
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hui Yang | -- | 2667 | 2022-10-19 14:39:44 | | | |
| 2 | Lindsay Dong | -3 word(s) | 2664 | 2022-10-20 10:03:38 | | |
Video Upload Options
Food allergy is a repeatable atopic disorder resulting from exposure to specific food allergens that can lead to life-threatening allergic reactions. Food allergens are digested and absorbed in the gut, mainly in the small intestine, which contains symbiotic microbiota. With the co-evolution of humans and microbiota, symbiotic microbes inevitably have a considerable impact on human health. The human intestinal microbiota, especially the ileum and colon, plays an important role in the intestinal mucosal immunity, by promoting local homeostatic interactions and regulating the immune response to food allergens in the peripheral mucosa
1. Food Allergy

2. Effect of Intestinal Microbiota on Mucosal Immune System
3. Influence of Dietary Intakes on Intestinal Microbiota Composition
There is a high-fat diet (HFD) alters the composition of the intestinal microbiota. For example, HFD-fed mice showed increased abundances of Firmicutes, Proteobacteria and Actinobacteria, while decreased abundances of Bacteroidetes phylum, Bifidobacterium, and Akkermansia genera compared to normal mice [12]. Meanwhile, HFD consumption in humans resulted in increased abundances of Blautia, Alistipes, Bilophila, several genera of the group Gammaproteobacteria, and decreased abundances of Roseburia, Clostridium, and Bacteroidess spp. [13]. Interestingly, gender also influenced the changes in the microbiota after HFD intake with higher abundances of Campylobacter, Blautia, Flavonifractor and Erysipelatoclostridium, while the abundances of Anaerotruncus, Eisenbergiella, Clostridiales (FamilyXIIIUCG_001) and Lachnospiraceae were higher in males [14]. Protein is a necessary ingredient in dietary and plays an important role in maintaining host health. It is worth noting that a large number of studies have confirmed that dietary protein intake also regulated the diversity of intestinal microbiota, and there are close relationships between different dietary protein sources and intestinal microbiota profiles. As for plant proteins intake, the abundances of Bifidobacterium and Lactobacillus increased; However, with the intake of animal protein, the abundances of Bifdobacterium, Roseburia, and Eubacterium rectale decreased, and the levels of Bacteroides, Bilophila, and Alistipes markedly increased [15]. Among them, Roseburia spp. is the most important and abundant bacteria that are involved in butyrate production in the intestine; Bifidobacterium is commonly found in infants’ intestine and increases in these bacteria have been suggested to relieve symptoms of IgE or Th2 allergy [16]. Similarly, animal protein-based diets have been documented to result in increased relative abundances of Enterococcus, Streptococcus, Turicibater, Escherichia, Peptostreptococcaceae, as well as Ruminococcaceaea in mice; in contrast, plant proteins-based diets enriched Bifidobacteriaceae, Desulfovibrionaceae, and Coriobacteriaceae families in mice. Notably, diets based on both animal and plant proteins showed increased abundances of lactobacilli, Lachnospiraceae, and Erysipelotrichaceae [17]. Intake of a fiber-rich diet increased the abundances of Bifidobacterium, Prevotellaceae, and Lachnospiraceae in mice, while decreasing the abundances of Porphyromonadaceae and Lactobacilli [18]. Vitamins also have the potential to modulate the intestinal microbiota.
4. Relationship between the Intestinal Microbiota and Food Allergy
4.1. Changes in the Intestinal Microbiota in Patients with Food Allergy
Growing evidence suggests that gut dysbiosis, an imbalance in the intestinal microbiota, plays a decisive role in the development of food allergy. Briefly, the intestinal microbiota composition of people with food allergy differs significantly from that of healthy people, and these differences are more evident in infants and children (Table 1).
