
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Grażyna Chwatko | -- | 5310 | 2022-10-20 11:38:15 | | | |
| 2 | Amina Yu | + 7 word(s) | 5317 | 2022-10-21 03:26:15 | | |
Video Upload Options
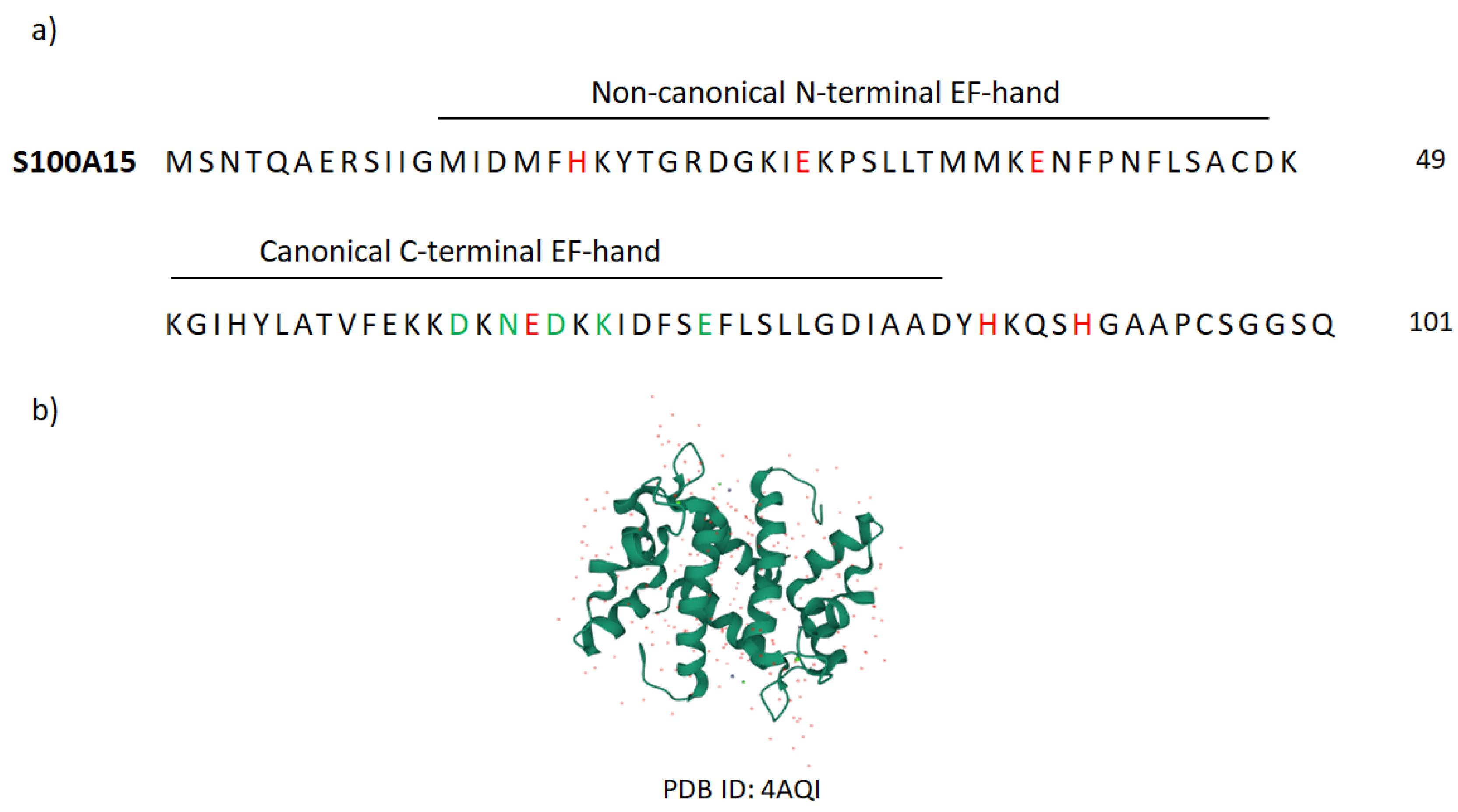
Very well-known AMP-family members are S100 proteins that constitute the largest, multigenic, and calcium-binding protein family in vertebrates. Over 20 types of these proteins have been identified, of which 13 are expressed in the normal or diseased human epidermis. The name of the S100 proteins is due to their biochemical characteristics, namely, they are 100% soluble in saturated ammonium sulfate at neutral pH. S100 proteins are small, acidic proteins with a molecular weight of 9–13 kDa. They are produced as monomers, but exist in cells as anti-parallel homo- and heterodimers, in which monomers are held together by non-covalent bonds and are oriented by a two-fold axis of rotation. Dimers can further associate to form higher-order multimers. Each S100 monomer consists of two helix–loop–helix structural motifs that are Ca2+-binding domains termed EF-hands.
