Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniel de Moraes Ferreira Jorge | -- | 1134 | 2022-10-19 01:46:53 | | | |
| 2 | Conner Chen | Meta information modification | 1134 | 2022-10-19 04:33:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jorge, D.D.M.F.; Huber, S.C.; Rodrigues, B.L.; Fonseca, L.F.D.; Azzini, G.O.M.; Parada, C.A.; Paulus-Romero, C.; Lana, J.F.S.D. The Mechanisms of Action of Pulsed Radiofrequency. Encyclopedia. Available online: https://encyclopedia.pub/entry/29979 (accessed on 07 February 2026).
Jorge DDMF, Huber SC, Rodrigues BL, Fonseca LFD, Azzini GOM, Parada CA, et al. The Mechanisms of Action of Pulsed Radiofrequency. Encyclopedia. Available at: https://encyclopedia.pub/entry/29979. Accessed February 07, 2026.
Jorge, Daniel De Moraes Ferreira, Stephany Cares Huber, Bruno Lima Rodrigues, Lucas Furtado Da Fonseca, Gabriel Ohana Marques Azzini, Carlos Amilcar Parada, Christian Paulus-Romero, José Fábio Santos Duarte Lana. "The Mechanisms of Action of Pulsed Radiofrequency" Encyclopedia, https://encyclopedia.pub/entry/29979 (accessed February 07, 2026).
Jorge, D.D.M.F., Huber, S.C., Rodrigues, B.L., Fonseca, L.F.D., Azzini, G.O.M., Parada, C.A., Paulus-Romero, C., & Lana, J.F.S.D. (2022, October 19). The Mechanisms of Action of Pulsed Radiofrequency. In Encyclopedia. https://encyclopedia.pub/entry/29979
Jorge, Daniel De Moraes Ferreira, et al. "The Mechanisms of Action of Pulsed Radiofrequency." Encyclopedia. Web. 19 October, 2022.
Copy Citation
Radiofrequency energy is a common treatment modality for chronic pain. While there are different forms of radiofrequency-based therapeutics, the common concept is the generation of an electromagnetic field in the applied area, that can result in neuromodulation (pulsed radiofrequency—PRF) or ablation. Radiofrequency (RF) energy-based procedures, whether conventional, ablative or pulsed, represent a technique commonly performed for chronic pain in a variety of musculoskeletal conditions.
pulsed radiofrequency
orthobiologics
neuromodulation
1. Introduction
Radiofrequency (RF) energy-based procedures, whether conventional, ablative or pulsed, represent a technique commonly performed for chronic pain in a variety of musculoskeletal conditions [1][2][3].
Pulsed radiofrequency (PRF) is derived from conventional RF with the aim of a less destructive RF-based treatment to be applied to the afferent nerve pathways of injured tissues [4]. PRF creates an electromagnetic field with the aim of functionally disrupting the neuronal membrane, which modulates gene expression, affecting the release of cytokines [5]. The application of PRF is based on the delivery of a train of sinusoidal electrical bursts (5–20 ms length) in the radiofrequency range (500 kHz) at a repetitive rate of a few hertz (2–5 Hz) [6] (Figure 1).