| Allergens Involved | Study Population | Age | Changes of Gut Microbiota in Food Allergy | Reference |
|---|---|---|---|---|
| Milk, eggs, wheat, nut, peanuts, fish, shrimp, soybeans | 166 infants | 0 to 1 year | ↑ Enterobacteriaceae/Bacteroidaceae ratio ↓ Ruminococcaceae |
[19] |
| Milk, eggs, wheat, nut, peanuts, fish, shrimp, soybeans | 49 healthy infants 38 infants with food allergy |
1 week to 12 months of age | ↑ Bifidobacterium spp. — Lactobacillus spp. and Clostridium perfringens |
[20] |
| Milk, eggs, wheat, nut, peanuts, fish, shrimp, soybean | 34 infants with food allergy 45 healthy controls |
2 to 11 years | ↑ Firmicutes and Fusobacteria ↓ Bacteroidetes, Proteobacteria and Actinobacteria ↑ Clostridium sensustricto and Anaerobacter ↓ Bacteroides and Clostridium XVIII |
[21] |
| Egg white, cow’s milk, wheat, peanut, soybean, gluten | 23 children with food allergy 22 healthy children |
6 to 24 months | ↑ Sphingomonas, Sutterella, Bifidobacterium, Collinsella, Clostridium sensustricto, Clostridium IV, Enterococcus, Lactobacillus, Roseburia, Faecalibacterium, Ruminococcus, Subdoligranulum, and Akkermansia, ↓ Bacteroides, Parabacteroides, Prevotella, Alistipes, Streptococcus, and Veillonella |
[22] |
| Egg, soybean, sesame, milk, shrimp, crab, peanut, wheat | 4 children with food allergy 4 healthy children |
18 months to 6 years | ↓ Dorea and Akkermansia ↑ Lachnospira, Veillonella, and Sutterella |
[23] |
| Egg | 141 children with egg allergy | 3 to 16 months | Lachnospiraceae, Streptococcaceae, and Leuconostocaceae families were differentially abundant in children with egg allergy | [24] |
| Cow milk | 226 children with milk allergy | 3 to 16 months | Clostridia and Firmicutes could be studied as probiotic candidates for milk allergy therapy | [25] |
| Peanuts, tree nuts, shellfish | 1879 participants was 81.5%, ranging from 2.5% for peanuts to 40.5% for seasonal. | mean age, 45.5 years; 46.9% male | higher Bacteroidales and reduced Clostridiales taxa in nut and seasonal allergies | [26] |
| Egg, crab, shrimp | 256 children with food allergy | 4 to 12 years | Bifidobacterium lactis can effectively alleviate allergic reactions on food-specific IgE of food in children | [27] |
In summary, these findings suggest that the intestinal microbiota is critical for modulating food allergy and suggest that regulating the microbiota community composition may be therapeutically relevant for food allergy. So far, research on the characteristics of the intestinal microbiota in patients with food allergy is still in its infancy, and no specific bacterial taxa has been identified that may be associated with the occurrence of food allergy. Additionally, the main limitation of all these studies is that the number of patients with sensitization or food allergy is small, such that statistical analyses of the effects of potential confounding variables, such as delivery modes, breastfeeding, diet, antibiotic intake, and pets, have not been possible. In addition, several studies have focused on sensitization to food rather than evaluating people with a history of allergic reactions to food or a confirmatory food challenge coupled with skin and/or serum IgE food-specific testing.
4.2. Relationship between Intestinal Microbial Metabolites and Food Allergy
The intestinal microbiota is involved in developing and regulating host physiology and immunity by directly participating in the decomposition of dietary components to synthesize and re-synthesize metabolites. Dietary fiber, composed of indigestible carbohydrates extracted from plant polysaccharides and oligosaccharides, is the main source of nutrients for intestinal bacteria, and their fermentation leads to the production of SCFAs (mainly acetic, butyric, and propionic acid). SCFAs are key intermediaries regulating mucosal and systemic immune homeostasis. Intestinal IgA, regulated by the intestinal microbiota, also plays an important role in maintaining intestinal homeostasis and normal function. The SCFAs metabolite, acetate, has been proved to promote intestinal IgA responses mediated by the “metabolite-sensing” receptor, GPR43 [28]. Treatment of mice with the SCFA, propionate, resulted in bone marrow hematopoietic alterations characterized by enhanced generation of macrophages and DC precursors via GPR41, which inhibits Th2 effector cells, thus alleviating allergic inflammation [29]. Moreover, GPR109a is required for butyrate-mediated Treg cells induction and IL-18 in the formation of immune tolerance to food allergens [30].
Emerging evidence has shown that microbiota-derived tryptophan and bile-acid metabolites also play vital roles in food allergy. Tryptophan is an essential amino acid that is degraded to serotonin, kynurenine, or indole in the gut. Supplementation of D-tryptophan produced by Bifidobacterium and Lactobacillus has been shown to inhibit allergic inflammation in the lungs by increasing intestinal microbial diversity and promoting Treg cell production [31].