1. S100 Proteins as Biomarkers and Therapeutic Targets
2. Koebnerisin (S100A15)
3. The Function of Koebnerisin in Other Immune-Mediated Inflammatory Diseases
References
- Benoit, S.; Toksoy, A.; Ahlmann, M.; Schmidt, M.; Sunderkötter, C.; Foell, D.; Pasparakis, M.; Roth, J.; Goebeler, M. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br. J. Dermatol. 2006, 155, 62–66.
- Foell, D.; Seeliger, S.; Vogl, T.; Koch, H.G.; Maschek, H.; Harms, E.; Sorg, C.; Roth, J. Expression of S100A12 (EN-RAGE) in cystic fibrosis. Thorax 2003, 58, 613–617.
- Turnier, J.L.; Fall, N.; Thornton, S.; Witte, D.; Bennett, M.R.; Appenzeller, S.; Klein-Gitelman, M.S.; Grom, A.A.; Brunner, H.I. Urine S100 proteins as potential biomarkers of lupus nephritis activity. Arthritis Res. Ther. 2017, 19, 242.
- Zhang, J.; Zhang, K.; Jiang, X.; Zhang, J. S100A6 as a Potential Serum Prognostic Biomarker and Therapeutic Target in Gastric Cancer. Dig. Dis. Sci. 2014, 59, 2136–2144.
- Wilsmann-Theis, D.; Wagenpfeil, J.; Holzinger, D.; Roth, J.; Koch, S.; Schnautz, S.; Bieber, T.; Wenzel, J. Among the S100 proteins, S100A12 is the most significant marker for psoriasis disease activity. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1165–1170.
- Zibert, J.R.; Skov, L.; Thyssen, J.P.; Jacobsen, G.K.; Grigorian, M. Significance of the S100A4 protein in psoriasis. J. Investig. Dermatol. 2010, 130, 150–160.
- Hamza, A.; Hassan, E.; Donia, H.; Maamon, Y. Serum calprotectin as a predictive biomarker in the treatment of psoriasis vulgaris with methotrexate. J. Egypt. Women’s Dermatol. Soc. 2019, 16, 112.
- Duvetorp, A.; Söderman, J.; Assarsson, M.; Skarstedt, M.; Svensson, Å.; Seifert, O. Observational study on swedish plaque psoriasis patients receiving narrowband-UVB treatment show decreased S100A8/A9 protein and gene expression levels in lesional psoriasis skin but no effect on S100A8/A9 protein levels in serum. PLoS ONE 2019, 14, e0213344.
- Wolf, R.; Ruzicka, T.; Yuspa, S.H. Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: Highly homologous but distinct in regulation and function. Amino Acids 2011, 41, 789–796.
- Wolf, R.; Mascia, F.; Dharamsi, A.; Howard, O.M.Z.; Cataisson, C.; Bliskovski, V.; Winston, J.; Feigenbaum, L.; Lichti, U.; Ruzicka, T.; et al. Gene from a psoriasis susceptibility locus primes the skin for inflammation. Sci. Transl. Med. 2010, 2, 61ra90.
- Batycka-Baran, A.; Hattinger, E.; Zwicker, S.; Summer, B.; Zack Howard, O.M.; Thomas, P.; Szepietowski, J.C.; Ruzicka, T.; Prinz, J.C.; Wolf, R. Leukocyte-derived koebnerisin (S100A15) and psoriasin (S100A7) are systemic mediators of inflammation in psoriasis. J. Dermatol. Sci. 2015, 79, 214–221.
