Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laura Grațiela Vicaș | -- | 4013 | 2022-10-18 19:31:11 | | | |
| 2 | Rita Xu | Meta information modification | 4013 | 2022-10-19 05:18:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.; Marian, E. Physico-Chemical Properties of Sodium Alginate. Encyclopedia. Available online: https://encyclopedia.pub/entry/29966 (accessed on 01 March 2026).
Frent OD, Vicas LG, Duteanu N, Morgovan CM, Jurca T, Pallag A, et al. Physico-Chemical Properties of Sodium Alginate. Encyclopedia. Available at: https://encyclopedia.pub/entry/29966. Accessed March 01, 2026.
Frent, Olimpia Daniela, Laura Gratiela Vicas, Narcis Duteanu, Claudia Mona Morgovan, Tunde Jurca, Annamaria Pallag, Mariana Eugenia Muresan, Sanda Monica Filip, Roxana-Liana Lucaciu, Eleonora Marian. "Physico-Chemical Properties of Sodium Alginate" Encyclopedia, https://encyclopedia.pub/entry/29966 (accessed March 01, 2026).
Frent, O.D., Vicas, L.G., Duteanu, N., Morgovan, C.M., Jurca, T., Pallag, A., Muresan, M.E., Filip, S.M., Lucaciu, R., & Marian, E. (2022, October 18). Physico-Chemical Properties of Sodium Alginate. In Encyclopedia. https://encyclopedia.pub/entry/29966
Frent, Olimpia Daniela, et al. "Physico-Chemical Properties of Sodium Alginate." Encyclopedia. Web. 18 October, 2022.
Copy Citation
The macromolecules of natural origin have attracted the attention of many researchers as essential to protect the structures of unstable drug substances. After analyzing the studies carried out by various authors, researchers found that these molecules are used for both investigational and therapeutic purposes. This requires the design of certain drug delivery formulations knowing the nature of the macromolecule, its target organ, the required dose and the route of delivery. Therefore, researchers consider it important to use sodium alginate to optimize the delivery of drug substances for maximum therapeutic performance in the body after administration.
sodium alginate
microencapsulation
microparticles
1. Introduction
Sodium alginate is a natural polysaccharide with a linear structure, is biodegradable, biocompatible and safe for the body, provides strength and flexibility to the tissue, and can be used industrially because it has gelling, viscous and stabilizing properties and the ability to retain water. Alginate can be synthesized from the cell wall of various species of brown algae: Laminaria hyperborea, Ecklonia maxima, Ascophyllum nodosum, Eisenia bicyclis and Macrocystis pyrifera ecc., and from various species of bacteria: Azotobacter and Pseudomonas. From these sources, alginate extracted from brown algae has commercial importance for the food, pharmaceutical, cosmetic industries, etc. [1][2][3][4][5].
The extraction of alginates from brown algae is carried out in alkaline medium with sodium carbonate, sodium hydroxide or aluminum hydroxide, in several stages after the collected algae have been dried and shredded. The extract obtained is subjected to precipitation with sodium chloride or calcium and to the filtration operation, the precipitate formed (sodium/calcium alginate) is converted into alginic acid by treatment with diluted clorhydric acid, and the alginic acid is converted into a dry sodium alginate powder. The alginate obtained in order to be used must undergo chemical treatments to remove impurities (e.g., heavy metals, endotoxins, proteins, carbohydrates and polyphenols) and then turned into powder. In order for alginate to be able to be used in the biomedical and pharmaceutical field, it must be safe for the body and biocompatible, that is, it must have high purity. A crude alginate purified by a multistage extraction method is devoid of or contains impurities in a low amount and can be taken orally without causing a response from the immune system [2][3][6][7][8][9].
Nowadays, the development of microencapsulated pharmaceutical forms has become an attractive and widely used field in pharmaceutical technology because over time, they have proven to be safe and effective drug release systems. The emergence of new processible, biocompatible, biodegradable and nontoxic biomaterials for the body in the field of medicine have made it possible to develop much more efficient and much more advantageous pharmaceutical systems than classical pharmaceutical forms. Thus, the category of new high-performance materials includes anionic natural polymers, such as sodium alginate, that are considered advantageous microencapsulation materials and which according to studies can influence the kinetics of the release of the drug from the matrix, according to their degradation in the body [10][11]. When developing particulated pharmaceutical forms, some of the main objectives of the formulation are to maintain the rate of release of the medicinal substance at an effective therapeutic level, with controlled speed and release time, and to protect the medicinal substances from gastrointestinal, enzymatic degradation, etc., and from the action of external factors [12].