4.3. Potential Mechanisms of Intestinal Microbiota in Regulating Food Allergy
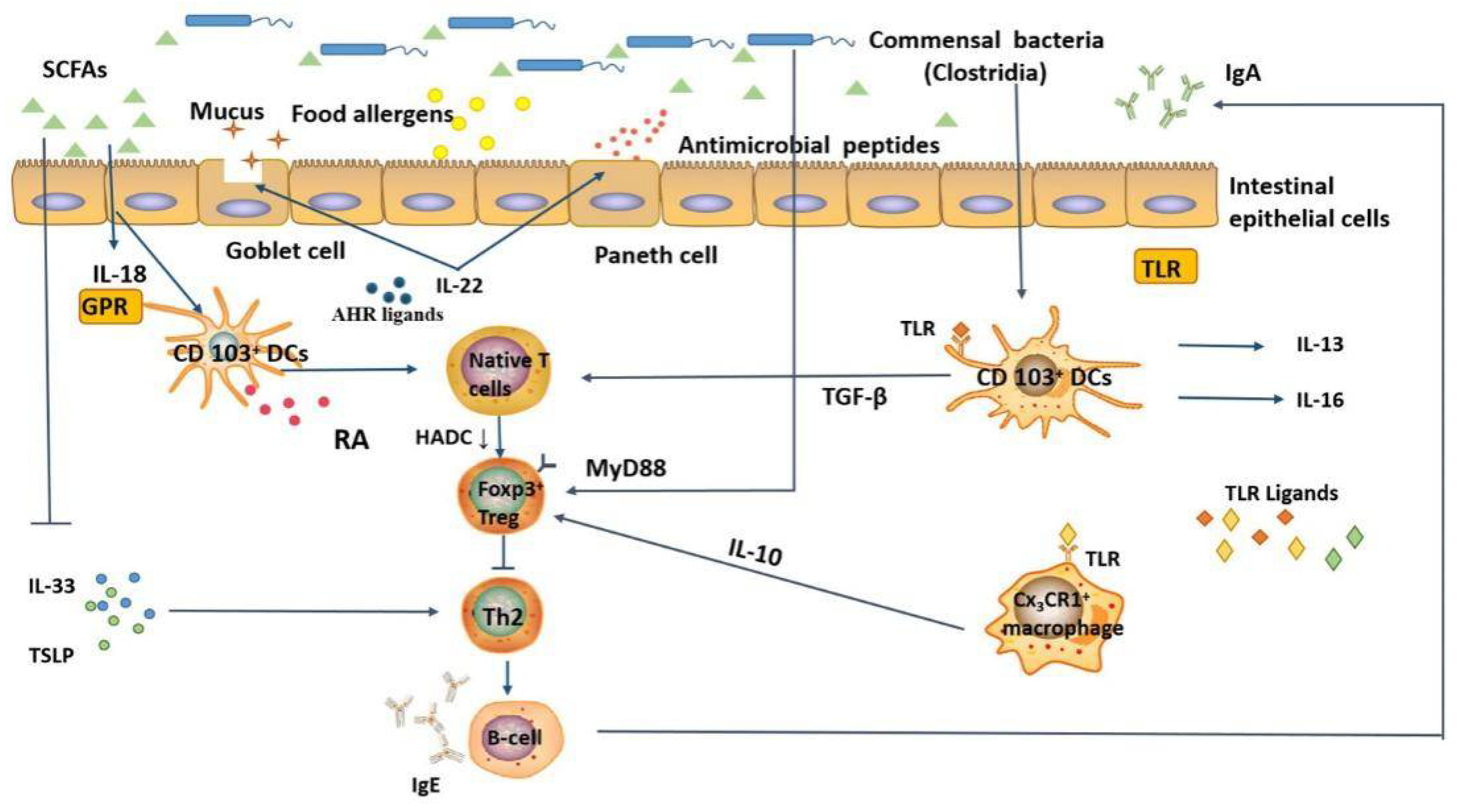
5. The Role of Probiotics in Relieving Allergy
Probiotics are active microbiota that positively impact human health when administered appropriately. They regulate the microbiological balance, inhibit the growth of harmful bacteria, lower blood lipids and cholesterol concentrations, maintain normal immunity of the gut mucosa, and regulate blood pressure [32]. Probiotics mainly include lactic acid bacteria, Bifidobacteria, and yeast, with lactic acid bacteria being the primary probiotic bacteria. The probiotics with the greatest potential in promoting the development of the immune system are those belonging to the genus Lactobacillus and Bifidobacterium. At present, the use of probiotics for the prevention and treatment of allergic diseases mainly focuses on maintaining the Th1/Th2 cell balance, improving intestinal barrier function, and maintaining the balance of intestinal microbiota. Reportedly, the addition of Lacticaseibacillus rhamnosus, but not Lacticaseibacillus casei or Bifidobacterium, has been reported to be effective in accelerating the induction of oral tolerance in individuals with milk allergy [33]. Other studies have shown that supplementation with Lacticaseibacillus rhamnosus promote the abundance of butyrate-producing strains such as Lachnospiraceae and Ruminoccaceae, suggesting that probiotics can increase the abundances of tolerance-promoting microbes and promote immune tolerance to food allergens [34].