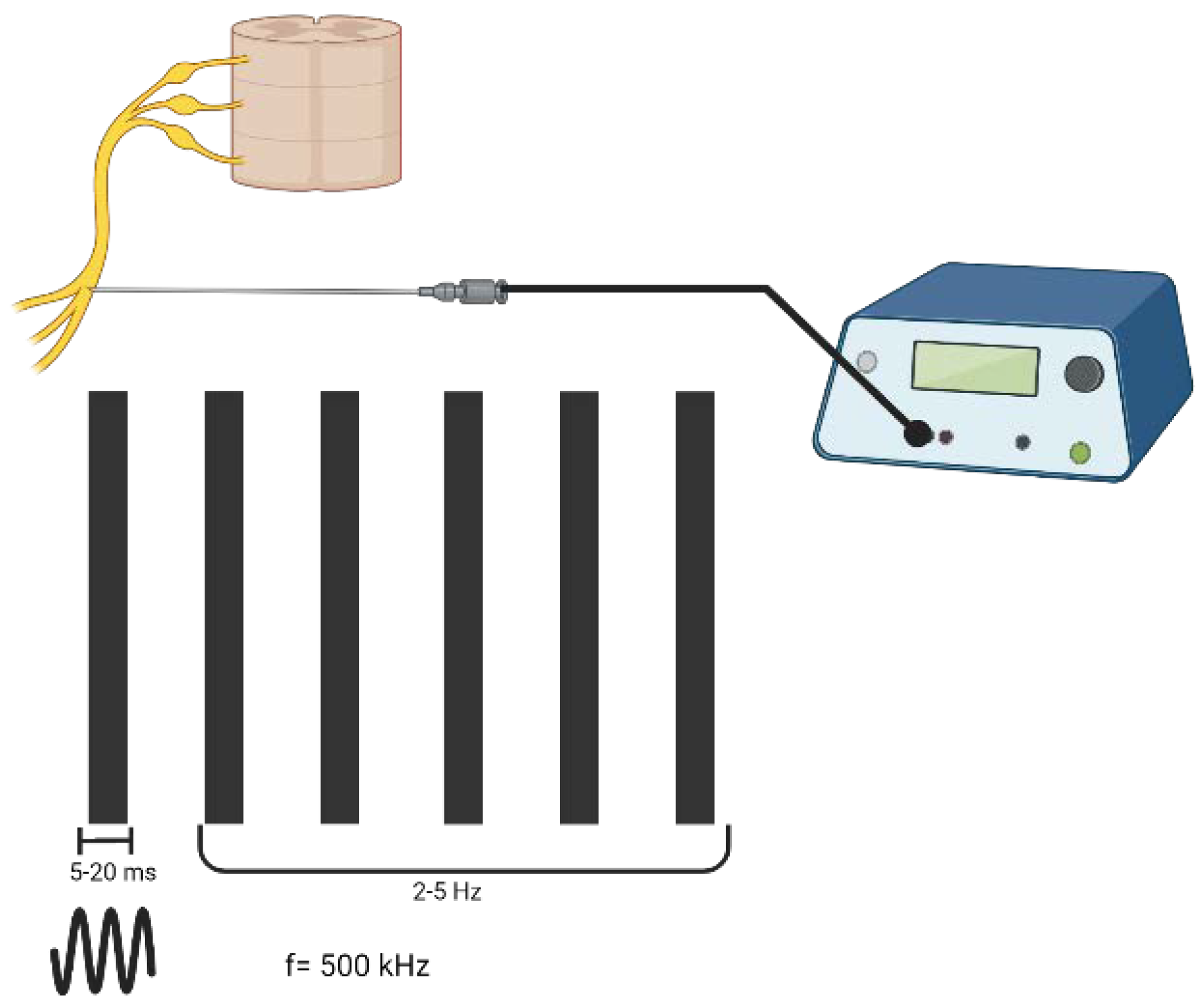
Figure 1. The parameters of pulsed radiofrequency; the way waveforms of electricity are delivered to tissues. Created with BioRender.com, accessed on 6 September 2022.
The changes produced by electrical fields are selective for small unmyelinated and lightly myelinated nerve fibers, producing a motor-sparing effect [5]. Histological evaluations show that PRF promotes transient endoneural edema that can persist for up to 1 week following treatment [7]. The pain relief commonly seen after PRF treatment can last up to several months [8]. Currently, the most common pathologies treated with PRF are radicular pain, occipital and trigeminal neuralgia, and shoulder and knee pain [9].
Although both techniques have been reported to be effective (pulsed and ablative RF), there are some limitations more related to the ablative RF, such as failure to completely denervate the nerve, disrupt nociceptive sensation, worsening of symptoms due to aberrant neuronal regeneration, neuroma formation, and reduced motor function, neuritis, paresthesia and deafferentation pain syndrome [10].
2. Pulsed Radiofrequency: Mechanisms of Action
Some studies demonstrated that the analgesic effect of PRF is not related to thermal effects or to permanent physical neural damage [11][12]. This effect could be due to a neuromodulatory-type process, which alters the synaptic transmission or the excitability of C-fibers [5][13]. These fibers are responsible for pain and temperature sensations and are involved in most neuropathic pain syndromes [14].