- Wolf, R.; Lewerenz, V.; Büchau, A.S.; Walz, M.; Ruzicka, T. Human S100A15 splice variants are differentially expressed in inflammatory skin diseases and regulated through Th1 cytokines and calcium. Exp. Dermatol. 2007, 16, 685–691.
- Salem, S.A.M.; Harvy, M.; Abdelaal, W.; El-Hagry, O.O.; El-Khateeb, E.A.; Emam, H.M.E.S.; El Nemr, R. Study of serum levels and skin expression of S100B protein inpsoriasis. An. Bras. Dermatol. 2017, 92, 323.
- Chamcheu, J.C.; Pal, H.C.; Siddiqui, I.A.; Adhami, V.M.; Ayehunie, S.; Boylan, B.T.; Noubissi, F.K.; Khan, N.; Syed, D.N.; Elmets, C.A.; et al. Prodifferentiation, anti-inflammatory and antiproliferative effects of delphinidin, a dietary anthocyanidin, in a full-thickness three-dimensional reconstituted human skin model of psoriasis. Skin Pharmacol. Physiol. 2015, 28, 177–188.
- Gambichler, T.; Bechara, F.G.; Scola, N.; Rotterdam, S.; Altmeyer, P.; Skrygan, M. Serum levels of antimicrobial peptides and proteins do not correlate with psoriasis severity and are increased after treatment with fumaric acid esters. Arch. Dermatol. Res. 2012, 304, 471–474.
- Anderson, K.S.; Wong, J.; Polyak, K.; Aronzon, D.; Enerbäck, C. Detection of psoriasin/S100A7 in the sera of patients with psoriasis. Br. J. Dermatol. 2009, 160, 325–332.
- Salama, R.H.M.; Al-Shobaili, H.A.; Al Robaee, A.A.; Alzolibani, A.A. Psoriasin: A novel marker linked obesity with psoriasis. Dis. Markers 2013, 34, 33–39.
- Oesterle, A.; Hofmann Bowman, M.A. S100A12 and the S100/Calgranulins: Emerging Biomarkers for Atherosclerosis and Possibly Therapeutic Targets. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2496–2507.
- Shiotsu, Y.; Mori, Y.; Nishimura, M.; Sakoda, C.; Tokoro, T.; Hatta, T.; Maki, N.; Iida, K.; Iwamoto, N.; Ono, T.; et al. Plasma S100A12 Level Is Associated with Cardiovascular Disease in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 718.
- Wittkowski, H.; Frosch, M.; Wulffraat, N.; Goldbach-Mansky, R.; Kallinich, T.; Kuemmerle-Deschner, J.; Frühwald, M.C.; Dassmann, S.; Pham, T.-H.; Roth, J.; et al. S100A12 is a novel molecular marker differentiating systemic-onset juvenile idiopathic arthritis from other causes of fever of unknown origin. Arthritis Rheum. 2008, 58, 3924–3931.
- Foell, D.; Kane, D.; Bresnihan, B.; Vogl, T.; Nacken, W.; Sorg, C.; FitzGerald, O.; Roth, J. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology 2003, 42, 1383–1389.
- Chen, H.I.; Fernig, D.G.; Rudland, P.S.; Sparks, A.; Wilkinson, M.C.; Barraclough, R. Binding to Intracellular Targets of the Metastasis-Inducing Protein, S100A4 (p9Ka). Biochem. Biophys. Res. Commun. 2001, 286, 1212–1217.
- Chow, K.H.; Park, H.J.; George, J.; Yamamoto, K.; Gallup, A.D.; Graber, J.H.; Chen, Y.; Jiang, W.; Steindler, D.A.; Neilson, E.G.; et al. S100A4 is a biomarker and regulator of glioma stem cells that is critical for mesenchymal transition in glioblastoma. Cancer Res. 2017, 77, 5360–5373.
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 proteins in health and disease. Biochim. Biophys. Acta-Mol. Cell Res. 2020, 1867, 118677.
