| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David Ross Fraser | + 3606 word(s) | 3606 | 2020-11-11 10:53:37 | | | |
| 2 | Rita Xu | -1247 word(s) | 2359 | 2020-11-17 05:03:05 | | |
Video Upload Options
Vitamin D, unlike the micronutrients, vitamins A, E, and K, is largely obtained not from food but by the action of solar ultraviolet light on its precursor, 7-dehydrocholesterol, in skin. With the decline in UV light intensity in winter, most skin production of vitamin D occurs in summer. Since no defined storage organ or tissue has been found for vitamin D, it has been assumed that an adequate vitamin D status in winter can only be maintained by oral supplementation. Skeletal muscle cells have now been shown to provide an extravascular conservation site for 25-hydroxyvitamin D, which cycles between blood and muscle accounting for its long residence time in blood. In winter, this conservation mechanism appears to be upregulated by parathyroid hormone; a process that appears to have evolved to maintain adequate vitamin D status when vitamin D supply falls.
1. Characterization of Vitamin D Status
The concentration in blood serum or plasma of 25-hydroxyvitamin D [25(OH)D], which is the most plentiful vitamin D metabolite, has become established as the definitive indicator of vitamin D status [1][2]. When the concentration falls below a generally agreed level, (usually 50 nmol/L), vitamin D status is said to be insufficient or deficient in the same way that status is defined for other small molecules derived from the environment, such as vitamins A, E, and K. However, there are three substantial differences between this measurement of vitamin D status and measurements of these fat-soluble micronutrients. The first is that 25(OH)D concentration in blood varies with season. Since it is derived from vitamin D produced in skin by the action of solar ultraviolet radiation on 7-dehydrocholesterol, the 25(OH)D levels rise during the months of summer and fall during winter, particularly in those people who live far from the equator [3][4]. In temperate geographical regions in winter, the intensity of solar UV light is very low in the vitamin D-producing wavelength range of 290–320 nm [5][6]. Are the lower values of 25(OH)D concentration in winter really an indication of deficiency or insufficiency of vitamin D, if this is a universal feature of populations?
The second difference with vitamins A, E, and K is that there is no apparent storage organ or tissue for vitamin D or 25(OH)D. Although vitamin D is found in adipose tissue, suggesting that this is a storage site [7][8], it can only be released when stored fatty acids are mobilized to supply energy [9][10][11]. Thus, sequestered vitamin D in adipocytes should not be regarded as a functional store, ready to be transported to the liver and converted to 25(OH)D, whenever circulating levels of this metabolite decline.
The third unique feature is that 25(OH)D has a very long residence time in blood. The half-life is very variable between 15 and 50 days [12] with a mean value in a recent study of 89 days [13]. In contrast, other hormonal steroids in blood, including the vitamin D hormone, 1,25-dihydroxyvitamin D (1,25(OH)2D), are cleared in minutes or hours after entering the circulation [14][15]. An explanation for this long residence time of 25(OH)D is not readily apparent, particularly because the vitamin D binding protein (DBP) in blood, to which 25(OH)D is tightly bound, has a comparatively short half-life of only 1–3 days and is continuously being replenished from synthesis and secretion by the liver [16][17][18]. Therefore, for 25(OH)D to be retained in blood for such a long time, it either has to transfer from one DBP molecule to another in the circulation or else it repeatedly passes to and from some extravascular site, binding to a new DBP molecule with each cycle. The DBP concentration in blood is in vast excess to that of 25(OH)D with only 1%–5% of the protein molecules having a 25(OH)D molecule bound to the single, high affinity, specific binding site [19].
2. Evidence for Conservation of 25(OH)D
The discovery that maternal 25(OH)D in rats was transported across the placenta and accumulated in the skeletal muscle of fetuses suggested that skeletal muscle might have a functional role in conserving this vitamin D metabolite [20]. Although the concentration of 25(OH)D in the muscle of sheep and cattle is only about 0.1–0.3 µg/100 g [21], muscle represents 30%–38% of body mass in humans [22] and, thus, total 25(OH)D in total skeletal muscle could be comparable to that in the circulation (Table 1).
