Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thiago Luis Scudeler | -- | 4491 | 2022-10-19 18:49:23 | | | |
| 2 | Sirius Huang | + 128 word(s) | 4619 | 2022-10-20 07:24:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Oliveira, L.L.H.D.; Correia, V.M.; Nicz, P.F.G.; Soares, P.R.; Scudeler, T.L. Myocardial Infarction with Non-Obstructive Coronary Arteries. Encyclopedia. Available online: https://encyclopedia.pub/entry/30243 (accessed on 07 February 2026).
Oliveira LLHD, Correia VM, Nicz PFG, Soares PR, Scudeler TL. Myocardial Infarction with Non-Obstructive Coronary Arteries. Encyclopedia. Available at: https://encyclopedia.pub/entry/30243. Accessed February 07, 2026.
Oliveira, Lucas Lentini Herling De, Vinícius Machado Correia, Pedro Felipe Gomes Nicz, Paulo Rogério Soares, Thiago Luis Scudeler. "Myocardial Infarction with Non-Obstructive Coronary Arteries" Encyclopedia, https://encyclopedia.pub/entry/30243 (accessed February 07, 2026).
Oliveira, L.L.H.D., Correia, V.M., Nicz, P.F.G., Soares, P.R., & Scudeler, T.L. (2022, October 19). Myocardial Infarction with Non-Obstructive Coronary Arteries. In Encyclopedia. https://encyclopedia.pub/entry/30243
Oliveira, Lucas Lentini Herling De, et al. "Myocardial Infarction with Non-Obstructive Coronary Arteries." Encyclopedia. Web. 19 October, 2022.
Copy Citation
Myocardial infarction with non-obstructive coronary arteries (MINOCA) is a heterogeneous group of conditions that include both atherosclerotic (coronary plaque disruption) and non-atherosclerotic (spontaneous coronary artery dissection, coronary artery spasm, coronary artery embolism, coronary microvascular dysfunction, and supply–demand mismatch) causes resulting in myocardial damage that is not due to obstructive coronary artery disease. Failure to identify the underlying cause may result in inadequate and inappropriate therapy in these patients.
MINOCA
myocardial infarction
coronary artery disease
1. Introduction
Patient is admitted to the emergency department with chest pain, ischemic electrocardiogram (ECG), and highly positive troponin. The patient is sent to the cath lab, but no coronary obstructions were detected.
Myocardial infarction with nonobstructive coronary arteries (MINOCA) is defined as an acute myocardial infarction (AMI) without significant coronary artery obstruction on angiography (>50%) or without specific imaging findings.
MINOCA was first described by Gross and Steinberg in 1939 [1]. Recent studies have found a prevalence of MINOCA of 2–11% in AMI patients [2][3][4][5][6]. However, prevalence varied widely across the studies.
The prognosis of patients with MINOCA is far from benign. Patients with MINOCA are at increased risk for adverse cardiovascular events such as AMI and death [3][7][8]. However, it is difficult to establish an accurate prognostic assessment of patients with MINOCA, as the prognosis can be influenced by the cause, as well as the degree of myocardial damage associated with AMI.
MINOCA may present with or without ST-segment elevation and, in general, patients have lower increases in cardiac troponin than patients with obstructive coronary artery disease [9][10].
A myriad of conditions can lead to MINOCA, and the mechanisms involved are both atherosclerotic and non-atherosclerotic, although the underlying cause of AMI is not always apparent. Therefore, cardiovascular imaging tests have a critical role in assessing patients with MINOCA.
2. Diagnostic Criteria
The term MINOCA refers exclusively to ischemic conditions, such as epicardial vasospasm and non-obstructive atherosclerotic plaque instability. However, several non-ischemic diseases have similar presentations to MINOCA. Therefore, the term MINOCA is frequently used as a descriptive diagnosis until further evaluation confirms an ischemic mechanism or unravels an alternate diagnosis, such as Takotsubo cardiomyopathy (TCM) or myocarditis.
Recently, the European Society of Cardiology [11] and the American Heart Association [12] defined MINOCA according to specific criteria (Table 1).
Table 1. Diagnostic criteria for myocardial infarction with non-obstructive coronary arteries.
|
|
|
AMI = acute myocardial infarction; ECG = electrocardiogram; TCM = Takotsubo cardiomyopathy; MINOCA = myocardial infarction with non-obstructive coronary arteries.
Therefore, the diagnostic criteria are very useful in exposing the ischemic nature of MINOCA.
3. Epidemiology
Both acute myocardial infarction with obstructive coronary artery disease (AMI-CAD) and MINOCA are more prevalent in men. However, women are more likely to present with MINOCA than men, representing 24% of the AMI-CAD population and 43% of the MINOCA population [9].
Patients with MINOCA tend to be somewhat younger than AMI-CAD patients, with a median age at presentation of 58 years [9]. The prevalence of traditional cardiovascular risk factors seems similar in both groups, with the exception of hypercholesterolemia, which is less frequent in MINOCA patients [9].
The Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study evaluated the clinical characteristics and outcomes of young patients (age between 18 and 55 years) with MINOCA versus AMI-CAD [2]. The registry showed an incidence of MINOCA of 11%. MINOCA was more common in women and less associated with traditional risk factors than AMI-CAD (8.7% versus 1.3%; p < 0.001). Moreover, similarly to the data presented by Pasupathy et al. [9], the VIRGO study showed that MINOCA patients presented less frequently with ST-segment-elevation myocardial infarction (STEMI) compared to patients with AMI-CAD (21% vs. 52%, p < 0.001).
The prognosis of patients with MINOCA is not benign, but the long-term mortality after MINOCA is lower than that in patients with AMI-CAD. Annual mortality varies from 1.15% to 3.5% [7][9]. Other studies have shown an increased risk of new AMI and hospitalization for heart failure [13][14].
Pelliccia et al. showed that reduced left ventricular ejection fraction, nonobstructive coronary artery disease, use of beta blockers during follow-up, and ST-segment depression on the admission electrocardiogram are significant predictors of long-term prognosis in patients with MINOCA [7].
4. Physiopathology
MINOCA is a heterogeneous entity with various mechanisms responsible for the acute presentation. The adequate mechanism by which ischemia happens is a temporary suspension of blood flow to the myocardium, which usually takes place in the epicardial arteries, but which may also happen in the microvasculature [15].
The American Heart Association 2019 statement categorized the causes of MINOCA as atherosclerotic or non-atherosclerotic [12]. Atherosclerotic causes encompass plaque disruption while non-atherosclerotic causes encompass epicardial coronary vasospasm, coronary microvascular dysfunction, spontaneous coronary artery dissection, and supply-demand mismatch.
5. Atherosclerotic Causes
Coronary Plaque Disruption
Studies have shown that approximately 38–40% of patients with MINOCA have some evidence of plaque disruption, including plaque rupture, erosion, or calcified nodules when intracoronary imaging is performed [16][17].
Plaque rupture is defined as fibrous cap discontinuity leading to a communication between plaque cavity and the coronary lumen [18]. Plaque erosion is defined as a thrombus contiguous to the luminal surface of a plaque without signs of rupture [19]. Calcified nodule is defined based on optical coherence tomography (OCT) imaging criteria as a signal-poor region with poorly delineated borders that protrudes into the arterial lumen [20].
An autopsy series of 800 cases of sudden coronary death found a prevalence of plaque rupture of 55–60%, plaque erosion of 30–35%, and plaque erosion of 2–7% [21].
This mechanism of coronary plate disruption can lead to in situ obstruction or to embolization of atherosclerotic debris and platelet aggregates, causing AMI. The coronary angiography does not evidence obstructive lesions possibly because of the endogenous fibrinolytic system [22] or due to the superimposed vasospasm on an unstable plaque, with normalization by the time of the catheterization (Figure 1). However, the angiographic appearance may suggest plaque disruption; for example, haziness or a small filling defect [12]. However, intravascular imaging can accurately diagnose plaque disruption, preferably with OCT or, to a lesser extent, with intravascular ultrasound (IVUS).
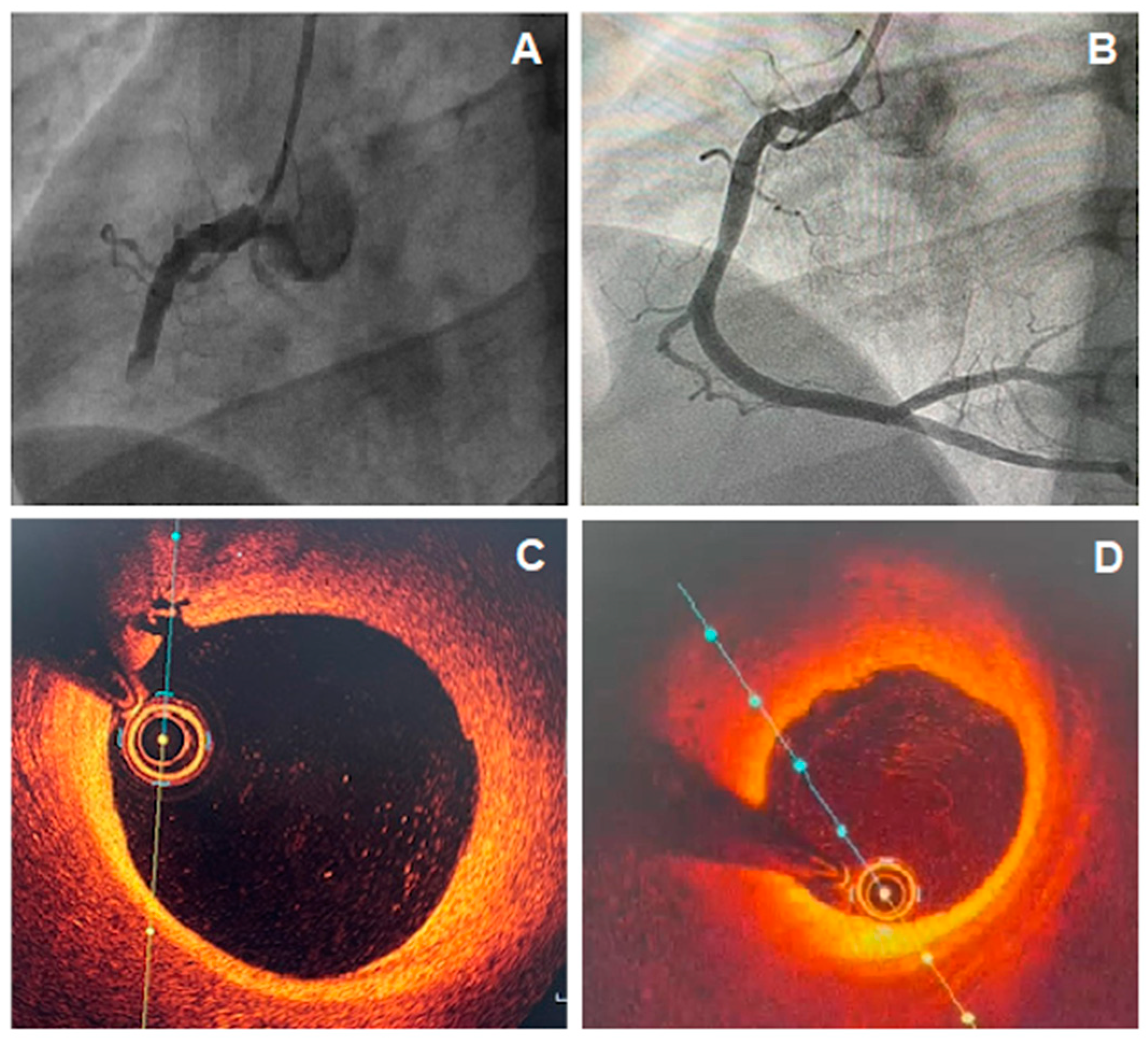
Figure 1. (A) Coronary angiography showing total occlusion of the right coronary artery (RCA); (B) Coronary angiography of RCA, 5 days after recanalization and intracoronary thrombolytic administration, showing non-obstructive atherosclerotic plaque; (C,D) optical coherence tomography (OCT) images showing a lipid plaque with signs of inflammation and intimal rupture.