2. Chemical Structure of Alginate
According to the information of Phillips G.O. and Williams P.A. and of Lee K.Y. and Mooney D.J., until 1958, information about the chemical structure of alginate suggested that sodium alginate is predominantly made up only of β-D-manuronic fractions, but it was later observed that α-L-glucuronic acid fractions are also present in its structure. The ratio in which the two fractions are present in its structure varies according to the natural source from which it was extracted [3][9][13].
Sodium alginate is considered to be a polyanionic copolymer which structurally is the sodium salt of alginic acid, an acid consisting of several successive groups of the two uronic acids: β-D-manuronic acids (M) and α-L-glucuronic (G), linearly linked to each other by 1–4 glycosidic bonds [2][4][5][14][15][16].
It has the chemical formula (C6H7NaO6)n and an average molecular weight of 216.121 g/mol [17]. By a partial hydrolysis reaction in acidic medium, the alginate molecule can be cleaved into three successive fractions: manuronics (MMMMM), glucuronics (GGGGG) and a mixture of manuronic fractions with glucuronics (MGMGMG), as shown in Figure 1 [9][18].
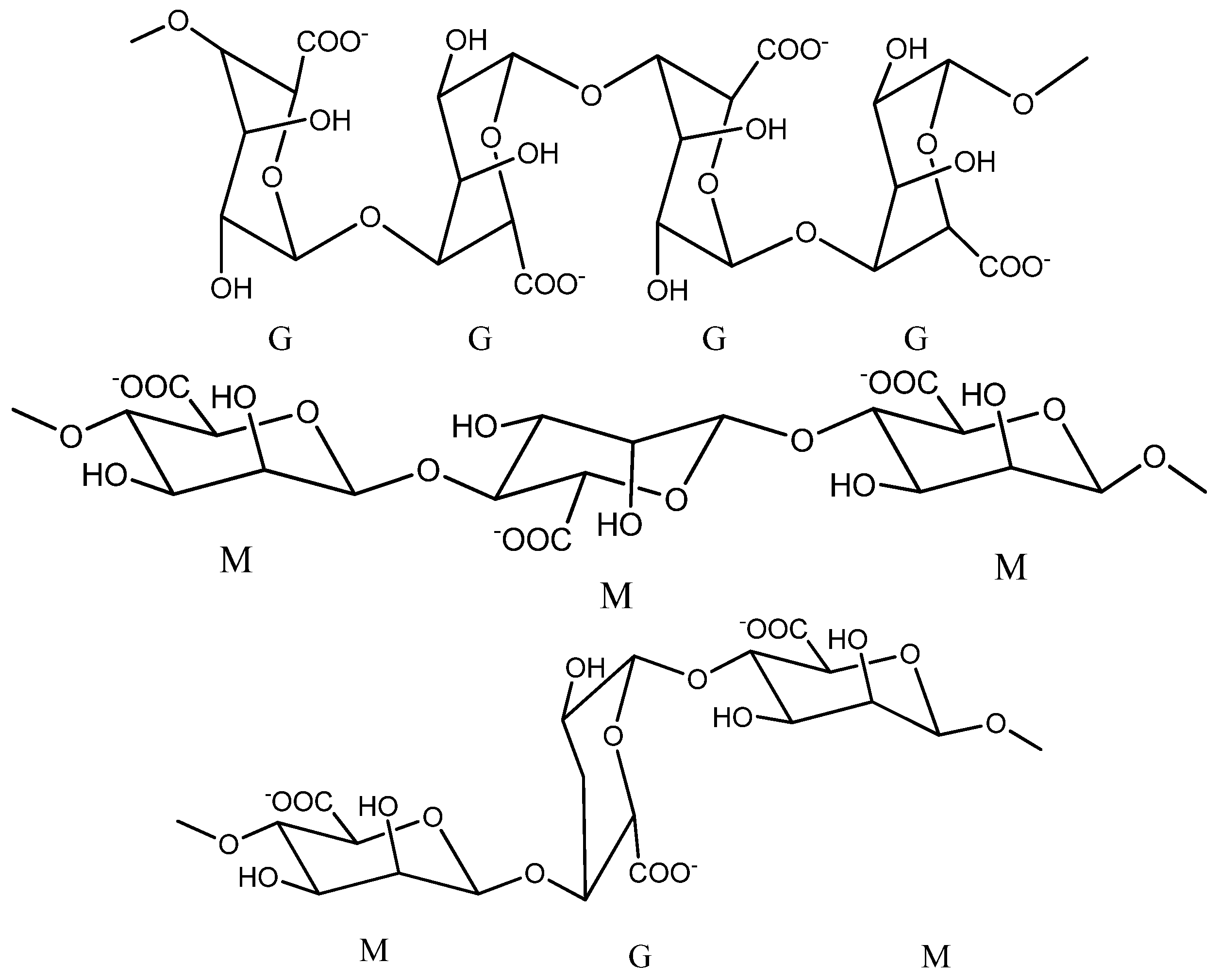
Figure 1. Chemical structure of sodium alginate (Chemdraw scheme).