- Morizane, S.; Gallo, R.L. Antimicrobial peptides in the pathogenesis of psoriasis. J. Dermatol. 2012, 39, 225–230.
- Gauglitz, G.G.; Bureik, D.; Zwicker, S.; Ruzicka, T.; Wolf, R. The antimicrobial peptides psoriasin (s100a7) and koebnerisin (S100A15) suppress extracellular matrix production and proliferation of human fibroblasts. Skin Pharmacol. Physiol. 2015, 28, 115–123.
- Gläser, R.; Meyer-Hoffert, U.; Harder, J.; Cordes, J.; Wittersheim, M.; Kobliakova, J.; Fölster-Holst, R.; Proksch, E.; Schröder, J.M.; Schwarz, T. The Antimicrobial Protein Psoriasin (S100A7) Is Upregulated in Atopic Dermatitis and after Experimental Skin Barrier Disruption. J. Investig. Dermatol. 2009, 129, 641–649.
- Qin, W.; Ho, L.; Wang, J.; Peskind, E.; Pasinetti, G.M. S100A7, a Novel Alzheimer’s Disease Biomarker with Non-Amyloidogenic α-Secretase Activity Acts via Selective Promotion of ADAM-10. PLoS ONE 2009, 4, e4183.
- LinMei; XiaBairong; QinLing; ChenHong; LouGe S100A7 Regulates Ovarian Cancer Cell Metastasis and Chemoresistance Through MAPK Signaling and Is Targeted by miR-330-5p. DNA Cell Biol. 2018, 37, 491–500.
- Tian, T.; Li, X.; Hua, Z.; Ma, J.; Wu, X.; Liu, Z.; Chen, H.; Cui, Z.; Tian, T.; Li, X.; et al. S100A7 promotes the migration, invasion and metastasis of human cervical cancer cells through epithelial–mesenchymal transition. Oncotarget 2017, 8, 24964–24977.
- Padilla, L.; Dakhel, S.; Adan, J.; Masa, M.; Martinez, J.M.; Roque, L.; Coll, T.; Hervas, R.; Calvis, C.; Llinas, L.; et al. S100A7: From mechanism to cancer therapy. Oncogene 2017, 36, 6749–6761.
- Cook, M.G.; Massi, D.; Szumera-Ciećkiewicz, A.; Van den Oord, J.; Blokx, W.; van Kempen, L.C.; Balamurugan, T.; Bosisio, F.; Koljenović, S.; Portelli, F.; et al. An updated European Organisation for Research and Treatment of Cancer (EORTC) protocol for pathological evaluation of sentinel lymph nodes for melanoma. Eur. J. Cancer 2019, 114, 1–7.
- Xiong, T.; Pan, F.; Li, D. Expression and clinical significance of S100 family genes in patients with melanoma. Melanoma Res. 2019, 29, 23.
- Jury, C.S.; Mcallister, E.J.; Mackie, R.M. Rising levels of serum S100 protein precede other evidence of disease progression in patients with malignant melanoma. Br. J. Dermatol. 2000, 143, 269–274.
- Sedaghat, F.; Notopoulos, A. S100 protein family and its application in clinical practice. Hippokratia 2008, 12, 198.
- Faries, M.B.; Gupta, R.K.; Ye, X.; Lee, C.; Yee, R.; Leopoldo, Z.; Essner, R.; Foshag, L.J.; Elashoff, D.; Morton, D.L. A Comparison of 3 Tumor Markers (MIA, TA90IC, S100B) in Stage III Melanoma Patients. Cancer Investig. 2009, 25, 285–293.
- Strobel, K.; Skalsky, J.; Kalff, V.; Baumann, K.; Seifert, B.; Joller-Jemelka, H.; Dummer, R.; Steinert, H.C. Tumour assessment in advanced melanoma: Value of FDG-PET/CT in patients with elevated serum S-100B. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1366–1375.
- Ionita, M.G.; Vink, A.; Dijke, I.E.; Laman, J.D.; Peeters, W.; Van Der Kraak, P.H.; Moll, F.L.; De Vries, J.P.P.M.; Pasterkamp, G.; De Kleijn, D.P.V. High levels of myeloid-related protein 14 in human atherosclerotic plaques correlate with the characteristics of rupture-prone lesions. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1220–1227.