Preclinical studies have proposed several biological effects of PRF. The mechanisms of action may consist of morphological changes in the inner structures of axons [15], molecular effects, including alterations in cellular activity [16], gene expression [8][17][18], an increase in the expression of inflammatory cytokines [15] and inhibition of extracellular signal-regulated kinases [19]. Recently, it was demonstrated that PRF may have a long-term depression effect on neuropathic pain [20]. These reported mechanisms help to elucidate how PRF inhibits the transmission of pain signals in a biological pathway. However, the biophysical mechanisms by which this electric field may act are not clearly elucidated yet [6]. One of the main effects reported after a PRF treatment is the increase in cytosolic free calcium concentration, an important messenger involved in both short and long-term cellular processes. This mechanism could link PRF effects to a direct consequence on electric fields [6].
In addition, it was hypothesized that PRF could cause neural membrane permeabilization due to a mild electroporation process leading to a Ca2+ influx. Once the cell is exposed to high electric fields for a short period, the cell membrane increases its permeability to ions and molecules, a process called electroporation. Accordingly, PRF could be applied to different regions of afferent nervous pathways, so this influx could occur in the same fashion in all aspects of the targeted neuron [21].
PRF is not limited to targeting afferent nerves, which was observed after the use of intra-articular PRF, and, as a result, it was verified that PRF promotes an analgesic effect. Therefore, it was suggested that PRF could present a local anti-inflammatory effect due to an effect on the immune system, which may ultimately impact the nociceptive process [22][23].
It was reported that PRF has the ability to decrease levels of interleukin-1 (IL-1), metalloproteinase-3 (MMP-3), and tumor necrosis (TNF-α) in the synovial fluid of severe osteoarthritic (OA) patients. This was demonstrated by the observation of clinical improvements, resulting in better outcomes in comparison to the use of betamethasone. The effect on the immune response could be explained by the inhibition of immune cells and pro-inflammatory cytokines. Therefore, as inflammatory cytokines are regulated, PRF stimulates a greater level of cascade reaction-stop amplification of the inflammatory reaction and avoids the common inflammatory wind-up phenomenon. This may explain the mechanisms of long-term pain mitigation after using PRF [24]. Given its ability to impact immune cells, there are case reports considering the intravenous route to apply RF in order to treat unresolved immune issues [22].
Preclinical studies demonstrated a rapid onset (within 3 h) increase in c-FOS expression, a specific marker for cellular activity, which lasted one week after PRF stimulus. c-FOS inhibition of excitatory C fibers is a possible mechanism involved in analgesia [25]. In vitro, PRF stimuli induce a transient decrease in excitatory postsynaptic potential, with recovery in a fast and complete way in hippocampal organotypic slices [26].
Pertaining to ultrastructural changes in the sciatic nerve, a preclinical study reported extensive mitochondrial swelling and hyperplasia. Functional recovery of the animals suggests that this mechanism is a compensatory response that helps the recovery and regeneration of lesioned nerve fibers. In the treated animals, it was observed that the macrophages contained intracellular cholesterol crystals and necrotic tissue, suggesting that PRF promoted an inflammatory response to clear the lesion area, as an early response to PRF [17].
Up until now, the literature has been trying to elucidate the mechanisms of action underlying the use of PRF. Well-designed randomized controlled and in vitro trials are needed in order to clarify the mechanism of action further for procedures at frequencies and temperatures used in current clinical practice and how they modify central and peripheral components of pain pathways. Additionally, it is important to note that clinical outcomes could be influenced by lesion parameters (sensory and motor stimulation thresholds), lesion duration, electrode position, and local tissue properties [27].
Another possible effect of PRF involves changes in the oxygen molecule. The recombination of oxygen (geminate recombination or cage recombination) competes with the final separation of the radicals (escape reaction with the possibility of forming products different from those of cage recombination). The ratio of the cage to escape reaction yields will critically depend on the rate of spin evolution, which, on the other hand, depends on an external magnetic field [28].