6. Non-Atherosclerotic Causes
6.1. Spontaneous Coronary Artery Dissection (SCAD)
The first case of SCA was described by Pretty in 1931 [23]. Since then, SCAD continues to be misdiagnosed, underdiagnosed, and incorrectly managed, which may harm patients with SCAD.
SCAD is defined as a separation of the layers of an epicardial coronary-artery wall by intramural hemorrhage, with or without an intimal tear [24]. This intramural hemorrhage may progress in a way that it causes obstruction of the blood flow with subsequent myocardial ischemia. Its pathogenesis is not understood to its full extent, but hypotheses lie mainly on spontaneous intramural hemorrhage formation in the arterial wall or coronary flow mediated expansion after an intimal tear. This disease is of growing interest, as it has been more diagnosed with intravascular imaging methods [25][26][27].
Eleid et al. showed that coronary artery tortuosity may be a marker for or a potential mechanism for SCAD [28].
Other predisposing factors would include fibromuscular dysplasia (FMD), postpartum status, multiparity (≥4 births), connective tissue disorders, systemic inflammatory conditions, and hormonal therapy [29][30][31].
SCAD most commonly occurs in patients with few or no traditional cardiovascular risk factors [32][33].
Recent studies have shown that SCAD occurs overwhelmingly in women [34][35] and is the most common cause of pregnancy-associated AMI [36][37]. A recent analysis of a US administrative database found a prevalence of 1.81 SCAD events per 100,000 pregnancies during pregnancy or in the 6-week postpartum period [38]. The left main or left anterior descending artery has been described as the most commonly affected [37][39]. The pathophysiological mechanism involved in this phenomenon is not fully understood, but it is possibly associated with numerous conditions such as hormonal changes of pregnancy that may lead to alterations in the architecture of the arterial wall [40][41].
In addition to hormonal influences, other situations are associated with SCAD such as underlying arteriopathies, genetic factors, inherited or acquired arteriopathies, or systemic inflammatory diseases, often compounded by environmental precipitants or stressors [33].
SCAD can be classified based on angiographic appearance into four types (Figure 2) [24][26]. Type 1 angiographic SCAD describes those with evident arterial wall stain with multiple radiolucent lumens. Type 2 A refers to a segment with diffuse narrowing (typically >20 mm) with “normal” segment proximally and distally. Type 2 B refers to diffuse narrowing extending to the distal end of the vessel. Type 3 refers to short segment of stenosis (<20 mm in length) that mimics atherosclerosis. Type 4 is characterized by dissection leading to an abrupt total occlusion, usually of a distal coronary segment.
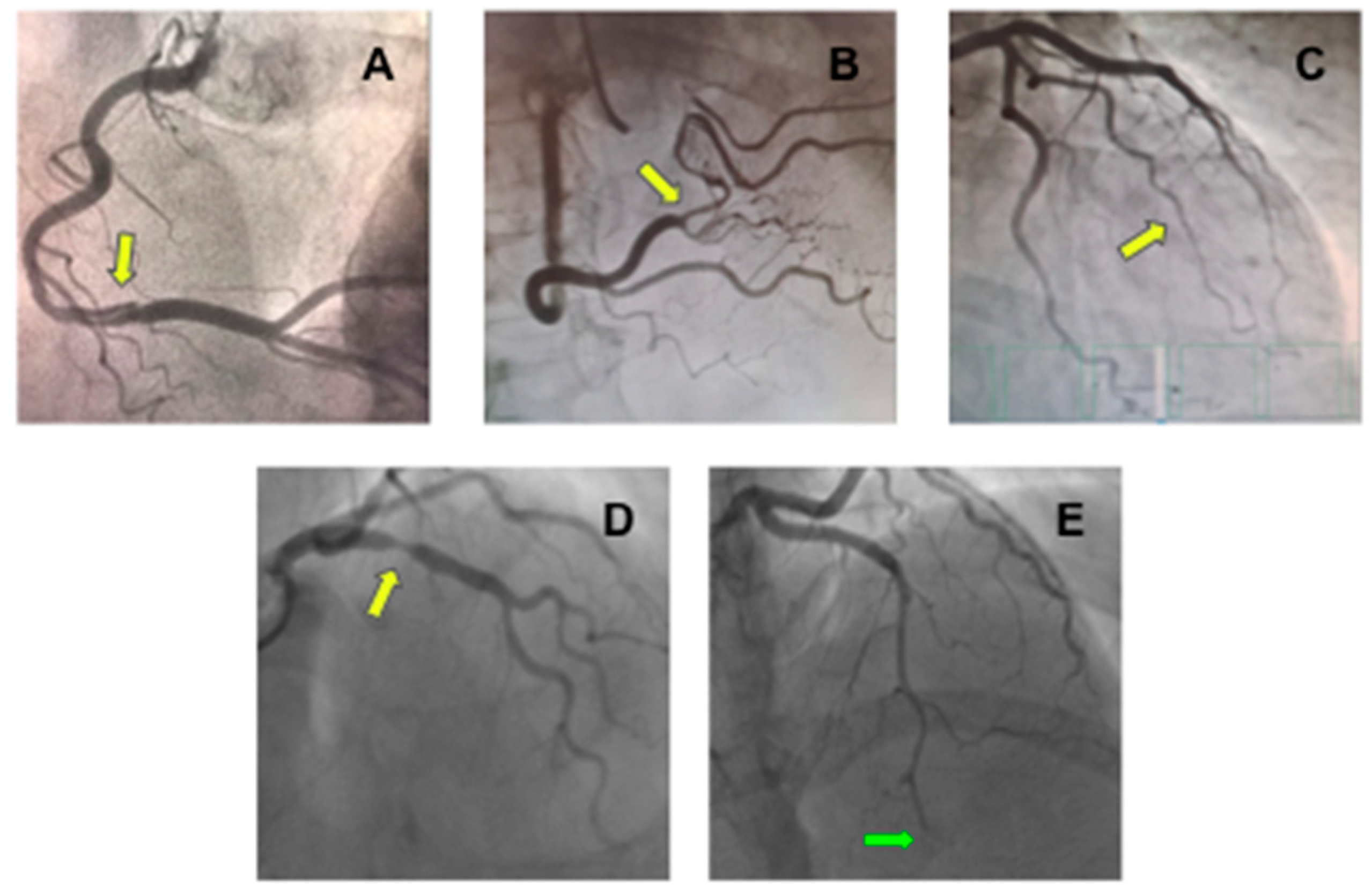
Figure 2. Coronary angiography showing different types of spontaneous coronary artery dissection (SCAD). (A) An image of double lumen at right coronary artery (yellow arrow) compatible with type 1 SCAD; (B) Stenosis at right coronary artery with distal vessel caliber normalization (yellow arrow) compatible with type 2A SCAD; (C) Long stenosis from mid-to-distal first marginal branch of the left circumflex coronary (yellow arrow) compatible with type 2B SCAD; (D) Focal stenosis at right coronary artery (yellow arrow) compatible at type 3 SCAD; and (E) Stenosis from mid-to-distal left anterior descending coronary artery (green arrow) with an abrupt total occlusion (green arrow) compatible with type 4 SCAD.
Observational studies have shown that the majority of patients (70–97%) have angiographic healing weeks to months after a conservatively managed index episode [35][42][43].
The time course of healing remains uncertain, but it can be detected within days [44].
The diagnosis of SCAD is usually possible with coronary angiography alone, but intravascular imaging such as IVUS or OCT is paramount for detecting more challenging SCAD cases (Figure 3). However, while OCT images in SCAD are characteristic, IVUS images require closer scrutiny to discriminate between plaque disruption and SCAD, given the lower spatial resolution of IVUS [45]. However, OCT could further aggravate the dissection or exacerbate a new intimate tear due to contrast injection. In addition, the more recent high-definition IVUS (HD IVUS) has better spatial resolution, which helps with the diagnosis of SCAD [46].

Figure 3. Flowchart for the diagnosis of spontaneous coronary artery dissection (SCAD). CCTA: Coronary Computed Tomography Angiography; IVUS: Intravascular ultrasound; OCT: Optical coherence tomography; IC: intracoronary; PCI: percutaneous coronary intervention.