Table 1. Comparison of total body 25(OH)D in blood plasma and skeletal muscle
|
|
Blood Plasma Volume (L) |
Muscle Mass (kg) |
|
Total for 70 kg human |
2.7–4.3 L [23] |
21–26.6 kg ## [21] |
|
25(OH)D concentration |
20 µg/L ### |
1–3 µg/kg #### [22] |
|
Total body 25(OH)D |
54–86 µg |
21–80 µg |
## Assuming body mass of 70 kg, ### Minimum adequate status 25(OH)D concentration, #### Assuming human muscle has similar 25(OH)D concentrations to sheep and cattle.
In addition, studies in adolescent children [24] found that plasma 25(OH)D concentration was positively correlated with total body lean mass in which the main component is skeletal muscle. Furthermore, there are now several published findings of a positive relationship between the intensity of physical exercise and the concentration of 25(OH)D in blood, e.g., [25][26], particularly in winter when there would be little exposure to solar UV light during outdoor exercise [24]. Muscle biopsies from sheep grazing outdoors in winter showed a significantly higher concentration of 25(OH)D than biopsies from sheep outdoors at the end of summer (Table 2).
Table 2. Plasma and muscle concentrations of 25(OH)D in outdoor grazing sheep at latitude 33.9° S. Mean values ± Standard Error of the Mean (SEM)
|
Season |
Plasma 25(OH)D3 |
Muscle 25(OH)D |
|
End of summer values (n = 5) |
10.67 ± 1.65 ng/mL |
0.84 ± 0.18 µg/100 g tissue |
|
End of winter values (n = 5) |
5.36 ± 0.71 ng/mL * |
1.82 ± 0.39 µg/100 g tissue * |
* Winter values are significantly different from summer values by the t-test p < 0.05.
3. Skeletal Muscle Cell Uptake of DBP
The key discovery that pointed to a role for muscle in maintaining vitamin D status came from studying the properties of muscle cells in vitro [27]. The cell membrane was found to contain the proteins megalin and cubilin. These proteins, like those in the renal tubule cells [28][29] and in hepatic stellate cells [30], transport DBP from the extracellular fluid into the cell cytoplasm. The vitamin D binding protein has two specific, high-affinity binding sites. One is for vitamin D and its metabolites with the highest affinity being for 25(OH)D (Kd < 1 nmol/L). The other binding site is specific for filamentous actin [31][32]. A commonly held theory postulates that the actin-binding site of DBP functions to bind actin if released into blood from damaged cells and, thus, protects against intravascular coagulation [33][34]. Yet it has been known for over 30 years that DBP becomes bound to actin in skeletal muscle [35]. Some of the internalized DBP could be bound to actin in actomyosin, via its specific actin-binding site, but much of the remainder is bound to actin dispersed throughout the cytoplasm. Both megalin and DBP have also been found in human muscle biopsies [36].
The role attributed to megalin/cubilin in renal proximal tubule cells is the recovery of DBP that leaks into the glomerular filtrate. This process is credited with conserving 25(OH)D even though only 1%–5% of the retrieved DBP molecules would be transporting 25(OH)D [19]. The observation that genetically modified global megalin-knockout mice become vitamin D deficient has, therefore, been interpreted as evidence that renal megalin uptake of DBP prevents loss of DBP-bound 25(OH)D in urine [28]. A more plausible explanation is that muscle conservation of 25(OH)D is completely abolished in megalin-knockout mice so that the residence time of 25(OH)D in blood would be no greater than that of DBP itself. The main site of DBP clearance from blood has also been attributed to the kidney [32]. Yet, the uptake and proteolysis of DBP in the much larger mass of total body skeletal muscle would appear to be a more likely explanation for its short residence time in the circulation.