Studies show that the proportion of the two fractions M and G and the length of the chains in the alginate structure may vary from one species of brown algae to another, so the alginate that is extracted from Laminaria digitata and Ascophyllum nodosums presented a ratio of 1.16:1.82 between the two fractions M and G [19]. Glucuronic chains give alginate many advantages, such as their possibility to participate in cross-linking with calcium ions and the possibility of forming gels with superior mechanical properties [9].
3. Physico-Chemical Properties of Sodium Alginate
From the physico-chemical point of view, sodium alginate is presented in the form of a solid powder that is white or slightly yellowish and hydrophilic, dissolves easily in water and has the ability to form gels in the presence of divalent ions, all of which make alginate a useful material for the delivery of medicines and cellular immobilization [5][6].
The physico-chemical properties of alginates (mechanical properties, swelling and diffusion capacity) are influenced by several characteristics: the composition and arrangement of the two groups of uronic acid in the structure, the molecular weight of the polymer, the type of functional groups in the structure and the concentration of the reticular agent used [2][7][20]. The characteristics of alginates may vary depending on the natural source from which it was extracted and the season and the geographical location from which the plant was harvested [6].
3.1. Physico-Chemical Properties
3.1.1. Molecular Weight
Commercial sodium alginate has a high molecular weight between 32,000 and 400,000. It has long M and G chains in the structure and a polydispersion index that varies between 1.5 and 3 (Mw/Mn). Studies show that the viscosity of alginate solutions is influenced by the molecular mass and pH of the reaction mass, so the viscosity increases with a decrease in pH and reaches a maximum around pH 3–3.5, because at this value, the carboxyl groups in its structure become protonated and can form hydrogen bonds. Increasing the molecular weight of alginate increases the rate of gelling and the physical properties of gels (tensile strength, elasticity, viscosity).
However, sometimes too much of an increase in molecular weight can lead to a very viscous solution of alginate, which is undesirable in certain situations [9][21][22]. For example, in the preparation of alginate hydrogels used as a cell immobilization matrix (in the case of vaccines), if the alginate solutions used are too viscous, the viability of the cells during the hydrogel formation process may be reduced by the high shear forces applied when mixing them with alginate. Cell membranes in general are highly sensitive to mixing, and sometimes strong mixing can cause cell death [23].
3.1.2. Solubility
The solubility of sodium alginate in cold water is slower and leads to obtaining a viscous solution. It is insoluble in alcohol, hydroalcoholic solutions with alcohol content above 30%, chloroform and ether [4]. Studies show that its solubility depends on the pH, molecular weight, ionic strength, nature of the ions present in the structure and concentration [24]. The pKa value of guluronic acid is 3.6, while that of manuronic acid is 3.3. Compared to sodium alginate, calcium alginate is insoluble in water and organic solvents, but is soluble in sodium citrate [22].
3.1.3. Stability
Sodium alginate is compatible with most anionic substances and with few cationic substances, and it shows higher stability against external factors if it is conditioned in the form of a dry powder than in the form of a solution. With acids, sodium alginate gradually forms a gel of alginic acid at low pH values; at elevated pH values, alginic acid dissolves and restores its original viscosity. In alkaline environment, sodium alginate can withstand short periods of time, since pH values higher than 11 reduce its viscosity. In the short-term, sodium alginate can withstand high temperatures, so it can be sterilized, but in the long term, the high temperature in sterilization can reduce the degree of viscosity [25].