Coronary computed tomography angiography (CCTA) has been utilized both in the initial diagnosis of SCAD and to assess healing. However, CCTA diagnostic criteria for SCAD need further refinement. During the acute SCAD episode, dissection planes are infrequently identified (<15%) by CCTA; abrupt luminal changes and sleeve-like hematomas within the coronary artery wall are more often observed [47][48]. Limitations of CCTA include low spatial resolution for small vessels and with a diameter < 2.5 mm and for the middle and distal portions of the coronary arteries, motion artifact, and unknown sensitivity and specificity [45]. Therefore, CCTA can have its place for proximal SCAD but very few for mid and distal SCAD which is the preferred location for SCAD.
6.2. Coronary Artery Spasm (CAS)
Vasospastic angina (VSA) was first described by Prinzmetal [49][50]. Recent studies have shown a high prevalence of epicardial (26–37%) and microvascular spasm (33–34%) in patients undergoing coronary angiography [51][52].
Therefore, CAS can occur at the level of the epicardial arteries as well as in the coronary microcirculation. Current standardized diagnostic criteria for microvascular spasm include reproduction of the patient’s angina symptoms and ischemic ECG changes in the absence of epicardial spasm during intracoronary spasm provocation testing using, for example, acetylcholine [53]. Of note, it is important to mention that it is difficult to identify the mechanism of microvascular dysfunction that triggers microvascular angina. Therefore, it is essential to distinguish between an impaired microcirculatory vasodilatory capacity, which can be diagnosed by measuring coronary flow reserve or microvascular resistance, and microvascular spasm determined by intracoronary acetylcholine administration.
CAS usually occurs at a localized segment of an epicardial artery (Figure 4), but sometimes involves two or more segments of the same (multifocal spasm) or of different (multivessel spasm) coronary arteries.
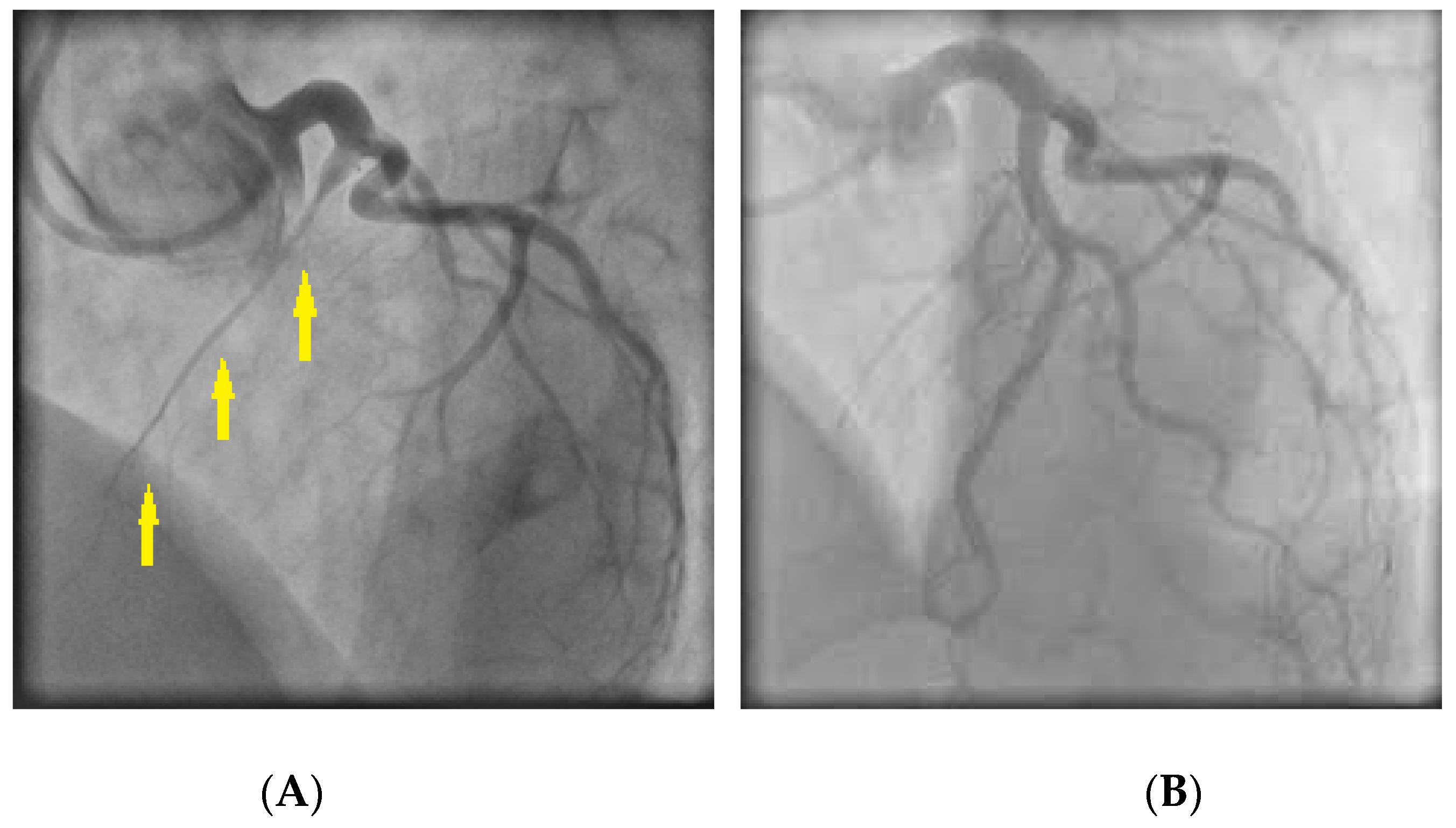
Figure 4. (A) Coronary angiography revealing spasm in the right coronary artery (arrows); (B) Response of the stenotic region to intracoronary nitroglycerin administration.
Of interest, myocardial bridging, per se, is unlikely to cause MINOCA [54]. However, it can predispose the affected artery to spasm [55][56].
Proposed mechanisms to constitute the substrate for CAS susceptibility include: (1) endothelial dysfunction and (2) primary hyperreactivity of vascular smooth muscle cells [57].
The endothelium plays a crucial role in the physiological regulation of coronary vascular tone, mainly through the release of vasodilating substances, the most important of which is nitric oxide (NO). Therefore, significant endothelial damage can impair vasodilation, favoring CAS in response to vasoconstrictor stimuli [58]. Various vasoactive stimuli (e.g., acetylcholine, serotonin, histamine) cause vasodilation by inducing nitric oxide (NO) release from the endothelium, but at the same time can cause vasoconstriction by direct stimulation of vascular smooth muscle cells. Thus, in the presence of endothelial dysfunction, its release into the vessel wall can lead to vasoconstriction or coronary spasm.
Additionally, there is consistent evidence to suggest that, in patients with variant angina, a primary nonspecific hyperreactivity of vascular smooth muscle cells in the coronary artery wall is the main abnormality responsible for coronary spasm. The pathogenic role of local hyperreactivity of vascular smooth muscle cells is suggested by the observation that vasoconstrictor stimuli that induce spasm in localized coronary segments of patients with variant angina are unable to induce spasm in other coronary segments of the same patients [59] and in patients with other forms of angina (in particular, stable angina) [60][61].
Furthermore, in patients with variant angina, CAS can be triggered by various stimuli that act through different receptors and cellular mechanisms [62][63], suggesting an intracellular and post-receptor location of the alteration responsible for the hyperreactivity.
Patients with VSA typically have recurrent episodes of angina with no clear relation to exercise and metabolic demand (Figure 5). Classically, these episodes are accompanied by ST deviations on ECG and prompt relief with nitrates. CAS attacks can occur both in patients with and without obstructive atherosclerosis [64].
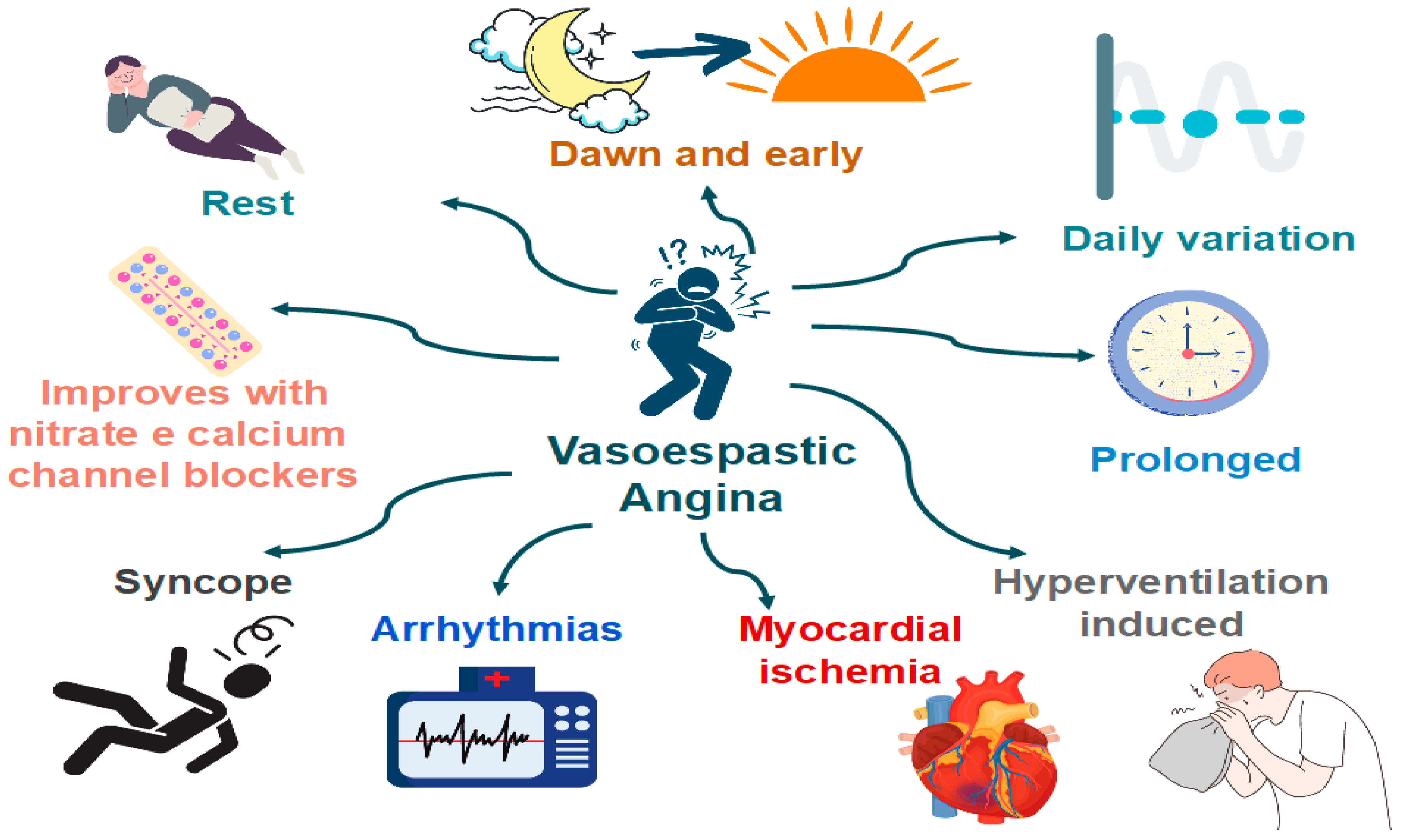
Figure 5. Clinical manifestations of coronary artery spasm (CAS).