Thus, the reaction kinetics become magnetic field dependent and this could explain the phenomena that result in the PRF mechanism of action. The radical pair mechanism is a plausible way in which weak magnetic field variations can affect chemical reactivity, allowing radical pairs containing substances that can function as chemical/biological magnetic sensors [28][29].
References
- Cohen, S.P.; Van Zundert, J. Pulsed Radiofrequency: Rebel Without Cause. Reg. Anesth. Pain Med. 2010, 35, 8–10.
- Lord, S.M.; Bogduk, N. Radiofrequency Procedures in Chronic Pain. Best Pract. Res. Clin. Anaesthesiol. 2002, 16, 597–617.
- Orhurhu, V.; Urits, I.; Orman, S.; Viswanath, O.; Abd-Elsayed, A. A Systematic Review of Radiofrequency Treatment of the Ankle for the Management of Chronic Foot and Ankle Pain. Curr. Pain Headache Rep. 2019, 23, 4.
- Sluijter, E.M.; Imani, F. Evolution and Mode of Action of Pulsed Radiofrequency. Anesthesiol. Pain Med. 2013, 2, 139–141.
- Chua, N.H.L.; Vissers, K.C.; Sluijter, E.M. Pulsed Radiofrequency Treatment in Interventional Pain Management: Mechanisms and Potential Indications—A Review. Acta Neurochir. 2011, 153, 763–771.
- Mercadal, B.; Vicente, R.; Ivorra, A. Pulsed Radiofrequency for Chronic Pain: In Vitro Evidence of an Electroporation Mediated Calcium Uptake. Bioelectrochemistry 2020, 136, 107624.
- Podhajsky, R.J.; Yasufumi, S.; Shinichi, K.; Myers, R.R. The Histologic Effects of Pulsed and Continuous Radiofrequency Lesions at 42 °C to Rat Dorsal Root Ganglion and Sciatic Nerve. Spine 2005, 30, 1008–1013.
- Vallejo, R. Pulsed Radiofrequency Modulates Pain Regulatory Gene Expression Along The Nociceptive Pathway. Pain Physician 2013, 5, E601–E613.
- Vanneste, T.; Lantschoot, A.V.; Koen, V.B.; Zundert, J.V. Pulsed Radiofrequency in Chronic Pain. Curr. Opin. Anaesthesiol. 2017, 30, 577–582.
- Hsu, M. Significance of Clinical Treatments on Peripheral Nerve and Its Effect on Nerve Regeneration. J. Neurol. Disord. 2014, 2, 168.
- Tun, K.; Berker, C.; Ahmet, G.G.; Erkan, K.; Sargon, F.; Ibrahim, T.; Ayhan, C.; Yucel, K. Ultrastructural Evaluation of Pulsed Radiofrequency and Conventional Radiofrequency Lesions in Rat Sciatic Nerve. Surg. Neurol. 2009, 72, 496–500.
- Protasoni, M.; Reguzzoni, M.; Sangiorgi, S.; Reverberi, C.; Borsani, E.; Rodella, L.F.; Dario, A.; Tomei, G.; Dell’Orbo, C. Pulsed Radiofrequency Effects on the Lumbar Ganglion of the Rat Dorsal Root: A Morphological Light and Transmission Electron Microscopy Study at Acute Stage. Eur. Spine J. 2009, 18, 473–478.
- Hata, J.; Perret-Karimi, D.; DeSilva, C.; Leung, D.; Betesh, N.; Luo, Z.D.; Dawodu, S.; Sinavsky, K.; Stokes, O.J.; English, S. Pulsed Radiofrequency Current in the Treatment of Pain. Crit. Rev. Phys. Rehabil. Med. 2011, 23, 213–240.
- Orstavik, K. Pathological C-Fibres in Patients with a Chronic Painful Condition. Brain 2003, 126, 567–578.
- Choi, S.; Choi, H.J.; Cheong, Y.; Chung, S.H.; Park, H.K.; Lim, Y.J. Inflammatory responses and morphological changes of radiofrequency-induced rat sciatic nerve fibers. Eur. J. Pain 2014, 18, 192–203.