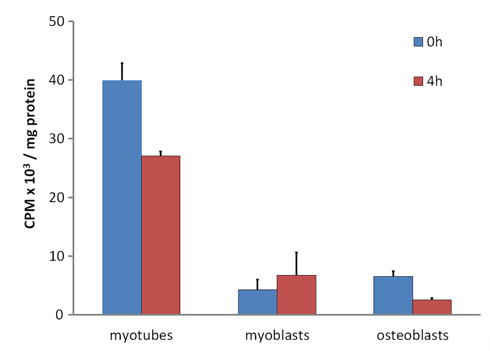
In vivo experiments with 131I-labelled DBP in rabbits revealed that DBP had a short residence time in skeletal muscle and soon underwent proteolysis [16]. DBP is a member of the albuminoid family of proteins. It is synthesized and secreted by the liver with similar characteristics to blood albumin [32]. Because albumin in the extracellular fluid in skeletal muscle is only about one-third the concentration in plasma [37], the extracellular DBP concentration will likewise be decreased to about 1.5 to 2 µmol/L when compared to the blood concentration of 5–6 µmol/L [38]. Thus, much of the 25(OH)D in the extracellular fluid will have dissociated from the low concentration of DBP and would be able to diffuse into muscle cells where it would bind to the internalized DBP in the cell cytoplasm. In comparison with control cells such as osteoblasts, 25(OH)D, is retained within myotubes (the differentiated cell type derived from myoblasts in culture, equivalent to myocytes in vivo) for a considerably longer time (Figure 1). When cytoplasmic DBP undergoes proteolysis in vivo, the bound 25(OH)D is released and would diffuse from the myocyte and return to the circulation, once again being bound to the plentiful DBP. This repeated uptake and release of 25(OH)D by the total mass of skeletal muscle cells would account for the apparent long residence time of 25(OH)D in blood plasma.
Figure 1. (Data reformatted from Reference [27]). Retention of tritiated-25(OH)D3 in C2 myotubes, C2 myoblasts and MG63 osteoblasts. Cells were incubated for 16 h with [26,27-3H]25-hydroxyvitamin D3 (Perkin Elmer, Waltham, MA, USA) in Dulbecco's Modified Eagle’s Medium (DMEM) supplemented with serum replacement (Sigma-Aldrich, St. Louis, MO, USA) followed by 3x washes with ice cold Phosphate Buffered Saline (PBS). At this point, 0 h or 4 h later, cells were harvested, lysed, and assayed for protein by the bicinchoninic acid assay (Thermo-Scientific, Bannockburn, IL, USA) or radioactivity counted by liquid scintillation counting (see Reference [27]). Data as mean counts per minute (CPM) ± Standard Error of the Mean (SEM) (n = 3 wells/group).
An alternative explanation for the long half-life of 25(OH)D in blood could be that there is continuous entry of newly synthesized 25(OH)D, perhaps from parent vitamin D trapped in adipose tissue. This possible steady input could be replacing a steady loss of 25(OH)D from blood, and, thus, account for an apparently long half-life. However, the long residence time in blood of tritium-labelled 25(OH)D in both mice [27] and humans [12] demonstrates that it is persistence of the same molecules in blood that is the real explanation. This phenomenon can only be explained by recycling of 25(OH)D from some extravascular region and skeletal muscle is the only candidate for that region.
Nevertheless, experiments investigating the uptake and retention of 25(OH)D in cultured muscle cells in vitro, have shown that raising the concentration of DBP in the culture medium blocks the uptake of 25(OH)D into the cells (Table 3). The higher the concentration of DBP, the lower the concentration of unbound 25(OH)D. This observation fits with the interpretation that it is unbound 25(OH)D that enters muscle cells, rather than that which might be carried in, bound to DBP, via the megalin/cubilin protein internalization process. The fact that only 1%–5% of DBP in the circulation is transporting a specifically bound 25(OH)D molecule also indicates that transport on DBP across the cell membrane cannot be the mechanism for the intracellular accumulation of 25(OH)D.
Table 3. Uptake of [3H]-25(OH)D3 by differentiated myotubes after 4 h in the presence of varying concentrations of D-binding protein (DBP) (Data reformatted from Reference [27]).
|
DBP Concentration |
0 |
1 nM |
10 nM |
100 nM |
|
[3H]25(OH)D cpm/mg cell protein |
1284 ± 147 |
1233 ± 67 |
778 ± 76 * |
402 ± 20 * |
* Significantly different from 1 nmol/L DBP p < 0.001.
