breur, G.J.; Akkus, O. In vivo response to electrochemically aligned collagen bioscaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 400–408.
- Gurkan, U.A.; Cheng, X.; Kishore, V.; Uquillas, J.A.; Akkus, O. Comparison of morphology, orientation, and migration of tendon derived fibroblasts and bone marrow stromal cells on electrochemically aligned collagen constructs. J. Biomed. Mater. Res. A 2010, 94A, 1070–1079.
- Xie, Y.; Zhang, F.; Akkus, O.; King, M.W. A collagen/PLA hybrid scaffold supports tendon-derived cell growth for tendon repair and regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022.
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502.
- Park, H.; Nazhat, S.N.; Rosenzweig, D.H. Mechanical activation drives tenogenic differentiation of human mesenchymal stem cells in aligned dense collagen hydrogels. Biomaterials 2022, 286, 121606.
- McClellan, P.; Ina, J.G.; Knapik, D.M.; Isali, I.; Learn, G.; Valente, A.; Wen, Y.; Wen, R.; Anderson, J.M.; Gillespie, R.J.; et al. Mesenchymal stem cell delivery via topographically tenoinductive collagen biotextile enhances regeneration of segmental tendon defects. Am. J. Sports Med. 2022, 50, 2281–2291.