3.2. Mechanical Properties
3.2.1. Viscosity
The viscosifying capacity of alginate is dependent on the molecular weight and concentration of the polymer, and gelling (affinity for cations) depends on the amount of glucuronic acid in the structure. Thus, in the structure, the higher the amount of glucuronic acid that is found, the more the solubility of alginate in water and the gelling capacity increases, resulting in a more resistant, viscous, strong and more stable gel [2][5][25][26]. According to studies, sodium alginate solutions are not Newtonian fluids but pseudoplastic fluids whose viscosity changes drastically when they are dissolved in water and diluted with water [4].
Studies show that the viscosity of alginate is dependent of temperature. The thermal and viscoelastic properties of alginate films can be studied using differential scanning calorimetry (DSC). DSC studies on various thermosensitive alginate gels obtained in the temperature range between 0 and 100 °C showed low rigidity at high temperature. It appears that at temperatures below 100 °C, the noncovalent bond between the adjacent polymeric groups kept the alginate intact under oscillatory conditions of deformation, but this equilibrium was interrupted by a constant magnetic stirring [2].
Commercially used sodium alginate has varying degrees of viscosity, and the resulting 1% aqueous solutions have viscosities that can vary from 20 to 400 cP (centipoise) and 0.02–0.4 PaS (pascals per second) at 20 °C [22].
3.2.2. Mucoadhesion
Alginate has good mucoadhesive properties due to the presence of free carboxyl and hydroxyl groups in the structure. In the physiological environment, electrostatic repulsive forces occur between alginate and mucin due to negative charges of sialic acid, sulfate groups in the mucus structure and anionic carboxylic groups of alginates. This suggests that the bioadhesion between mucin and alginate is achieved through intra- and intermolecular hydrogen bonds. Studies claim that the mechanism of mucoadhesion follows several stages: the first stage consists of intimate contact with the mucosa when wetting and swelling of the polymer occurs, and the last stage consisting in the formation of hydrogen bonds through the processes of interpenetration of the mucin with the polymer chains [6]. This property is an advantage in administration of medication to mucous membranes because it increases the contact time and adhesion of the drug to the site of action and also increases the bioavailability of medicines [4].
3.3. Biological Properties
The FDA (Federal Drug Administration) has approved the use of sodium alginate in the food, biomedical and pharmaceutical fields due to its biological properties, i.e., lack of toxicity and immunogenicity, biocompatibility and biodegradability [24].
Biocompatibility, Toxicity, Immunogenicity and Biodegradation
Studies shows that sodium alginate can be included as an excipient in various pharmaceutical forms intended for oral administration because it is safe, nontoxic and does not accumulate in the body. Due to its chelating capacity, it can bind to various heavy metals present in the intestine protecting the body from their effects. However, when it is intended to be used in implantology or intravenous administration, the factors that can influence its biocompatibility and immunogenicity should be taken into account, such as the chemical composition (ratio of G/M groups), purification process, nature, quantity and impact of residual contaminants. Many studies claim that the use of commercial alginate by parenteral route can cause fibrosis and immune response. In order for alginate to be safe for the body and to be used in the biomedical field, it must be prepared and purified very carefully by decontamination methods during the extraction process in order to remove all traces of heavy metals, endotoxins, proteins and phenolic compounds with immunogenic potency [24][27]. The enzymatic degradation of alginate in mammals is not possible due to the absence of alginase, an enzyme involved in the process of undoing the polymer chains, and medium- or high-molecular weight alginates cannot be eliminated renally entirely because they are filtered more slowly by the kidneys. Taking into account the problem of biodegradation, studies show that alginate can be degraded by oxidative way, ionic reticular, etc., if it is subjected to structural changes [24].
3.4. Other Properties
3.4.1. Ionic Reticular Capacity of Alginate with Ca2+ Ions
Alginate can form, by ionic reticulation with polyvalent cations, three-dimensional gels which have a rigid, orderly and strong structure. Agulhon P. et al., showed that the reticulation that is made between alginate and alkaline-earth cations is of an electrostatic nature, and that between alginate and the cations of transitional metals, it is covalent. This is due to the interaction of free carboxyl or hydroxyl groups of the G fractions in the alginate structure with bivalent/polyvalent cations under controlled temperature conditions [2][6][7][9][28]. The affinity of polyvalent ions to alginate is different following the order: trivalent cations > Pb2+ > Cu2+ > Cd2+ > Ba2+ > Sr2+ > Ca2+. Studies show that of the bivalent ions, Ba2+ and Sr2+ can form stronger micro-/nanoparticles of alginate than Ca2+ ions, although Ca2+ ions are the most used even if they do not have the highest interaction power. Ca2+ ions are the most preferred for the development of microparticles because they are the safest for the body, and through reticulation, they form an adequate network of gel of Ca-alginate in mild conditions [6][12]. The use of Pb2+, Cu2+ and Cd2+ is limited due to their toxicity [6]. In the literature, gelling is presented as an “egg box” type of network that is formed when Ca2+ ions replace the Na+ ions in the alginate structure, binds crosswise and is antiparallel to two alginate molecules [29], as shown in Figure 2.