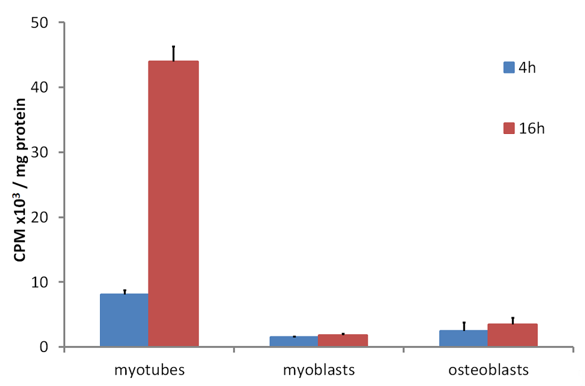
The accumulation of tritium-labelled 25(OH)D over 16 h by cultures of differentiated mouse skeletal myotube cells compared to the very low uptake by undifferentiated myoblasts and osteoblasts [39] demonstrated that some internal 25(OH)D-specific binding sites were present in the myotubes but not in the other two control cell types (Figure 2). Since the culture medium during the time of these incubations did not contain DBP, the specific binding inside myotubes would have been to DBP, which had been internalized from the culture medium while the myotubes were differentiating from myoblasts. When myotubes were cultured with fluorescently labelled DBP for up to 24 h and then observed by confocal microscopy, the fluorescent protein was clearly seen within the cells (Figure 3). Some of the fluorescence was in linear streaks along the long axis of the myotubes, suggesting that some of the labelled DBP was bound to actin in the actomyosin contractile elements. However, the fluorescent pattern was also distributed generally within the cell, indicating that much of the DBP was bound to the abundant actin, known to be distributed throughout the myotube cytoplasm [40].
Figure 2. (Data reformatted from Reference [27]) Time dependent tritiated-25(OH)D uptake in C2 myotubes and myoblasts and MG63 osteoblasts. Cells were incubated for 4 or 16 h with [26,27-3H]25-hydroxyvitamin D3 in DMEM supplemented with serum replacement and followed by 3x washes with ice cold PBS. At each time point, cells were harvested, lysed, and assayed for protein by the bicinchoninic acid assay or radioactivity counted by liquid scintillation counting (see Reference [27]). Data shown as means ± SEM (n = 3 wells/group).
It now appears that the megalin/cubilin protein internalization mechanism in myocytes is not limited to DBP because other extracellular proteins, such as albumin, have been found in human skeletal muscle [36] and have also been demonstrated to be incorporated into myotube cells in culture by this mechanism (unpublished results). Thus, the endocytosis of albumin by megalin/cubilin in muscle cells is similar to this property of megalin/cubilin in renal proximal tubule cells [29]. The function of the uptake of other extracellular proteins by skeletal muscle cells in vivo is a matter of speculation. Since skeletal muscle has a high rate of protein turnover, particularly when undergoing regular physical exercise [41], the internalized proteins, including DBP, could, after proteolysis, be supplying essential amino acids for protein resynthesis. It is, therefore, conceivable that, because DBP in myotube cultures is capable of retaining 25(OH)D for many hours (Figure 1) [27], the DBP bound to cytoplasmic actin is protected from the proteolysis that other internalized proteins undergo. Nevertheless, when the cytoplasmic DBP is eventually broken down, the retained 25(OH)D would be released and could then diffuse out of the cell, bind to extracellular DBP, and return to the circulation.
References
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Hassan Murad, M.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. Clin. Endocrinol. Metab. 2011, 96, 1911–1930.
- Rosen, C.J.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; Kovacs, C.S.; et al. IOM Committee Members Respond to Endocrine Society Vitamin D Guideline. Clin. Endocrinol. Metab. 2012, 97, 1146–1152.
- T.; Try, K.; Strømme, J.H. The vitamin D status of man at 70 degrees north Scand. J. Clin. Lab. Investig. 1980, 40, 227–232.
- Parviainen, M.T.; Koskinen, T. Vitamin A, D and E status in a Finnish population—A multivitamin study. Nutr. Clin. Nutr. 1983, 37, 397–403.
- Nowson, C.A.; McGrath, J.J.; Ebeling, P.R.; Haikerwal, A.; Daly, R.M.; Sanders, K.M.; Seibel, M.J.; Mason, R.S.; Working Group of Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia and Osteoporosis Australia. Vitamin D and health in adults in Australia and New Zealand: A position statement. J. Aust. 2012, 196, 686–687.
- Holick, M.F. Vitamin D deficiency. Engl. J. Med. 2007, 357, 266–281.