Features that are specific to VSA (versus classical angina) [64]:
- Angina occurs predominantly at rest and may occur more frequently from midnight to early morning;
- Effort and exercise tolerance are usually preserved;
- Hyperventilation can precipitate VSA [65];
- Episodes appear in “clusters;”
- VSA often has a more rapid response to sublingual nitroglycerin.
The diagnosis of VSA typically requires the documentation of CAS. Coronary angiography may be normal due to the self-limiting nature of the episodes. Therefore, the diagnosis of VSA may require provocative stimulus using acetylcholine, ergonovine, or methylergonovine (Figure 6). A positive provocative test for CAS must induce all of the following in response to the provocative stimulus: (1) reproduction of the usual chest pain, (2) ischemic ECG changes, and (3) >90% vasoconstriction on angiography [64].
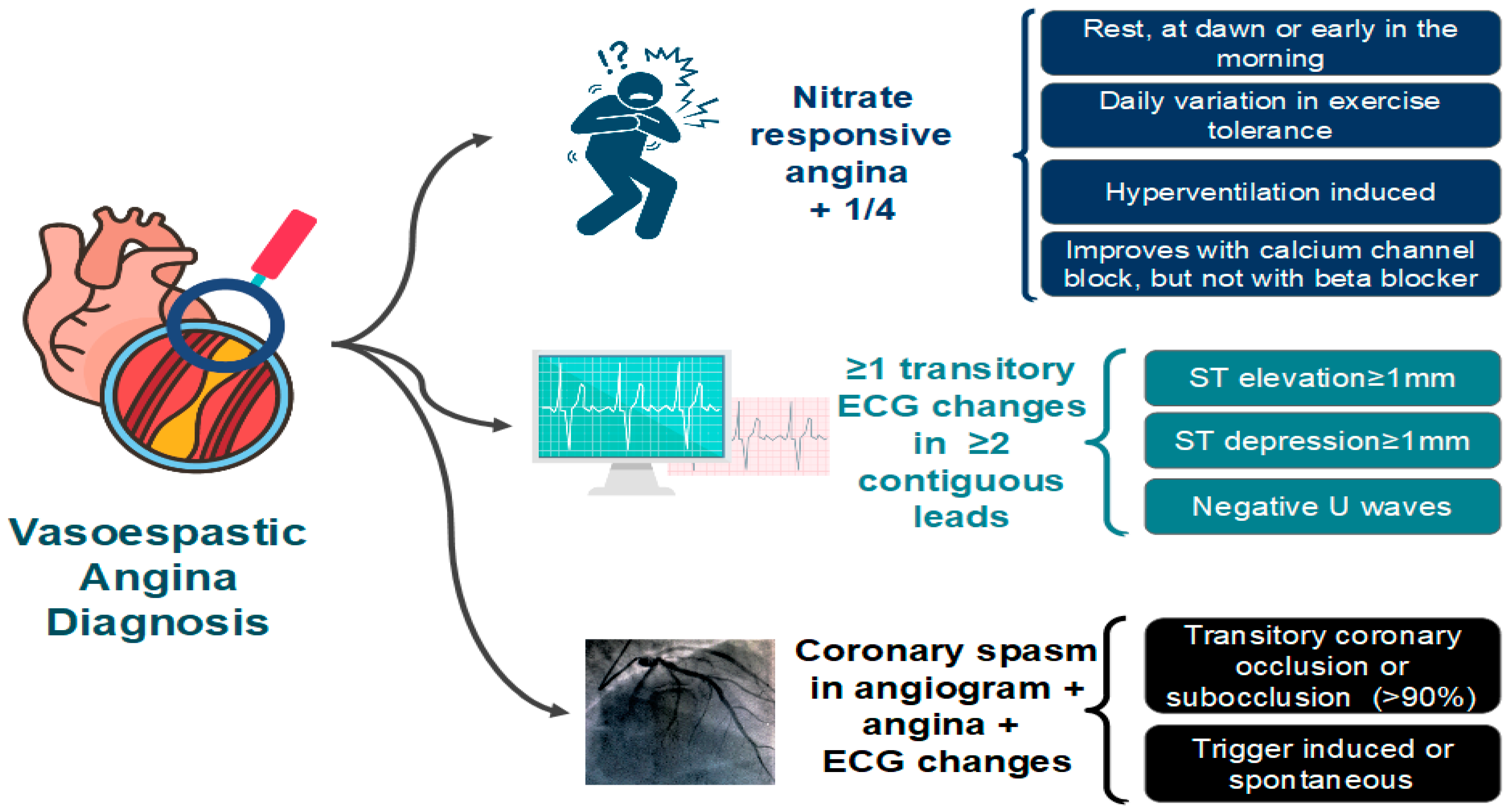
Figure 6. Diagnostic criteria for vasospastic angina (VSA) proposed by the Coronary Vasomotion Disorders International Study Group (COVADIS). ECG: electrocardiogram [64].
Although accurate [66], provocative tests for CAS are associated with risks. The risk of death is low, but the incidence of cardiac arrhythmias is relatively high (6.8%) [67]. A contemporary study analyzed 921 patients undergoing intracoronary acetylcholine testing, and no deaths or serious complications were reported [68]. Also, only 1% of patients had any kind of complications, namely non-sustained ventricular tachycardia, fast paroxysmal atrial fibrillation, bradycardia with hypotension and catheter-induced vasospasm. Although complications related to provocative tests may exist, none of them are associated with increased morbidity and mortality.
Montone et al. evaluated 80 patients suspected of a vasomotor etiology with acetylcholine or ergonovine intracoronary provocative tests [69]. Out of 37 positive tests, only two patients (5.4%) presented with arrhythmic complications (self-limited bradycardia). The authors reported a rate of complications comparable to that of spontaneous vasomotor angina episodes. Taking a closer look at the Montone series, the rate of test positivity was significant, which highlights the importance of this etiology in the pathogenesis of MINOCA: 37 out of 80 selected patients had a positive test; 24 had epicardial spasm and 13 had microvascular spasm.
The significance of this vasomotor change is likely similar to that found in ruptured plaques and evidence of a mechanism by which MINOCA is plausible, but a definitive causal association is probably not possible in all cases.
6.3. Coronary Artery Embolism/Thrombosis
Coronary artery embolism (CE) is an uncommon cause of AMI and the precise diagnosis remains challenging for the physician. A recent retrospective analysis suggested that up to 3% of AMI might result from CE [70].
The National Cerebral and Cardiovascular Center group proposed diagnostic criteria to define CE (Table 2) [70]:
Table 2. Coronary embolism diagnostic criteria.
| Major criteria |
|
| Minor criteria |
|
| Definite CE |
|
| Probable CE |
|
| A diagnosis of CE should not be made if there is: |
|
CMR = cardiac magnetic resonance; CE = coronary artery embolism. * Indicates multiple vessels within one coronary artery territory or multiple vessels in the coronary tree.
CE is divided into four groups: (1) direct; (2) paradoxical; (3) iatrogenic; and (4) hypercoagulable disorders, with some overlap among the categories.
Direct coronary emboli commonly originate from the left atrial appendage, left ventricle, the aortic or mitral valves, or the proximal coronary artery. Embolic tissue may be thrombus, valvular material, or even neoplasm.
Paradoxical emboli pass through a patent foramen ovale (PFO), atrial septal defect, or pulmonary arteriovenous malformations from the venous circulation into the systemic circulation. Most commonly the origin is from a deep vein (deep venous thrombosis).
Iatrogenic emboli may occur following interventional procedures, usually valve replacements and coronary intervention.
Hypercoagulable disorders that result in coronary thrombosis can be divided into inherited and acquired causes.
Inherited thrombophilia includes factor V Leiden, elevated factor VIII/von Willebrand factor, activated protein C resistance, protein C or S deficiency, prothrombin 20120A, and antithrombin deficiency. Acquired hypercoagulable states include thrombotic thrombocytopenic purpura (TTP), the autoimmune disorder antiphospholipid syndrome, heparin-induced thrombocytopenia (HIT), and myeloproliferative neoplasms.
The diagnosis of CE is based on the clinical presentation and the presence of risk factors. IVUS or OCT can help differentiate spontaneous coronary thrombosis or embolization from other MINOCA etiologies such as plaque rupture. Echocardiography, transesophageal echocardiography, and microbubble studies are helpful in finding the source of emboli. Thrombophilia can be investigated through specific tests.
6.4. Coronary Microvascular Dysfunction (CMD)
The term microvascular angina was initially proposed by Cannon and Epstein in 1988 to identify patients with myocardial ischemia triggered not by obstructive CAD, but by functional microvascular abnormalities [71]. Coronary microvascular dysfunction is also commonly referred to as syndrome X.
Microvascular circulation (vessels <0.5 mm in diameter) is not visualized on coronary angiography and represents approximately 70% of coronary resistance in the absence of obstructive CAD (Figure 7). The dysfunction affects only these vessels, and it is characterized by reduced coronary flow reserve (CFR) [72].