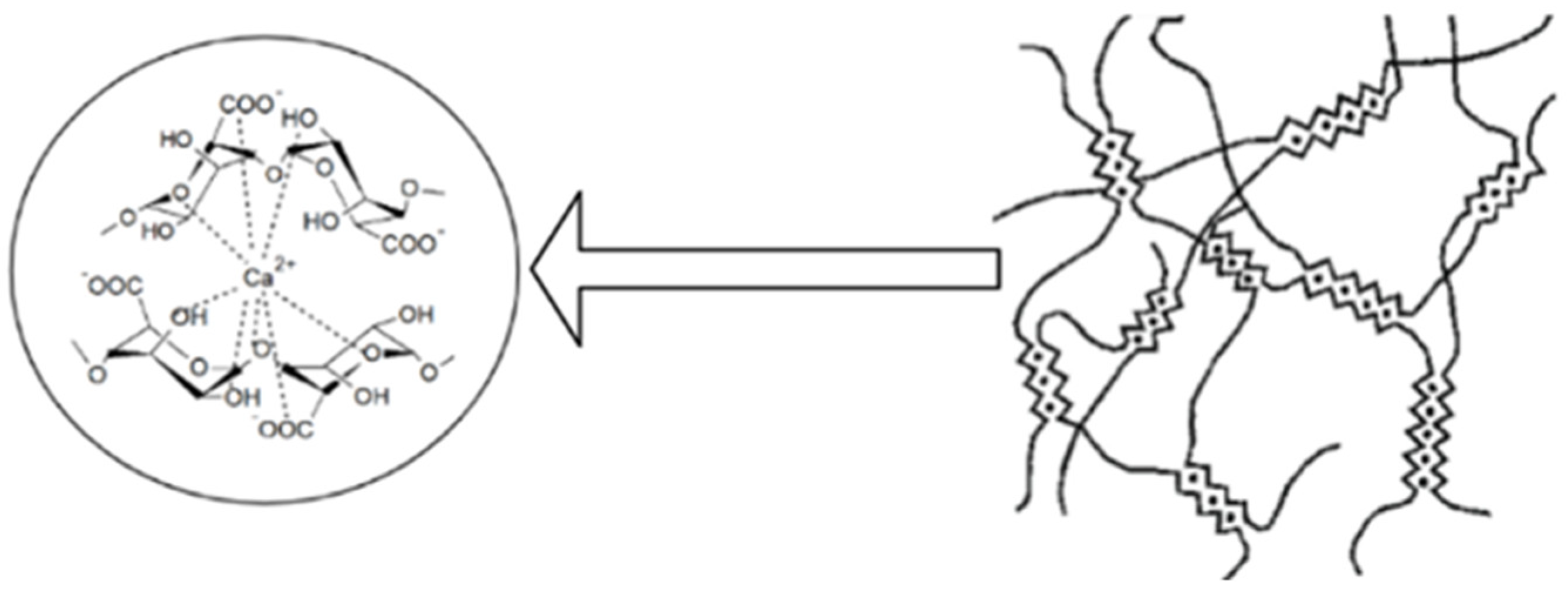
Figure 2. Formation of the three-dimensional network of the “egg box” type by the reticulating sodium alginate with Ca2+ ions (scheme made with the Biorender program).