- Mortensen, O.H.; Nielsen, A.R.; Erikstrup, C.; Plomgaard, P.; Fischer, C.P.; Krogh-Madsen, R.; Lindegaard, B.; Petersen, A.M.; Taudorf, S.; Pedersen, B.K. Calprotectin—A Novel Marker of Obesity. PLoS ONE 2009, 4, e7419.
- De Jong, H.K.; Achouiti, A.; Koh, G.C.K.W.; Parry, C.M.; Baker, S.; Faiz, M.A.; van Dissel, J.T.; Vollaard, A.M.; van Leeuwen, E.M.M.; Roelofs, J.J.T.H.; et al. Expression and Function of S100A8/A9 (Calprotectin) in Human Typhoid Fever and the Murine Salmonella Model. PLoS Negl. Trop. Dis. 2015, 9, e0003663.
- Konikoff, M.R.; Denson, L.A. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 524–534.
- Gebhardt, C.; Németh, J.; Angel, P.; Hess, J. S100A8 and S100A9 in inflammation and cancer. Biochem. Pharmacol. 2006, 72, 1622–1631.
- Prieto, D.; Sotelo, N.; Seija, N.; Sernbo, S.; Abreu, C.; Durán, R.; Gil, M.; Sicco, E.; Irigoin, V.; Oliver, C.; et al. S100-A9 protein in exosomes from chronic lymphocytic leukemia cells promotes NF-κB activity during disease progression. Blood 2017, 130, 777–788.
- Gunaldi, M.; Okuturlar, Y.; Gedikbasi, A.; Akarsu, C.; Karabulut, M.; Kural, A. Diagnostic importance of S100A9 and S100A12 in breast cancer. Biomed. Pharmacother. 2015, 76, 52–56.
- Topuz, M.F.; Binnetoglu, A.; Yumusakhuylu, A.C.; Sarı, M.; Baglam, T.; Gerin, F. Circulating calprotectin as a biomarker of laryngeal carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 2499–2504.
- Huang, C.H.; Kuo, C.J.; Liang, S.S.; Chi, S.W.; Hsi, E.; Chen, C.C.; Lee, K.T.; Chiou, S.H. Onco-proteogenomics identifies urinary S100A9 and GRN as potential combinatorial biomarkers for early diagnosis of hepatocellular carcinoma. BBA Clin. 2015, 3, 205–213.
- Yasar, O.; Akcay, T.; Obek, C.; Turegun, F.A. Significance of S100A8, S100A9 and calprotectin levels in bladder cancer. Scand. J. Clin. Lab. Investig. 2017, 77, 437–441.
- Bresnick, A.R. S100 proteins as therapeutic targets. Biophys. Rev. 2018, 10, 1617–1629.
- Chromy, B.A.; Gonzales, A.D.; Perkins, J.; Choi, M.W.; Corzett, M.H.; Chang, B.C.; Corzett, C.H.; McCutchen-Maloney, S.L. Proteomic analysis of human serum by two-dimensional differential gel electrophoresis after depletion of high-abundant proteins. J. Proteome Res. 2004, 3, 1120–1127.
- Allgöwer, C.; Kretz, A.L.; von Karstedt, S.; Wittau, M.; Henne-Bruns, D.; Lemke, J. Friend or Foe: S100 Proteins in Cancer. Cancers 2020, 12, 2037.
- MacK, G.S.; Marshall, A. Lost in migration. Nat. Biotechnol. 2010, 28, 214–229.
- Okada, M.; Tokumitsu, H.; Kubota, Y.; Kobayashi, R. Interaction of S100 proteins with the antiallergic drugs, olopatadine, amlexanox, and cromolyn: Identification of putative drug binding sites on S100A1 protein. Biochem. Biophys. Res. Commun. 2002, 292, 1023–1030.
- Shishibori, T.; Oyama, Y.; Matsushita, O.; Yamashita, K.; Furuichi, H.; Okabe, A.; Maeta, H.; Hata, Y.; Kobayashi, R. Three distinct anti-allergic drugs, amlexanox, cromolyn and tranilast, bind to S100A12 and S100A13 of the S100 protein family. Biochem. J. 1999, 338, 583.