Figure 7. Anatomy of the coronary arterial system and invasive diagnostic modalities to assess coronary microvascular function. FFR: fractional flow reserve; IMR: index of microvascular resistance; CFR: coronary flow reserve.
CFR is an invasive method that allows an integrated measurement of flow through the large epicardial arteries and coronary microcirculation, but once severe obstructive disease of the epicardial arteries is ruled out, reduced CFR is a marker of CMD. CFR is the ratio of hyperemic blood flow divided by resting blood flow and can be calculated using thermodilution or Doppler flow velocity. Overall, the prognostic value of CFR used a cutoff value < 2.0 [73]. The index of microcirculatory resistance (IMR) is calculated as the product of distal coronary pressure at maximal hyperemia multiplied by the hyperemic mean transit time. IMR ≥ 25 is representative of microvascular dysfunction [74][75][76]. Flow-limiting obstructive coronary artery disease can be assessed using Fractional Flow Reserve (FFR), which is the ratio of mean distal coronary pressure to mean aortic pressure at maximal hyperemia (abnormal FFR is defined as ≤0.80) [73]. FFR values > 0.8, CFR ≥ 2.0, and IMR < 25 represent absence of CMD and after vasoactive stimuli with acetylcholine with absence or reduction of coronary diameter < 90%, without angina and lack of ischemic ECG changes it is interpreted as pain non-cardiac and the opposite changes in the test allow the diagnosis of VSA. The FFR values > 0.8, CFR < 2.0, and IMR ≥ 25 represent the presence of CMD and after a vasoactive stimuli with acetylcholine with absence or reduction of coronary diameter < 90%, without angina and lack of ischemic ECG changes it is interpreted as microvascular angina and the opposite test result allows diagnosis of microvascular angina and VSA.
Recently, Rahman et al. described two endotypes of CMD: structural and functional [77]. In structural CMD patients have endothelial dysfunction, which leads to diminished peak resting coronary blood flow augmentation and increased demand during exercise the functional CMD is related to inefficient cardiac-coronary coupling during peak exercise and during rest leads to higher myocardial oxygen demand in the setting of exhausted vasodilatory reserve [78].
The Women’s Ischemia Syndrome Evaluation (WISE) study showed that the prevalence of microvascular dysfunction and nonobstructive CAD is high and is associated with relatively poor prognosis compared with women without evidence of microvascular dysfunction and nonobstructive CAD [79][80].
Recently, the COVADIS group proposed diagnostic criteria to define microvascular angina (Table 3) [53].
Table 3. Diagnostic criteria for the microvascular angina (MVA). Definitive MVA if all 4 criteria are present. Suspected MVA if symptoms of ischemia with no obstructive coronary artery disease are present (criteria 1 and 2) but only objective evidence of myocardial ischemia (criteria 3) or evidence of impaired coronary microvascular function (criteria 4) alone.
|
|
|
|
CAD = coronary artery disease; ECG = electrocardiogram; CCTA = coronary computed tomography angiography; FFR = fractional flow reserve; IMR = index of microcirculatory resistance; TIMI = thrombolysis in myocardial infarction.
The mechanism of microvascular dysfunction may be endothelium-dependent or endothelium-independent [81]. Endothelium-dependent dysfunction is a consequence of an imbalance between relaxing factors, such as NO, and constricting factors, such as endothelin. Endothelium-independent dysfunction is based on myocyte tone [81]. In addition, enhanced coronary vasoconstrictive reactivity and increased coronary microvascular resistance secondary to structural factors (e.g., luminal narrowing, vascular remodeling, vascular rarefaction, and extramural compression) are also involved in microvascular dysfunction [82].
Assessment of microvascular dysfunction includes invasive methods such as CFR, index of microvascular resistance (IMR), and absolute coronary blood flow measured, and non-invasive methods such as positron emission tomography (PET), CMR, and Doppler echocardiography.
The concept behind CFR lies in the prerogative that the vessels in the coronary tree have the potential to heighten their flow in response to vasodilator stimuli. This potential is the so-called flow reserve. In ischemic territories, endogenous mechanisms will lead to a basally more dilated coronary bed, and therefore with less flow reserve [83]. Measuring CFR attempts to evaluate the potential to increase blood flow in response to specific stimuli. Both epicardial obstructions and microvascular dysfunction may lead to lower CFR, thus CFR is only applicable to diagnose the latter in the absence of significant epicardial obstruction. CFR is calculated using thermodilution as resting mean transit time divided by hyperemic mean transit time. However, CFR has some limitations such as: (1) low specificity for microvascular dysfunction; (2) it does not have a clearly defined normality value; (3) is affected by hemodynamic variables at rest. In the Women’s Ischemia Syndrome Evaluation (WISE) study, a total of 47% of women had diminished coronary flow velocity reserve (CFVR), suggestive of microvascular dysfunction [84].
The index of microcirculatory resistance (IMR) was firstly developed by Fearon et al. and is calculated from estimates of maximal distal coronary flow during hyperemia and pressure [85]. Ng et al. showed that IMR is superior to CRF for assessing the coronary microcirculation by virtue of being more reproducible and less hemodynamically dependent than CFR [86] since it is not dependent on resting values. Moreover, IMR is not affected by epicardial stenosis severity [87]. Other indices can be used to assess CMD, such as hyperemic microvascular resistance [88], resistive reserve ratio [89], and microvascular resistance reserve (MRR) [90].
Recently, the novel technique to quantify absolute coronary flow and resistance through intracoronary continuous thermodilution has been developed. Morris et al. has demonstrated that this new method provides a comprehensive coronary physiological assessment of flow, pressure and resistance, across the entire coronary circulation, without the need for additional hardware, catheters, wires, or infusions [91].
However, the body of evidence concerning coronary flow and flow reserve measurement among the MINOCA population is currently limited. Similarly, the role and the clinical implications of continuous thermodilution-derived indexes within MINOCA patients are not yet established [92].
6.5. Supply–Demand Mismatch
Stable CAD typically does not cause myocardial necrosis because the obstruction grade is fixed. However, in states of intense demand, these obstructions may lead to critical hypoperfusion, with the development of AMI and necrosis. In extreme demand scenarios, this can occur even in the absence of obstructive coronary lesions (Figure 8).
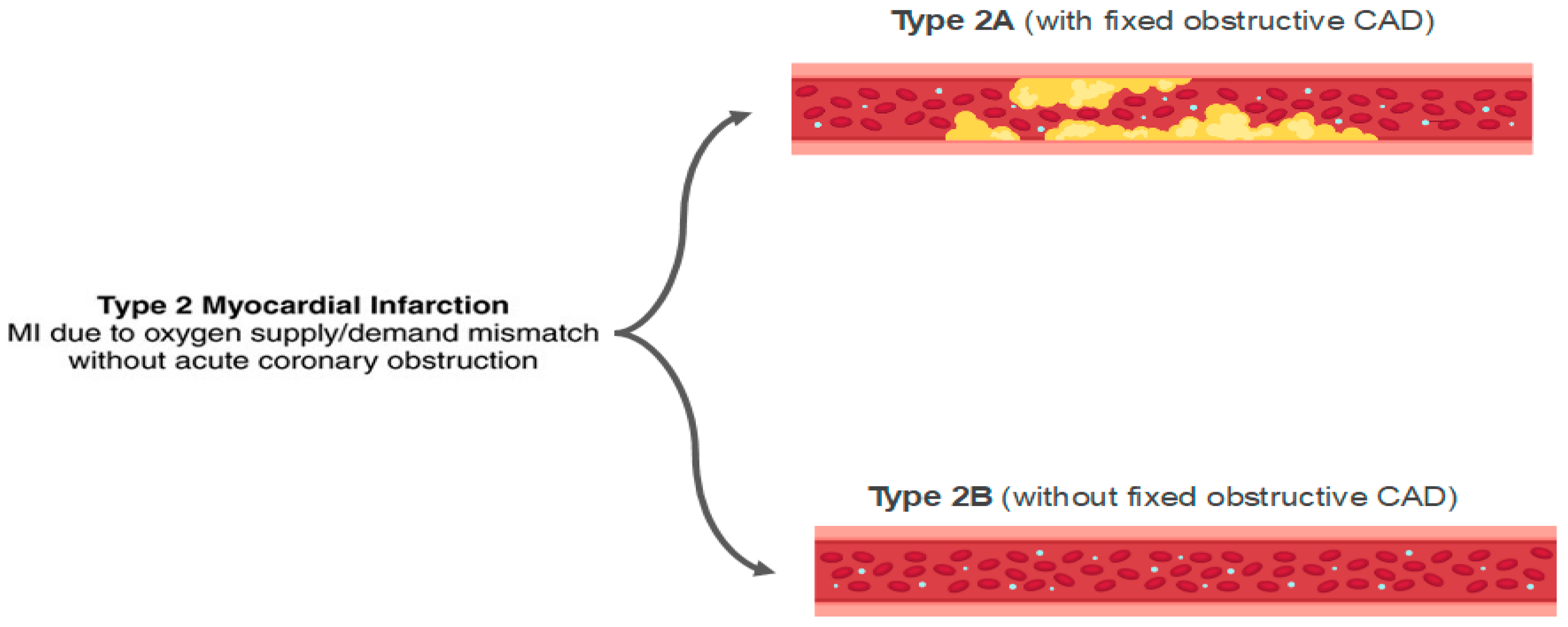
Figure 8. Classification of type 2 acute myocardial infarction (AMI). MI: myocardial infarction; CAD: coronary artery disease.
Approximately 50% of patients with type II AMI do not have significant coronary artery disease, and they can be classified as MINOCA [93].
The common causes of demand and supply mismatch are hypotension, tachyarrhythmia, and hypoxia [76].
Along with the widespread introduction of high-sensitivity troponin assays, the detection of abnormal levels of these cardiac biomarkers in hospitalized patients has been frequent [94].