Many probiotics have been shown to have potential immunomodulatory effects in immune cell models. Although in vitro cell models have some limitations, they have played a crucial role in the preliminary screening of the effects of different bacteria and metabolic components on the immune response.
Fermentation can improve the nutritional value of food by increasing the bioavailability of nutrients and reducing the amount of antinutritional factors. Moreover, fermented food can be used as a functional ingredient with high protein digestibility and probiotics.
Probiotics can be used as an effective tool for allergy prevention and alleviation. Bifidobacterium species have been shown to prevent and treat egg allergic reactions by promoting the synthesis of Tregs and inhibiting Th2 cells, to increase the number of CD103+ DCs in gut-associated lymphoid tissue CD103+ DCs [35].
References
- Pratap, K.; Taki, A.C.; Johnston, E.B.; Lopata, A.L.; Kamath, S.D. A Comprehensive Review on Natural Bioactive Compounds and Probiotics as Potential Therapeutics in Food Allergy Treatment. Front. Immunol. 2020, 11, 996.
- De Martinis, M.; Sirufo, M.M.; Viscido, A.; Ginaldi, L. Food Allergy Insights: A Changing Landscape. Arch. Immunol. Ther. Exp. 2020, 68, 1–15.
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117.
- Jakobsson, H.E.; Rodríguez-Piñeiro, A.M.; Schütte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Bäckhed, F.; Hansson, G.C.; Johansson, M.E.V. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015, 16, 164–177.
- Kwon, O.; Han, T.-S.; Son, M.-Y. Intestinal Morphogenesis in Development, Regeneration, and Disease: The Potential Utility of Intestinal Organoids for Studying Compartmentalization of the Crypt-Villus Structure. Front. Cell Dev. Biol. 2020, 8, 593969.
- Rotkiewicz, T.; Rotkiewicz, Z.; Depta, A.; Kander, M. Effect of Lactobacillus acidophilus and Bifidobacterium sp. on the course of Cryptosporidium parvum invasion in new-born piglets. Bull.-Vet. Inst. Pulawy 2001, 45, 187–196.
- Nielsen, D.S.G.; Jensen, B.B.; Theil, P.K.; Nielsen, T.S.; Knudsen, K.E.B.; Purup, S. Effect of butyrate and fermentation products on epithelial integrity in a mucus-secreting human colon cell line. J. Funct. Foods 2018, 40, 9–17.
- Knudsen, K.E.B.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Nielsen, D.S.G.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499.
- Diao, H.; Jiao, A.R.; Yu, B.; Mao, X.B.; Chen, D.W. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr. 2019, 14, 1–16.
- Sonner, J.K.; Keil, M.; Falk-Paulsen, M.; Mishra, N.; Rehman, A.; Kramer, M.; Deumelandt, K.; Röwe, J.; Sanghvi, K.; Wolf, L.; et al. Dietary tryptophan links encephalogenicity of autoreactive T cells with gut microbial ecology. Nat. Commun. 2019, 10, 1–14.
- Cui, B.; Liu, X.; Fang, Y.; Zhou, P.; Zhang, Y.; Wang, Y. Flagellin as a vaccine adjuvant. Expert Rev. Vaccines 2018, 17, 335–349.
- Coelho, O.G.L.; Cândido, F.G.; Alfenas, R.D.C.G. Dietary fat and gut microbiota: Mechanisms involved in obesity control. Crit. Rev. Food Sci. Nutr. 2019, 59, 3045–3053.
- Agans, R.; Gordon, A.; Kramer, D.L.; Perez-Burillo, S.; Rufián-Henares, J.A.; Paliy, O. Dietary Fatty Acids Sustain the Growth of the Human Gut Microbiota. Appl. Environ. Microbiol. 2018, 84, e01525-18.
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Lominchar, M.G.M.; Juan, C.S.; Larrosa, M. Microbiota Features Associated With a High-Fat/Low-Fiber Diet in Healthy Adults. Front. Nutr. 2020, 7, 583608.
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73.