However, the binding of alginate with calcium ions can be influenced by temperature in the sense that at low temperatures, the reticular capacity of alginate decreases. A slower reticulation leads to obtaining ordered gelatinous networks with improved mechanical properties. The mechanical properties of ionic reticulate alginate gels may also vary depending on its chemical structure: for example, gels obtained from alginate with a high content of G fractions are more rigid than those containing a small amount of M fractions [9]. Studies have shown that microencapsulated pharmaceutical forms consisting only of alginate and Ca2+ have some shortcomings compared to those consisting of two polymers and Ca2+ ions: the gelling process is formed instantly and cannot be controlled due to the increased solubility of alginate in water, the gels they form are not stable in the long term under physiological conditions, the polymeric matrix that is obtained is easily degraded in acidic medium, and it is porous and permeable, from which large amounts of the drug can be lost during preparation, making it difficult to control the release of the drug. Microparticles obtained by ionic reticulation from alginate and calcium ions are much more rigid, unlike microparticles obtained by coacervation from alginate and various natural or synthetic polymers that are much more flexible. By complexing sodium alginate with other natural polymers (e.g., chitosan) and with calcium ions, the physico-chemical properties of the preparations are considerably increased by increasing the stability of the dosage form, by limiting the loss of the medicinal substance and by improving the release profile of the active substance due to the decrease in the porosity of the pharmaceutical form [9][27][30][31][32][33][34].
Currently, reticulation of alginate has also been attempted with other natural polymers: gelatin [35], carrageenan [36], cellulose [37], pectin [38], acacia gum [39] and hyaluronic acid [40]; synthetic polymers: polyethylene glycol [41] and polyacrylamide [42][43]; proteins: ovalbumin [44]; polypeptides: poly L-glutamic acid [45], etc., to enhance its gelling properties and improve the final properties of pharmaceutical forms. Thus, by reticulating alginate with pectin in the presence of Ca2+ and by plasticizing with 10% glycerol, it is possible to obtain polymer films with low solubility in water that are flexible and have adequate swelling capacity [46]. Collagen reticulation proved to be advantageous because it managed to maintain the neural cells viable throughout the encapsulation process in the 3D network of the hydrogel [47]. Pires A.R.L. et al., managed, through an advantageous reticulation of alginate with chitosan and polydimethylsiloxane, to produce a bandage with wound-healing capacity, which was observed by thrombogenicity and hemolysis tests [48]. Babu V.R. et al., by reticulation of alginate with methylcellulose and glutaraldehyde, synthesized effective microspheres with controlled release of nifedipine [49].
3.4.2. Complex Coacervation Capacity of Alginate with Chitosan
Coacervation is a process of physicochemical microencapsulation [50] in which two different colloidal phases, one rich in polymeric particles, called the “coacervate phase”, and the other poor or totally devoid of polymeric particles, called the “equilibrium phase”, separate into coacervate microparticles when they come into contact with each other [51][52][53][54]. Liquid medicinal substances (in the form of emulsion), solids (in suspension form), hydrophilic or hydrophobic medicinal substances and living cells may be encapsulated in microparticles by the coacervation technology, provided that the active substances are insoluble or very poorly soluble in the polymeric matrix/coating and are compatible with the polymer used in microencapsulation [53]. According to the factor causing the desolvation, the polymeric systems involved in the reaction and the phase separation mechanisms, coacervation can be of two kinds: simple coacervation, which generally occurs in the presence of a single polymer through the dehydration mechanism caused by the addition of an electrolyte/salt/desolvating liquid to the reaction medium, or complex coacervation, which occurs in the presence of two or more incompatible polymers by an electrostatic reaction [52][53][54][55][56].
By simple coacervation, according to Figure 3, microencapsulated polymeric pharmacokinetic systems can be synthesized as follows: particles of medicinal substances are dispersed in a low-pH polymeric solution. A solution with high pH (8.5–9.0) of ammonium hydroxide, which is strongly hydrophilic, is added to the colloidal system in order to form the baking drops which are then adsorbed to the surface of the particles of the medicinal substance. The process of forming the microspheres is carried out under stirring, with high mixing speed, in order to avoid bonding and the formation of agglomerates, and then filtration. By this method, microparticles with the size ≤10 nm [52][55][57][58][59] can be obtained.