- Didriksen, A.; Burild, A.; Jakobsen, J.; Fuskevåg, O.M.; Jorde, R. Vitamin D3 increases in abdominal subcutaneous fat tissue after supplementation with vitamin D3. J. Endocrinol. 2015, 172, 235–241.
- Carrelli, A.; Bucovsky, M.; Horst, R.; Cremers, S.; Zhang, C.; Bessler, M.; Schrope, B.; Evanko, J.; Blanco, J.; Silverberg, S.J.; et al. Vitamin D Storage in Adipose Tissue of Obese and Normal Weight Women. Bone Miner. Res. 2017, 32, 237–242.
- Connors, M.H.; Sheikholislam, B.M.; Iria, J. Vitamin D Toxicity after Dieting in Hypoparathyroidism. Pediatrics 1976, 57, 794–796.
- Ziaie, H.; Razmjou, S.; Jomhouri, R.; Jenabi, A. Vitamin D Toxicity; Stored and Released from Adipose Tissue? Iran Med. 2016, 19, 597–600.
- Perticone, M.; Maio, R.; Sciacqua, A.; Suraci, E.; Pinto, A.; Pujia, R.; Zito, R.; Gigliotti, S.; Sesti, G.; Perticone, F. Ketogenic Diet-Induced Weight Loss is Associated with an Increase in Vitamin D Levels in Obese Adults. Molecules 2019, 24, 2499, doi:10.3390/molecules24132499.
- Clements, M.R.; Davies, M.; Fraser, D.R.; Lumb, G.A.; Mawer, E.B.; Adams, P.H. Metabolic inactivation of vitamin D is enhanced in primary hyperparathyroidism. Sci. 1987, 73, 659–664.
- Datta, P.; Philipsen, P.A.; Olsen, P.; Bogh, M.K.; Johansen, ; Schmedes, A.V.; Morling, N.; Wulfa, H.C. The half-life of 25(OH)D after UVB exposure depends on gender and vitamin D receptor polymorphism but mainly on the start level. Photochem. Photobiol. Sci. 2017, 16, 985–995.
- Mason, R.S.; Lissner, D.; Posen, S.; Norman, A.W. Blood levels of dihydroxylated vitamin D metabolites after an oral dose. Brit. Med. J. 1980, 280, 449–450.
- Schapiro, S.; Percin, C.J.; Kotichas, F.J. Half-life of plasma corticosterone during development. Endocrinology 1971, 89, 284–286.
- Haddad, J.G.; Fraser, D.R.; Lawson, D.E.M. Vitamin-D Plasma-Binding Protein—Turnover and Fate in the Rabbit. Clin. Investig. 1981, 67, 1550–1560.
- Kawakami, M.; Blum, C.B.; Ramakrishnan, R.; Dell, R.B.; Goodman, D.S. Turnover of the plasma binding protein for vitamin D and its metabolites in normal human subjects. Clin. Endocrinol. Metab. 1981, 53, 1110–1116.
- Abboud, M.; Rybchyn, M.S.; Rizk, R.; Fraser, D.R.; Mason, R.S. Sunlight exposure is just one of the factors which influence vitamin D status. Photobiol. Sci. 2017, 16, 302–313.
- Haddad, J.G. Purification, characterization and quantitation of the human serum binding protein for vitamin D and its metabolites. Methods Enzymol. 1980, 67, 449–459.
- Clements, M.R.; Fraser, D.R. Vitamin D supply to the rat fetus and neonate. Clin. Investig. 1988, 81, 1768–1773.
- Liu, J.; Greenfield, H.; Strobel, N.; Fraser, D.R. The influence of latitude on the concentration of vitamin D3 and 25-hydroxy-vitamin D3 in Australian red meat. Food Chem. 2013, 140, 432–435.
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. Appl. Physiol. 2000, 89, 81–88.
- Sawka, M.N.; Young, A.J.; Pandolf, K.B.; Dennis, R.C.; Valeri, C.R. Erythrocyte, plasma, and blood volume of healthy young men. Sci. Sports 1992, 24, 447–453.
- Foo, L.H.; Zhang, Q.; Zhu, K.; Ma, G.; Trube, A.; Greenfield, H.; Fraser, D.R. Relationship between vitamin D status, body composition and physical exercise of adolescent girls in Beijing. Int. 2009, 20, 417–425.