The Fourth Universal Definition of Myocardial Infarction delineates the principles by which the clinician may establish the differential diagnosis between AMI and myocardial injury (Figure 9) [95]. Any rise and/or fall in troponin level with at least one result over the 99th percentile is a myocardial injury. On the other hand, the alteration of cardiac biomarkers associated with ischemic findings (ECG, symptoms and imaging exams) defines the diagnosis of AMI. The diagnosis of a type 2 AMI, as opposed to myocardial injury, requires ischemic symptoms or signs and a rise or fall in troponin levels. The presence of CAD is not necessary for the diagnosis.
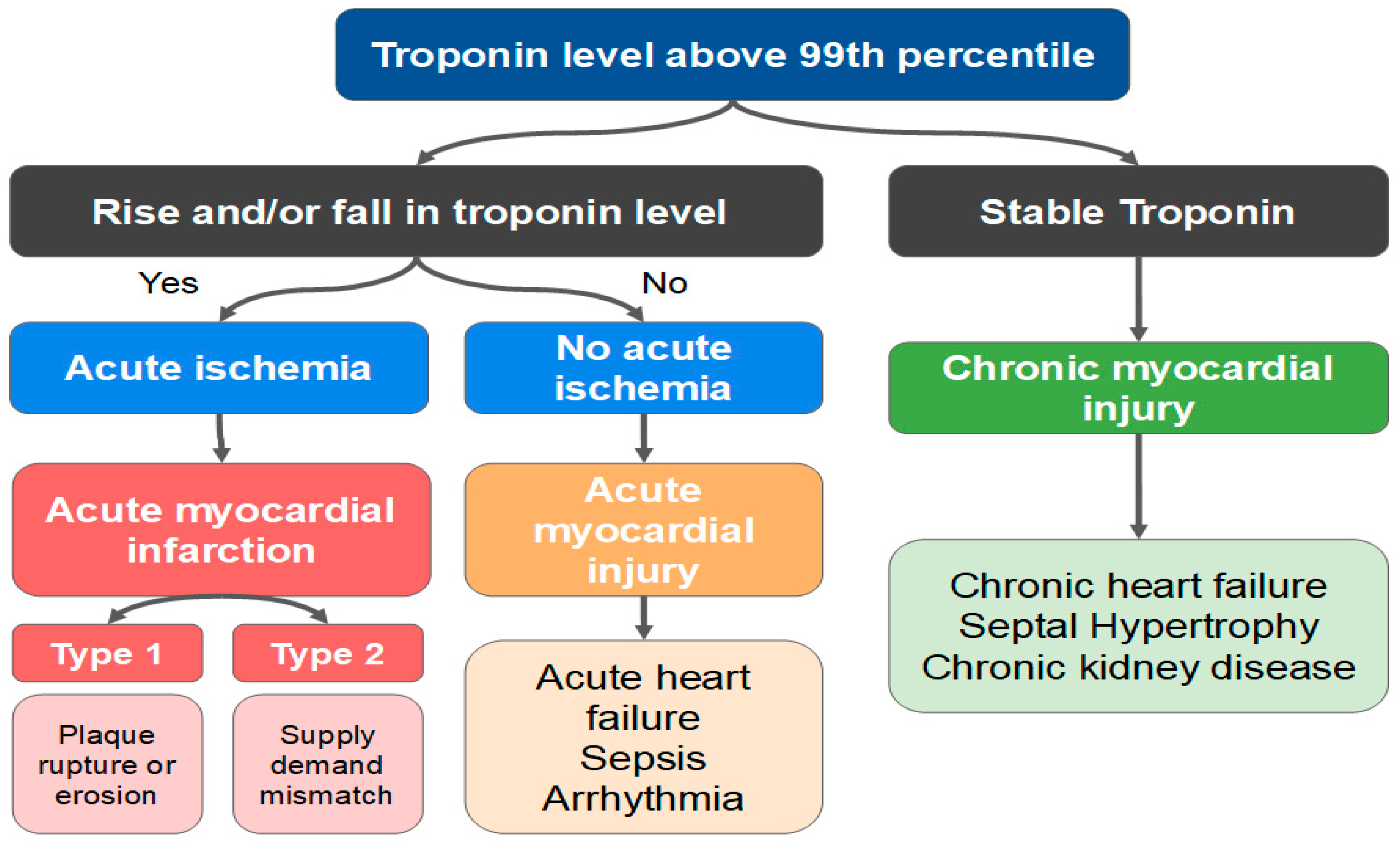
Figure 9. Differences between acute myocardial infarction (AMI) and acute/chronic myocardial injury.
Interestingly, several mechanisms not involving intracoronary thrombus are also categorized as type 2 AMI, such as SCAD, vasospasm, and microvascular dysfunction. Nevertheless, the classical clinical picture of an acute myocardial injury or a type 2 AMI is that of a patient in sepsis, or with severe tachycardia and/or hypertension, evolving with elevated biomarkers.
References
- Gross, H.; Steinberg, W.H. Myocardial infarction without significant lesions of coronary arteries. Arch. Intern. Med. 1939, 64, 249–267.
- Safdar, B.; Spatz, E.S.; Dreyer, R.P.; Beltrame, J.F.; Lichtman, J.H.; Spertus, J.A.; Reynolds, H.R.; Geda, M.; Bueno, H.; Dziura, J.D.; et al. Presentation, Clinical Profile, and Prognosis of Young Patients with Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): Results from the Study. J. Am. Heart Assoc. 2018, 7, e009174.
- Dreyer, R.P.; Tavella, R.; Curtis, J.P.; Wang, Y.; Pauspathy, S.; Messenger, J.; Rumsfeld, J.S.; Maddox, T.M.; Krumholz, H.M.; Spertus, J.A.; et al. Myocardial infarction with non-obstructive coronary arteries as compared with myocardial infarction and obstructive coronary disease: Outcomes in a Medicare population. Eur. Heart J. 2020, 41, 870–878.
- Kumar, J.; Kumar, R.; Armstrong, R.; Murphy, R.; Daly, C. 1 Incidence and prevalence of MINOCA (myocardial infarction with non-obstructive coronary arteries) in STEMI patients: Experience from Irish tertiary care centre. Heart 2021, 107, A1–A2.
- Kilic, S.; Aydın, G.; Çoner, A.; Doğan, Y.; Arican Özlük, Ö.; Çelik, Y.; Ungan, I.; Tascanov, M.B.; Düz, R.; Polat, V.; et al. Prevalence and clinical profile of patients with myocardial infarction with non-obstructive coronary arteries in Turkey (MINOCA-TR): A national multi-center, observational study. Anatol. J. Cardiol. 2020, 23, 176–182.
- Dees, D.; Rahimi, F.; Amann, M.; Nührenberg, T.G.; Löffelhardt, N.; Schmitz, R.; Valina, C.M.; Neumann, F.J.; Hochholzer, W. Prevalence and Causes of Myocardial Infarction with Non-Obstructive Coronary Arteries in a Contemporary Cohort of Patients with Suspected Myocardial Infarction. J. Clin. Med. 2021, 10, 5188.
- Pelliccia, F.; Pasceri, V.; Niccoli, G.; Tanzilli, G.; Speciale, G.; Gaudio, C.; Crea, F.; Camici, P.G. Predictors of mortality in myocardial infarction and nonobstructed coronary arteries: A systematic review and Meta-regression. Am. J. Med. 2020, 133, 73–83.
- Adams, C.; Sawhney, G.; Singh, K. Comparing pharmacotherapy in MINOCA versus medically managed obstructive acute coronary syndrome. Heart Vessel 2022, 37, 705–710.
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015, 131, 861–870.
- Tamis-Holland, J.E.; Jneid, H. Myocardial infarction with nonobstructive coronary arteries (MINOCA): It’s time to face reality! J. Am. Heart Assoc. 2018, 7, e009635.
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2017, 38, 143–153.
- Tamis-Holland, J.E.; Jneid, H.; Reynolds, H.R.; Agewall, S.; Brilakis, E.S.; Brown, T.M.; Lerman, A.; Cushman, M.; Kumbhani, D.J.; Arslanian-Engoren, C.; et al. Contemporary Diagnosis and Management of Patients with Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e891–e908.
- Eggers, K.M.; Hjort, M.; Baron, T.; Jernberg, T.; Nordenskjöld, A.M.; Tornvall, P.; Lindahl, B. Morbidity and cause-specific mortality in first-time myocardial infarction with nonobstructive coronary arteries. J. Intern. Med. 2019, 285, 419–428.
- Nordenskjöld, A.M.; Lagerqvist, B.; Baron, T.; Jernberg, T.; Hadziosmanovic, N.; Reynolds, H.R.; Tornvall, P.; Lindahl, B. Reinfarction in Patients with Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): Coronary Findings and Prognosis. Am. J. Med. 2019, 132, 335–346.
- Reynolds, H.R.; Srichai, M.B.; Iqbal, S.N.; Slater, J.N.; Macini, G.B.J.; Feit, F.; Pena-Sing, I.; Axel, L.; Attubato, M.J.; Yatskar, L.; et al. Mechanisms of Myocardial Infarction in Women without Angiographically Obstructive Coronary Artery Disease. Circulation 2011, 124, 1414–1425.
- Ouldzein, H.; Elbaz, M.; Roncalli, J.; Cagnac, R.; Carrié, D.; Puel, J.; Alibelli-Chermarin, M.-J. Plaque rupture and morphological characteristics of the culprit lesion in acute coronary syndromes without significant angiographic lesion: Analysis by intravascular ultrasound. Ann. Cardiol. Angeiol. 2012, 61, 20–26.
- Reynolds, H.R.; Maehara, A.; Kwong, R.Y.; Sedlak, T.; Saw, J.; Smilowitz, N.R.; Mahmud, E.; Wei, J.; Marzo, K.; Matsumura, M.; et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation 2021, 143, 624–640.
- Chen, Y.-C.; Huang, A.L.; Kyaw, T.S.; Bobik, A.; Peter, K. Atherosclerotic Plaque Rupture. Identifying the Straw That Breaks the Camel’s Back. Arterioscler. Thromb. Vasc. Biol. 2016, 36, e63–e72.
- Vergallo, R.; Ren, X.; Yonetsu, T.; Kato, K.; Uemura, S.; Yu, B.; Jia, H.; Abtahian, F.; Aguirre, A.D.; Tian, J.; et al. Pancoronary plaque vulnerability in patients with acute coronary syndrome and ruptured culprit plaque: A 3-vessel optical coherence tomography study. Am. Heart J. 2014, 167, 59–67.