- Chiou, J.W.; Fu, B.; Chou, R.H.; Yu, C. Blocking the Interactions between Calcium-Bound S100A12 Protein and the V Domain of RAGE Using Tranilast. PLoS ONE 2016, 11, e0162000.
- Cavalier, M.C.; Pierce, A.D.; Wilder, P.T.; Alasady, M.J.; Hartman, K.G.; Neau, D.B.; Foley, T.L.; Jadhav, A.; Maloney, D.J.; Simeonov, A.; et al. Covalent Small Molecule Inhibitors of Ca2+-Bound S100B. Biochemistry 2014, 53, 6628.
- Dulyaninova, N.G.; Hite, K.M.; Zencheck, W.D.; Scudiero, D.A.; Almo, S.C.; Shoemaker, R.H.; Bresnick, A.R. Cysteine 81 Is Critical for the Interaction of S100A4 and Myosin-IIA. Biochemistry 2011, 50, 7218.
- Vogt, A.; McDonald, P.R.; Tamewitz, A.; Sikorski, R.P.; Wipf, P.; Skoko, J.J.; Lazo, J.S. A cell-active inhibitor of mitogen-activated protein kinase phosphatases restores paclitaxel-induced apoptosis in dexamethasone-protected cancer cells. Mol. Cancer Ther. 2008, 7, 330–340.
- Brisson, M.; Nguyen, T.; Wipf, P.; Joo, B.; Day, B.W.; Skoko, J.S.; Schreiber, E.M.; Foster, C.; Bansal, P.; Lazo, J.S. Redox regulation of Cdc25B by cell-active quinolinediones. Mol. Pharmacol. 2005, 68, 1810–1820.
- Sack, U.; Walther, W.; Scudiero, D.; Selby, M.; Aumann, J.; Lemos, C.; Fichtner, I.; Schlag, P.M.; Shoemaker, R.H.; Stein, U. S100A4-induced cell motility and metastasis is restricted by the Wnt/β-catenin pathway inhibitor calcimycin in colon cancer cells. Mol. Biol. Cell 2011, 22, 3344.
- Dahlmann, M.; Kobelt, D.; Walther, W.; Mudduluru, G.; Stein, U. S100A4 in Cancer Metastasis: Wnt Signaling-Driven Interventions for Metastasis Restriction. Cancers 2016, 8, 59.
- Stein, U.; Arlt, F.; Smith, J.; Sack, U.; Herrmann, P.; Walther, W.; Lemm, M.; Fichtner, I.; Shoemaker, R.H.; Schlag, P.M. Intervening in β-Catenin Signaling by Sulindac Inhibits S100A4-Dependent Colon Cancer Metastasis. Neoplasia 2011, 13, 131.
- Brufsky, A. Trastuzumab-based therapy for patients with HER2-positive breast cancer: From early scientific development to foundation of care. Am. J. Clin. Oncol. Cancer Clin. Trials 2010, 33, 186–195.
- König, M.; Rharbaoui, F.; Aigner, S.; Dälken, B.; Schüttrumpf, J. Tregalizumab–A Monoclonal Antibody to Target Regulatory T Cells. Front. Immunol. 2016, 7, 11.
- Saif, M.W. Anti-VEGF agents in metastatic colorectal cancer (mCRC): Are they all alike? Cancer Manag. Res. 2013, 5, 103–115.
- Hofmann, K.; Clauder, A.K.; Manz, R.A. Targeting B cells and plasma cells in autoimmune diseases. Front. Immunol. 2018, 9, 835.
- Björk, P.; Björk, A.; Vogl, T.; Stenström, M.; Liberg, D.; Olsson, A.; Roth, J.; Ivars, F.; Leanderson, T. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009, 7, 0800–0812.
- Austermann, J.; Zenker, S.; Roth, J. S100-alarmins: Potential therapeutic targets for arthritis. Expert Opin. Ther. Targets 2017, 21, 739–751.