- Van Zundert, J.; Louw, A.J.A.; Joosten, E.A.J.; Kessels, A.G.H.; Honig, W.; Dederen, P.J.W.C.; Veening, J.G.; Vles, J.S.H.; Kleef, M.V. Pulsed and Continous Radiofrequency Current Adjacent to the Cervical Dorsal Root Ganglion of the Rat Induces Late Cellular Activity in the Dorsal Horn. Anesthesiology 2005, 102, 125–131.
- Liu, Y.; Feng, Y.; Zhang, T. Pulsed Radiofrequency Treatment Enhances Dorsal Root Ganglion Expression of Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels in a Rat Model of Neuropathic Pain. J. Mol. Neurosci. 2015, 57, 97–105.
- Jia, Z.; Ren, H.; Li, Q.; Ji, N.; Luo, F. Pulsed Radiofrequency Reduced Neuropathic Pain Behavior in Rats Associated With Upregulation of GDNF Expression. Pain Physician 2016, 19, 49–58.
- Yeh, C.; Wu, Z.; Chen, J.; Wong, C.; Huang, C.; Wang, J.; Chien, C. Association between Extracellular Signal-Regulated Kinase Expression and the Anti-Allodynic Effect in Rats with Spared Nerve Injury by Applying Immediate Pulsed Radiofrequency. BMC Anesthesiol. 2015, 15, 92.
- Huang, R.; Liao, C.; Tsai, S.; Yen, C.; Lin, C.; Chen, T.; Lin, W.; Chang, C.; Wen, Y. Rapid and Delayed Effects of Pulsed Radiofrequency on Neuropathic Pain: Electrophysiological, Molecular, and Behavioral Evidence Supporting Long-Term Depression. Pain Physician 2017, 20, E269–E283.
- Garcia-Sanchez, T.; Mercadal, B.; Polrot, M.; Muscat, A.; Sarnago, H.; Lucia, O.; Mir, L.M. Successful Tumor Electrochemotherapy Using Sine Waves. IEEE Trans. Biomed. Eng. 2020, 67, 1040–1049.
- Teixeira, A.; Sluijter, M.E. Intravenous Application of Pulsed Radiofrequency—4 Case Reports. Anesth. Pain Med. 2013, 3, 219–222.
- Schianchi, P.M.; Sluijter, M.E.; Balogh, S.E. The Treatment of Joint Pain with Intra-Articular Pulsed Radiofrequency. Anesthesiol. Pain Med. 2013, 3, 250–255.
- Yuan, Y.; Shen, W.; Han, Q.; Liang, D.; Chen, L.; Yin, Q.; Zhu, W.; Xu, H. Clinical Observation of Pulsed Radiofrequency in Treatment of Knee Osteoarthritis. Int. J. Clin. Exp. Med. 2016, 9, 20050–20055.
- Byrd, D.; Mackey, S. Pulsed Radiofrequency for Chronic Pain. Curr. Pain Headache Rep. 2008, 12, 37–41.
- Cahana, A.; Vutskits, L.; Muller, D. Acute Differential Modulation of Synaptic Transmission and Cell Survival during Exposure to Pulsed and Continuous Radiofrequency Energy. J. Pain 2003, 4, 197–202.
- Cahana, A.; Zundert, J.V.; Macrea, L.; Kleef, M.V.; Sluijter, E.M. Pulsed Radiofrequency: Current Clinical and Biological Literature Available. Pain Med. 2006, 7, 411–423.
- Brasil, L.J.; Marroni, N.; Schemitt, E.; Colares, J. Effects of Pulsed Radiofrequency on a Standard Model of Muscle Injury in Rats. Anesthesiol. Pain Med. 2020, 10, e97372.
- Usselman, R.J.; Hill, I.; Singel, D.J.; Martino, C.F. Spin Biochemistry Modulates Reactive Oxygen Species (ROS) Production by Radio Frequency Magnetic Fields. Editado por Jörg Langowski. PLoS ONE 2014, 9, e93065.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
19 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