- Dugan, K.E.; Maehara, A.; Kwong, R.Y.; Mahajan, A.M.; Reynolds, H.R. Calcified nodule as a cause of myocardial infarction with non-obstructive coronary artery disease. Int. J. Case Rep. Images 2016, 7, 388–391.
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1282–1292.
- Okafor, O.N.; Gorog, D.A. Endogenous Fibrinolysis: An Important Mediator of Thrombus Formation and Cardiovascular Risk. J. Am. Coll. Cardiol. 2015, 65, 1683–1699.
- Pretty, H.C. Dissecting aneurysm of coronary artery in a woman aged 42: Rupture. Br. Med. J. 1931, 1, 667.
- Kim, E.S.H. Spontaneous Coronary-Artery Dissection. N. Engl. J. Med. 2020, 383, 2358–2370.
- Tweet, M.; Gulati, R.; Williamson, E.E.; Vrtiska, T.J.; Hayes, S.H. Multimodality Imaging for Spontaneous Coronary Artery Dissection in Women. JACC Cardiovasc. Imaging 2016, 9, 436–450.
- Saw, J.; Mancini, G.B.J.; Humphries, K.; Fung, A.; Boone, R.; Starovoytov, A.; Aymong, E. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter. Cardiovasc. Interv. 2016, 87, E54–E61.
- Saw, J.; Starovoytov, A.; Humphries, K.; Sheth, T.; So, D.; Minhas, K.; Brass, N.; Lavoie, A.; Bishop, H.; Lavi, S.; et al. Canadian spontaneous coronary artery dissection cohort study: In-hospital and 30-day outcomes. Eur. Heart J. 2019, 40, 1188–1197.
- Eleid, M.F.; Guddeti, R.R.; Tweet, M.S.; Lerman, A.; Singh, M.; Best, P.J.; Prasad, M.; Rihal, C.S.; Hayes, S.N.; Gulati, R. Coronary artery tortuosity in spontaneous coronary artery dissection: Angiographic characteristics and clinical implications. Circ. Cardiovasc. Interv. 2014, 7, 656–662.
- Saw, J.; Aymong, E.; Sedlak, T.; Buller, C.E.; Starovoytov, A.; Ricci, D.; Robinson, S.; Vuurmans, T.; Gao, M.; Humphries, K.; et al. Spontaneous coronary artery dissection: Association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ. Cardiovasc. Interv. 2014, 7, 645–655.
- Michelis, K.C.; Olin, J.W.; Kadian-Dodov, D.; d’Escamard, V.; Kovacic, J.C. Coronary artery manifestations of fibromuscular dysplasia. J. Am. Coll. Cardiol. 2014, 64, 1033–1046.
- Saw, J.; Humphries, K.; Aymong, E.; Sedlak, T.; Prakash, R.; Starovoytov, A.; Mancini, G.B.J. Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J. Am. Coll. Cardiol. 2017, 70, 1148–1158.
- Tweet, M.S.; Hayes, S.N.; Pitta, S.R.; Simari, R.D.; Lerman, A.; Lennon, R.J.; Gersh, B.J.; Khambatta, S.; Best, P.J.M.; Rihal, C.S.; et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012, 126, 579–588.
- Hayes, S.N.; Kim, E.S.H.; Saw, J.; Adlam, D.; Arslanian-Engoren, C.; Economy, K.E.; Ganesh, S.K.; Gulati, R.; Lindsay, M.E.; Mieres, J.H.; et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement from the American Heart Association. Circulation 2018, 137, e523–e557.
- Nakashima, T.; Noguchi, T.; Haruta, S.; Yamamoto, Y.; Oshima, S.; Nakao, K.; Taniguchi, Y.; Yamaguchi, J.; Tsuchihashi, K.; Seki, A.; et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the Angina Pectoris—Myocardial Infarction Multicenter Investigators in Japan. Int. J. Cardiol. 2016, 207, 341–348.
- Rogowski, S.; Maeder, M.T.; Weilenmann, D.; Haager, P.K.; Ammann, P.; Rohner, F.; Joerg, L.; Rickli, H. Spontaneous coronary artery dissection: Angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter. Cardiovasc. Interv. 2017, 89, 59–68.
- Elkayam, U.; Jalnapurkar, S.; Barakkat, M.N.; Khatri, N.; Kealey, A.J.; Mehra, A.; Roth, A. Pregnancy-associated acute myocardial infarction: A review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014, 129, 1695–1702.
- Tweet, M.S.; Hayes, S.N.; Codsi, E.; Gulati, R.; Rose, C.H.; Best, P.J.M. Spontaneous coronary artery dissection associated with pregnancy. J. Am. Coll. Cardiol. 2017, 70, 426–435.
- Faden, M.S.; Bottega, N.; Benjamin, A.; Brown, R.N. A nationwide evaluation of spontaneous coronary artery dissection in pregnancy and the puerperium. Heart 2016, 102, 1974–1979.
- Cade, J.R.; Szarf, G.; de Siqueira, M.E.M.; Chaves, A.; Andréa, J.C.M.; Figueira, H.R.; Gomes, M.M.; Freitas, B.P.; Medeiros, J.F.; dos Santos, M.R.; et al. Pregnancy-associated spontaneous coronary artery dissection: Insights from a case series of 13 patients. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 54–61.
- Kamel, H.; Roman, M.J.; Pitcher, A.; Devereux, R.B. Pregnancy and the risk of aortic dissection or rupture: A cohort-crossover analysis. Circulation 2016, 134, 527–533.
- Saw, J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ. Cardiovasc. Interv. 2017, 10, e005119.
- Tweet, M.S.; Eleid, M.F.; Best, P.J.; Lennon, R.J.; Lerman, A.; Rihal, C.S.; Holmes, D.R., Jr.; Hayes, A.N.; Gulati, R. Spontaneous coronary artery dissection: Revascularization versus conservative therapy. Circ. Cardiovasc. Interv. 2014, 7, 777–786.
- Rashid, H.N.Z.; Wong, D.T.L.; Wijesekera, H.; Gutman, S.J.; Shanmugam, V.B.; Gulati, R.; Malaipan, Y.; Meredith, I.T.; Psaltis, P.J. Incidence and characterization of spontaneous coronary artery dissection as a cause of acute coronary syndrome: A single-centre Australian experience. Int. J. Cardiol. 2016, 202, 336–338.
- Prakash, R.; Starovoytov, A.; Heydari, M.; Mancini, G.B.; Saw, J. Catheter-induced iatrogenic coronary artery dissection in patients with spontaneous coronary dissection. JACC Cardiovasc. Interv. 2016, 9, 1851–1853.
- Hayes, S.N.; Tweet, M.S.; Adlam, D.; Kim, E.S.H.; Gulati, R.; Price, J.E.; Rose, C.H. Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 961–984.
- Song, L.; Mintz, G.S.; Kadohira, T.; Fall, K.N.; Brogno, D.A.; Maehara, A. Spontaneous coronary artery dissection with intra-adventitial hematoma detected by high-definition intravascular ultrasound. Coron. Artery Dis. 2016, 27, 707–708.
- Tweet, M.S.; Akhtar, N.J.; Hayes, S.N.; Best, P.J.; Gulati, R.; Araoz, P.A. Spontaneous coronary artery dissection: Acute findings on coronary computed tomography angiography. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 467–475.
- Pozo-Osinalde, E.; Garcia-Guimaraes, M.; Bastante, T.; Aguilera, M.C.; Rodríguez-Alcudia, D.; Rivero, F.; Hernández, S.; Jiménez-Borregero, L.J.; Alfonso, F. Characteristic findings of acute spontaneous coronary artery dissection by cardiac computed tomography. Coron. Artery Dis. 2020, 31, 293–299.
- Prinzmetal, M.; Kennamer, R.; Merliss, R.; Wada, T.; Bor, N. Angina pectoris I. A variant form of angina pectoris: Preliminary report. Am. J. Med. 1959, 27, 375–388.
- Prinzmetal, M.; Ekmekci, A.; Kennamer, R.; Kwoczynski, J.K.; Shubin, H.; Toyoshima, H. Variant Form of Angina Pectoris. JAMA 1960, 174, 1794–1800.
- Aziz, A.; Hansen, H.S.; Sechtem, U.; Prescott, E.; Ong, P. Sex-related differences in vasomotor function in patients with angina and unobstructed coronary arteries. J. Am. Coll. Cardiol. 2017, 70, 2349–2358.
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified medical therapy using invasive coronary function testing in angina: The CorMicA trial. J. Am. Coll. Cardiol. 2018, 72, 2841–2855.
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Merz, C.N.B.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20.
- Matta, A.; Nader, V.; Canitrot, R.; Delmas, C.; Bouisset, F.; Lhermusier, T.; Blanco, S.; Campelo-Parada, F.; Elbaz, M.; Carrie, D.; et al. Myocardial bridging is significantly associated to myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 501–507.
- Nam, P.; Choi, B.G.; Choi, S.Y.; Byun, J.K.; Mashaly, A.; Park, Y.; Jang, W.Y.; Kim, W.; Choi, J.Y.; Park, E.J.; et al. The impact of myocardial bridge on coronary artery spasm and long-term clinical outcomes in patients without significant atherosclerotic stenosis. Atherosclerosis 2018, 270, 8–12.
- Montone, R.A.; Gurgoglione, F.L.; Del Buono, M.G.; Rinaldi, R.; Meucci, M.C.; Iannaccone, G.; La Vecchia, G.; Camilli, M.; D’Amario, D.; Leone, A.M.; et al. Interplay Between Myocardial Bridging and Coronary Spasm in Patients with Myocardial Ischemia and Non-Obstructive Coronary Arteries: Pathogenic and Prognostic Implications. J. Am. Heart Assoc. 2021, 10, e020535.
- Lanza, G.A.; Careri, G.; Crea, F. Mechanisms of Coronary Artery Spasm. Circulation 2011, 124, 1774–1782.
- Vanhoutte, P.M.; Shimokawa, H. Endothelium-derived relaxing factor and coronary vasospasm. Circulation 1989, 80, 1–9.