- Sekimoto, R.; Fukuda, S.; Maeda, N.; Tsushima, Y.; Matsuda, K.; Mori, T.; Nakatsuji, H.; Nishizawa, H.; Kishida, K.; Kikuta, J.; et al. Visualized macrophage dynamics and significance of S100A8 in obese fat. Proc. Natl. Acad. Sci. USA 2015, 112, E2058–E2066.
- Dou, C.; Liu, Z.; Xu, M.; Jia, Y.; Wang, Y.; Li, Q.; Yang, W.; Zheng, X.; Tu, K.; Liu, Q. miR-187-3p inhibits the metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting S100A4. Cancer Lett. 2016, 381, 380–390.
- Yang, D.; Du, G.; Xu, A.; Xi, X.; Li, D. Expression of miR-149-3p inhibits proliferation, migration, and invasion of bladder cancer by targeting S100A4. Am. J. Cancer Res. 2017, 7, 2209.
- Fan, F.; Lu, J.; Yu, W.; Zhang, Y.; Xu, S.; Pang, L.; Zhu, B. MicroRNA-26b-5p regulates cell proliferation, invasion and metastasis in human intrahepatic cholangiocarcinoma by targeting S100A7. Oncol. Lett. 2018, 15, 386.
- Guo, Y.; Fu, W.; Chen, H.; Shang, C.; Zhong, M. miR-24 functions as a tumor suppressor in Hep2 laryngeal carcinoma cells partly through down-regulation of the S100A8 protein. Oncol. Rep. 2012, 27, 1097.
- Wolf, R.; Howard, O.M.Z.; Dong, H.-F.; Voscopoulos, C.; Boeshans, K.; Winston, J.; Divi, R.; Gunsior, M.; Goldsmith, P.; Ahvazi, B.; et al. Chemotactic Activity of S100A7 (Psoriasin) Is Mediated by the Receptor for Advanced Glycation End Products and Potentiates Inflammation with Highly Homologous but Functionally Distinct S100A15. J. Immunol. 2008, 181, 1499–1506.
- Wolf, R.; Mirmohammadsadegh, A.; Walz, M.; Lysa, B.; Tartler, U.; Remus, R.; Hengge, U.; Michel, G.; Ruzicka, T. Molecular cloning and characterization of alternatively spliced mRNA isoforms from psoriatic skin encoding a novel member of the S100 family. FASEB J. 2003, 17, 1–21.
- S100A7A-Protein S100-A7A-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q86SG5/entry (accessed on 5 September 2022).
- Ji, Y.Z.; Liu, S.R. Koebner phenomenon leading to the formation of new psoriatic lesions: Evidences and mechanisms. Biosci. Rep. 2019, 39, 1–12.
- Murray, J.I.; Tonkin, M.L.; Whiting, A.L.; Peng, F.; Farnell, B.; Cullen, J.T.; Hof, F.; Boulanger, M.J. Structural characterization of S100A15 reveals a novel zinc coordination site among S100 proteins and altered surface chemistry with functional implications for receptor binding. BMC Struct. Biol. 2012, 12, 16.
- Chessa, C.; Bodet, C.; Jousselin, C.; Wehbe, M.; Lévêque, N.; Garcia, M. Antiviral and Immunomodulatory Properties of Antimicrobial Peptides Produced by Human Keratinocytes. Front. Microbiol. 2020, 11, 1155.
- Büchau, A.S.; Hassan, M.; Kukova, G.; Lewerenz, V.; Kellermann, S.; Würthner, J.U.; Wolf, R.; Walz, M.; Gallo, R.L.; Ruzicka, T. S100A15, an antimicrobial protein of the skin: Regulation by E. coli through toll-like receptor 4. J. Investig. Dermatol. 2007, 127, 2596–2604.
- Hegyi, Z.; Zwicker, S.; Bureik, D.; Peric, M.; Koglin, S.; Batycka-Baran, A.; Prinz, J.C.; Ruzicka, T.; Schauber, J.; Wolf, R. Vitamin D analog calcipotriol suppresses the Th17 cytokine-induced proinflammatory S100 alarmins psoriasin (S100A7) and koebnerisin (S100A15) in psoriasis. J. Investig. Dermatol. 2012, 132, 1416–1424.