- Scragg, R.; Holdaway, I.; Jackson, R.; Lim, T. Plasma 25-hydroxyvitamin D3 and its relation to physical activity and other heart disease risk factors in the general population. Epidemiol. 1992, 2, 697–703.
- Bell, N.H.; Godsen, R.N.; Henry, D.P.; Shary, J.; Epstein, S. The effects of muscle-building exercise on vitamin D and mineral metabolism. Bone Miner. Res. 1988, 3, 369–373.
- Abboud, M.; Puglisi, D.A.; Davies, B.N.; Rybchyn, M.; Whitehead, N.P.; Brock, K.E.; Cole, L.; Gordon-Thomson, C.; Fraser, D.R.; Mason, R.S. Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology 2013, 154, 3022–3030.
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Melsen, F.; Christensen, E.I.; Willnow, T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999, 96, 507–515.
- Shiying, C.; Verroust, P.J.; Stiren, K.; Moestrup, S.K.; Christensen, E.I. Megalin/gp330 mediates uptake of albumin in renal proximal tubule. J. Physiol. 1996, 271, F900–F907.
- Gressner, O.A.; Lahme, B.; Gressner, A.M. Gc-globulin (vitamin D binding protein) is synthesized and secreted by hepatocytes and internalized by hepatic stellate cells through Ca(2+)-dependent interaction with the megalin/gp330 receptor. Chim. Acta 2008, 390, 28–37.
- Van Baelen, H.; Bouillon, R.; De Moor, P. Vitamin D binding protein (Gc-globulin) binds actin. Biol. Chem. 1980, 255, 2270–2272.
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Endocrinol. 2020, 10, 910, doi:10.3389/fendo.2019.00910.
- Goldschmidt-Clermont, P.J.; Van Baelen, H.; Bouillon, R.; Shook, T.E.; Williams, M.H.; Nel, A.E.; Galbraith, R.M. Role of group-specific component (vitamin D binding protein) in clearance of actin from the circulation in the rabbit. Clin. Investig. 1988; 81, 1519–1527.
- Otterbein, L.R.; Cosio, C.; Graceffa, P.; Dominguez, R. Crystal structures of the vitamin D-binding protein and its complex with actin: Structural basis of the actin-scavenger system. Natl. Acad. Sci. USA 2002, 99, 8003–8008.
- Haddad, J.G. Human serum binding protein for vitamin D and its metabolites (DBP): Evidence that actin is the DBP binding compound in human skeletal muscle. Biochem. Biophys. 1982, 213, 538–544.
- Brennan-Speranza, T.C.; Mor, D.; Mason, R.S.; Bartlett, J.R.; Duque, G.; Levinger, I.; Levinger, P. Skeletal muscle vitamin D in patients with end stage osteoarthritis of the knee. Steroid Biochem. Mol. Biol. 2017, 173, 180–184.
- Reed, R.K. Albumin concentration and colloid osmotic pressure of interstitial fluid collected by wick technique from rat skeletal muscle. Evaluation of the method. Acta Physiol. Scand. 1981, 112, 1–5.
- Haddad, J.G. Plasma vitamin D-binding protein (Gc globulin): Multiple tasks. Steroid Biochem. Mol. Biol. 1995, 53, 579–582.
- Mason, R.S.; Rybchyn, M.S.; Abboud, M.; Brennan-Speranza, T.C.; Fraser, D.R. The role of skeletal muscle in maintaining vitamin D status in winter. Dev. Nutr. 2019, 3, nzz087.
- Schiavon, C.R.; Zhang, T.; Zhao, B.; Moore, A.S.; Wales, P.; Andrade, L.R.; Wu, M.; Sung, T.-C.; Dayn, Y.; Feng, J.W.; et al. Actin chromobody imaging reveals sub-organellar actin dynamics. Methods 2020, doi:10.1038/s41592-020-0926-5.
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; Smeets, J.S.J.; Betz, M.W.; Senden, J.M.; Goessens, J.P.B.; Gijsen, A.P.; Rollo, I.; Verdijk, L.B.; van Loon, L.J.C. Dose-response effects of dietary protein on muscle protein synthesis during recovery from endurance exercise in young men: A double-blind randomized trial. J. Clin. Nutr. 2020, 112, 303–317.