- Cheng, R.; Yao, J.; Wan, Q.; Guo, J.; Pu, F.; Shi, L.; Hu, W.; Yang, Y.; Li, L.; Li, M.; et al. Oral administration of Bifidobacterium bifidum TMC3115 to neonatal mice may alleviate IgE-mediated allergic risk in adulthood. Benef. Microbes 2018, 9, 815–828.
- Kostovcikova, K.; Coufal, S.; Galanova, N.; Fajstova, A.; Hudcovic, T.; Kostovcik, M.; Prochazkova, P.; Jiraskova Zakostelska, Z.; Cermakova, M.; Sediva, B.; et al. Diet Rich in Animal Protein Promotes Pro-inflammatory Macrophage Response and Exacerbates Colitis in Mice. Front. Immunol. 2019, 10, 919.
- Hashemi, Z.; Fouhse, J.; Im, H.S.; Chan, C.B.; Willing, B.P. Dietary Pea Fiber Supplementation Improves Glycemia and Induces Changes in the Composition of Gut Microbiota, Serum Short Chain Fatty Acid Profile and Expression of Mucins in Glucose Intolerant Rats. Nutrients 2017, 9, 1236.
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.; Sears, M.R.; HayGlass, K.T.; Mandhane, P.J.; Turvey, S.; Subbarao, P.; Becker, A.B.; et al. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin. Exp. Allergy 2015, 45, 632–643.
- Qin, Y.; Wang, M.; Dong, P. Association Between Food Allergy and Microbiome Status in 1-Year-Old Infants in STRONG Kids 2 Cohort; Illinois Library: Urbana, IL, USA, 2018; Available online: https://hdl.handle.net/2142/99865 (accessed on 1 April 2018).
- Ling, Z.; Li, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Tong, X.; Yuan, L.; Wang, Y.; Sun, J.; Li, L.; et al. Altered Fecal Microbiota Composition Associated with Food Allergy in Infants. Appl. Environ. Microbiol. 2014, 80, 2546–2554.
- Chen, C.; Chen, K.-J.; Kong, M.; Chang, H.; Huang, J. Alterations in the gut microbiotas of children with food sensitization in early life. Pediatr. Allergy Immunol. 2016, 27, 254–262.
- Inoue, R.; Sawai, T.; Sawai, C.; Nakatani, M.; Romero-Pérez, G.A.; Ozeki, M.; Nonomura, K.; Tsukahara, T. A preliminary study of gut dysbiosis in children with food allergy. Biosci. Biotechnol. Biochem. 2017, 81, 2396–2399.
- Fazlollahi, M.; Chun, Y.; Grishin, A.; Wood, R.A.; Burks, A.W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.A.; Sicherer, S.H.; et al. Early-life gut microbiome and egg allergy. Allergy 2018, 73, 1515–1524.
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.; Sampson, H.; Sicherer, S.; et al. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130.
- Hua, X.; Goedert, J.J.; Pu, A.; Yu, G.; Shi, J. Allergy associations with the adult fecal microbiota: Analysis of the American Gut Project. eBioMedicine 2016, 3, 172–179.
- Liu, Q.; Jing, W.; Wang, W. Bifidobacterium lactis Ameliorates the Risk of Food Allergy in Chinese Children by Affecting Relative Percentage of Treg and Th17 Cells. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 4561038.
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017, 10, 946–956.
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166.
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139.
- Kepert, I.; Fonseca, J.; Müller, C.; Milger, K.; Hochwind, K.; Kostric, M.; Fedoseeva, M.; Ohnmacht, C.; Dehmel, S.; Nathan, P.; et al. D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J. Allergy Clin. Immunol. 2017, 139, 1525–1535.
- Al-Sahlany, S.T.; Niamah, A.K. Bacterial viability, antioxidant stability, antimutagenicity and sensory properties of onion types fermentation by using probiotic starter during storage. Nutr. Food Sci. 2022, 52, 901–916.
- Berni Canani, R.; Di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; Di Scala, C.; Granata, V.; Nocerino, R. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J. Allergy Clin. Immunol. 2017, 139, 1906–1913.e4.
- Berni Canani, R.; Sangwan, N.; Stefka, A.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016, 10, 742–750.
- Yang, B.; Xiao, L.; Liu, S.; Liu, X.; Luo, Y.; Ji, Q.; Yang, P.; Liu, Z. Exploration of the effect of probiotics supplementation on intestinal microbiota of food allergic mice. Am. J. Transl. Res. 2017, 9, 376–385.