- Kaski, J.C.; Tousoulis, D.; Gavrielides, S.; McFadden, E.; Galassi, A.R.; Crea, F.; Maseri, A. Comparison of epicardial coronary artery tone and reactivity in Prinzmetal’s variant angina and chronic stable angina pectoris. J. Am. Coll. Cardiol. 1991, 17, 1058–1062.
- Bertrand, M.E.; LaBlanche, J.M.; Tilmant, P.Y.; Thieuleux, F.A.; Delforge, M.R.; Carre, A.G.; Asseman, P.; Berzin, B.; Libersa, C.; Laurent, J.M. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary angiography. Circulation 1982, 65, 1299–1306.
- Kaski, J.C.; Maseri, A.; Vejar, M.; Crea, F.; Hackett, D. Spontaneous coronary artery spasm in variant angina results from a local hyperreactivity to a generalized constrictor stimulus. J. Am. Coll. Cardiol. 1989, 14, 1456.
- Yasue, H.; Horio, Y.; Nakamura, N.; Fujii, H.; Sonoda, R.; Kugiyama, K.; Obata, K.; Morikami, Y.; Kimura, T. Induction of coronary artery spasm by acetylcholine in patients with variant angina: Possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation 1986, 74, 955–963.
- Crea, F.; Chierchia, S.; Kaski, J.C.; Davies, G.J.; Margonato, A.; Miran, D.O.; Maseri, A. Provocation of CAS by dopamine in patients with active variant angina pectoris. Circulation 1986, 74, 262–269.
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Merz, C.N.B.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2017, 38, 2565–2568.
- Previtali, M.; Ardissino, D.; Barberis, P.; Panciroli, C.; Chimienti, M.; Salerno, J.A. Hyperventilation and ergonovine tests in Prinzmetal’s variant angina pectoris in men. Am. J. Cardiol. 1989, 63, 17–20.
- Zaya, M.; Mehta, P.K.; Merz, N.B. Provocative Testing for Coronary Reactivity and Spasm. J. Am. Coll. Cardiol. 2014, 63, 103–109.
- Takagi, Y.; Yasuda, S.; Takahashi, J.; Tsunoda, R.; Ogata, Y.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; Tanabe, Y.; et al. Clinical implications of provocation tests for coronary artery spasm: Safety, arrhythmic complications, and prognostic impact: Multicentre registry study of the Japanese Coronary Spasm Association. Eur. Heart J. 2013, 34, 258–267.
- Ong, P.; Athanasiadis, A.; Borgulya, G.; Vokshi, I.; Bastiaenen, R.; Kubik, S.; Hill, S.; Schäufele, T.; Mahrholdt, H.; Kaski, J.C.; et al. Clinical Usefulness, Angiographic Characteristics, and Safety Evaluation of Intracoronary Acetylcholine Provocation Testing Among 921 Consecutive White Patients with Unobstructed Coronary Arteries. Circulation 2014, 129, 1723–1730.
- Montone, R.A.; Niccoli, G.; Fracassi, F.; Russo, M.; Gurgoglione, F.; Cammà, G.; Lanza, G.A.; Crea, F. Patients with acute myocardial infarction and non-obstructive coronary arteries: Safety and prognostic relevance of invasive coronary provocative tests. Eur. Heart J. 2018, 39, 91–98.
- Shibata, T.; Kawakami, S.; Noguchi, T.; Tanaka, T.; Asaumi, Y.; Kanaya, T.; Nagai, T.; Nakao, K.; Fujino, M.; Nagatsuka, K.; et al. Prevalence, Clinical Features, and Prognosis of Acute Myocardial Infarction Attributable to Coronary Artery Embolism. Circulation 2015, 132, 241–250.
- Cannon, R.O., 3rd; Epstein, S.E. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am. J. Cardiol. 1988, 61, 1338–1343.
- Camici, P.G.; Crea, F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007, 356, 830–840.
- Kern, M.J.; Lerman, A.; Bech, J.W.; De Bruyne, B.; Eeckhout, E.; Fearon, W.F.; Higano, S.T.; Lim, M.J.; Meuwissen, M.; Piek, J.J.; et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: A scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation 2006, 114, 1321–1341.
- Melikian, N.; Vercauteren, S.; Fearon, W.F.; Cuisset, T.; MacCarthy, P.A.; Davidavicius, G.; Aarnoudse, W.; Bartunek, J.; Vanderheyden, M.; Wyffels, E.; et al. Quantitative assessment of coronary microvascular function in patients with and without epicardial atherosclerosis. EuroIntervention 2010, 5, 939–945.
- Luo, C.; Long, M.; Hu, X.; Huang, Z.; Hu, C.; Gao, X.; Du, Z. Thermodilution-Derived Coronary Microvascular Resistance and Flow Reserve in Patients with Cardiac Syndrome X. Circ. Cardiovasc. Interv. 2014, 7, 43–48.
- Solberg, O.G.; Ragnarsson, A.; Kvarsnes, A.; Endresen, K.; Kongsgârd, E.; Aakhus, S.; Gullestad, L.; Stavem, K.; Aaberge, L. Reference interval for the index of coronary microvascular resistance. EuroIntervention 2014, 9, 1069–1075.
- Rahman, H.; Ryan, M.; Lumley, M.; Modi, B.; McConkey, H.; Ellis, H.; Scannell, C.; Clapp, B.; Marber, M.; Webb, A.; et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation 2019, 140, 1805–1816.
- Rahman, H.; Demir, O.M.; Khan, F.; Ryan, M.; Ellis, H.; Mills, M.T.; Chiribiri, A.; Webb, A.; Perera, D. Physiological Stratification of Patients with Angina Due to Coronary Microvascular Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 2538–2549.
- Shaw, L.J.; Merz, N.B.; Pepine, C.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Mankad, S.; Sharaf, B.L.; et al. Insights From the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: Gender Differences in Traditional and Novel Risk Factors, Symptom Evaluation, and Gender-Optimized Diagnostic Strategies. J. Am. Coll. Cardiol. 2006, 47, S4–S20.
- Johnson, B.D.; Shaw, L.J.; Buchthal, S.D.; Merz, C.N.B.; Kim, H.-W.; Scott, K.N.; Olson, M.B.; Pepine, C.J.; Hollander, J.; Sharaf, B.; et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease; results from the WISE. Circulation 2004, 109, 2993–2999.
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J. Clin. Med. 2020, 9, 2880.
- Godo, S.; Suda, A.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Coronary Microvascular Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1625–1637.
- Ayub, M.T.; Kalra, D. Coronary Microvascular Dysfunction and the Role of Noninvasive Cardiovascular Imaging. Diagnostics 2020, 10, 679.
- Reis, S.E.; Holubkov, R.; Smith, A.J.C.; Kelsey, S.F.; Sharaf, B.L.; Reichek, N.; Rogers, W.J.; Merz, C.N.; Sopko, G.; Pepine, C.J.; et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: Results from the NHLBI WISE study. Am. Heart J. 2001, 141, 735–741.
- Fearon, W.F.; Balsam, L.B.; Farouque, H.M.; Caffarelli, A.D.; Robbins, R.C.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Novel index for invasively assessing the coronary microcirculation. Circulation 2003, 107, 3129–3132, Erratum in Circulation 2003, 108, 3165.
- Ng, M.K.; Yeung, A.C.; Fearon, W.F. Invasive assessment of the coronary microcirculation: Superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation 2006, 113, 2054–2061.
- Aarnoudse, W.; Fearon, W.F.; Manoharan, G.; Geven, M.; van de Vosse, F.; Rutten, M.; De Bruyne, B.; Pijls, N.H.J. Epicardial Stenosis Severity Does Not Affect Minimal Microcirculatory Resistance. Circulation 2004, 110, 2137–2142.
- Teunissen, P.F.; de Waard, G.A.; Hollander, M.R.; Robbers, L.F.; Danad, I.; Biesbroek, P.S.; Amier, R.P.; Echavarría-Pinto, M.; Quirós, A.; Broyd, C.; et al. Doppler-derived intracoronary physiology indices predict the occurrence of microvascular injury and microvascular perfusion deficits after angiographically successful primary percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2015, 8, e001786.
- Lee, S.H.; Lee, J.M.; Park, J.; Choi, K.H.; Hwang, D.; Doh, J.H.; Nam, C.W.; Shin, E.S.; Hoshino, M.; Murai, T.; et al. Prognostic Implications of Resistive Reserve Ratio in Patients with Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e015846.
- De Bruyne, B.; Pijls, N.H.J.; Gallinoro, E.; Candreva, A.; Fournier, S.; Keulards, D.C.J.; Sonck, J.; Van’t Veer, M.; Barbato, E.; Bartunek, J.; et al. Microvascular Resistance Reserve for Assessment of Coronary Microvascular Function: JACC Technology Corner. J. Am. Coll. Cardiol. 2021, 78, 1541–1549.
- Morris, P.D.; Gosling, R.; Zwierzak, I.; Evans, H.; Aubiniere-Robb, L.; Czechowicz, K.; Evans, P.C.; Hose, D.R.; Lawford, P.V.; Narracott, A.J.; et al. A novel method for measuring absolute coronary blood flow and microvascular resistance in patients with ischaemic heart disease. Cardiovasc. Res. 2021, 117, 1567–1577.
- Mangiacapra, F.; Viscusi, M.M.; Paolucci, L.; Nusca, A.; Melfi, R.; Ussia, G.P.; Grigioni, F. The Pivotal Role of Invasive Functional Assessment in Patients with Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA). Front. Cardiovasc. Med. 2021, 8, 781485.
- Lindahl, B.; Baron, T.; Albertucci, M.; Prati, F. Myocardial infarction with non-obstructive coronary artery disease. EuroIntervention 2021, 17, 875–887.
- McCarthy, C.; Murphy, S.; Cohen, J.A.; Rehman, S.; Jones-O’Connor, M.; Olshan, D.S.; Singh, A.; Vaduganathan, M.; Januzzi, J.L., Jr.; Wasfy, J.H. Misclassification of Myocardial Injury as Myocardial Infarction: Implications for Assessing Outcomes in Value-Based Programs. JAMA Cardiol. 2019, 4, 460–464.
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
2 times
(View History)
Update Date:
20 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