- Batycka-Baran, A.; Maj, J.; Wolf, R.; Szepietowski, J.C. The new insight into the role of antimicrobial proteins-alarmins in the immunopathogenesis of psoriasis. J. Immunol. Res. 2014, 2014, 628289.
- Awad, S.M.; Attallah, D.A.; Salama, R.H.; Mahran, A.M.; Abu El-Hamed, E. Serum levels of psoriasin (S100A7) and koebnerisin (S100A15) as potential markers of atherosclerosis in patients with psoriasis. Clin. Exp. Dermatol. 2018, 43, 262–267.
- Rodríguez-Cerdeira, C.; Cordeiro-Rodríguez, M.; Carnero-Gregorio, M.; López-Barcenas, A.; Martínez-Herrera, E.; Fabbrocini, G.; Sinani, A.; Arenas-Guzmán, R.; González-Cespón, J.L. Biomarkers of Inflammation in Obesity-Psoriatic Patients. Mediat. Inflamm. 2019, 2019, 7353420.
- Batycka-Baran, A.; Hattinger, E.; Marchenkov, A.; Koziol, M.; Bieniek, A.; Szepietowski, J.; Ruzicka, T.; Wolf, R. Koebnerisin (S100A15): A novel player in the pathogenesis of rosacea. J. Am. Acad. Dermatol. 2019, 80, 1753–1755.
- Batycka-Baran, A.; Koziol-Galczynska, M.; Bieniek, A.; Wolf, R.; Łaczmański; Szepietowski, J. C. Expression of koebnerisin (S100A15) and calgranulin A (S100A8) in lesional and perilesional skin in patients suffering from hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e402–e404.
- Zouboulis, C.C.; Nogueira da Costa, A.; Makrantonaki, E.; Hou, X.X.; Almansouri, D.; Dudley, J.T.; Edwards, H.; Readhead, B.; Balthasar, O.; Jemec, G.B.E.; et al. Alterations in innate immunity and epithelial cell differentiation are the molecular pillars of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 846–861.
- Batycka-Baran, A.; Matusiak, Ł.; Nowicka-suszko, D.; Szepietowski, J.C.; Baran, W. Increased serum levels of s100a4 and s100a15 in individuals suffering from hidradenitis suppurativa. J. Clin. Med. 2021, 10, 5320.
- Hattinger, E.; Zwicker, S.; Ruzicka, T.; Yuspa, S.H.; Wolf, R. Opposing functions of Psoriasin (S100A7) and Koebnerisin (S100A15) in epithelial carcinogenesis. Curr. Opin. Pharmacol. 2013, 13, 588.
- Yao, R.; Lopez-Beltran, A.; Maclennan, G.T.; Montironi, R.; Eble, J.N.; Cheng, L. Expression of S100 Protein Family Members in the Pathogenesis of Bladder Tumors. Anticancer Res. 2007, 27, 3051–3058.
- Briso, E.M.; Guinea-Viniegra, J.; Bakiri, L.; Rogon, Z.; Petzelbauer, P.; Eils, R.; Wolf, R.; Rincón, M.; Angel, P.; Wagner, E.F. Inflammation-mediated skin tumorigenesis induced by epidermal c-Fos. Genes Dev. 2013, 27, 1959.
- Chen, Y.C.; Lin, M.C.; Hsiao, C.C.; Zheng, Y.X.; Chen, K.D.; Sung, M.T.; Chen, C.J.; Wang, T.Y.; Lin, Y.Y.; Chang, H.C.; et al. Increased S100A15 expression and decreased DNA methylation of its gene promoter are involved in high metastasis potential and poor outcome of lung adenocarcinoma. Oncotarget 2017, 8, 45710–45724.
- Wolf, R.; Voscopoulos, C.; Winston, J.; Dharamsi, A.; Goldsmith, P.; Gunsior, M.; Vonderhaar, B.K.; Olson, M.; Watson, P.H.; Yuspa, S.H. Highly homologous hS100A15 and hS100A7 proteins are distinctly expressed in normal breast tissue and breast cancer. Cancer Lett. 2009, 277, 101.