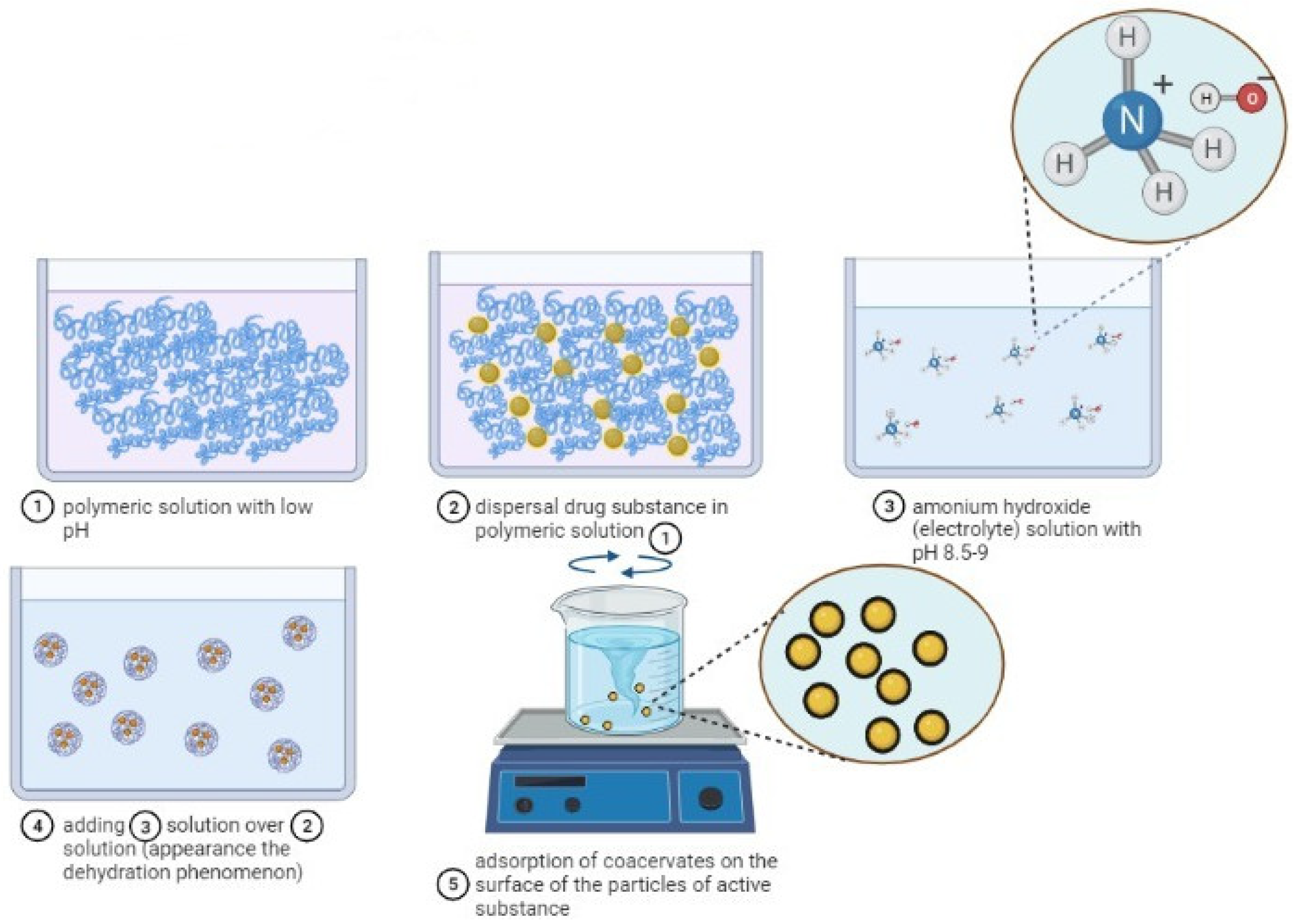
Figure 3. The mechanism of microparticles formation by simple coacervation (scheme made with the Biorender program).
By coacervation, the microparticles are formed according to a mechanism consisting of four stages, as can be seen in Figure 4, and are based on a three-phase system represented by the solvent, the active substance and the covering material:
At the first stage, the preparation of the aqueous solutions of the two polymers and the suspension or emulsification of the active substance solid or liquid in the solution of the anionic polymer forming a hydrophobic phase takes place;
Then, coacervation takes place by adding the hydrophobic phase to the droplets in the aqueous low-polymer (cationic) environment and by the separation of the phases by electrostatic interaction of the two polymeric media, favored by the reaction medium and pH;
At the third stage, the adsorption of the coacervate takes place at the surface of the particles of the active substance, forming a continuous gelatinous envelope around it;
Finally, the polymeric matrix solidification/hardening at high temperature (drying) and the separation of the microcapsules by centrifugation or filtration takes place [53][54].
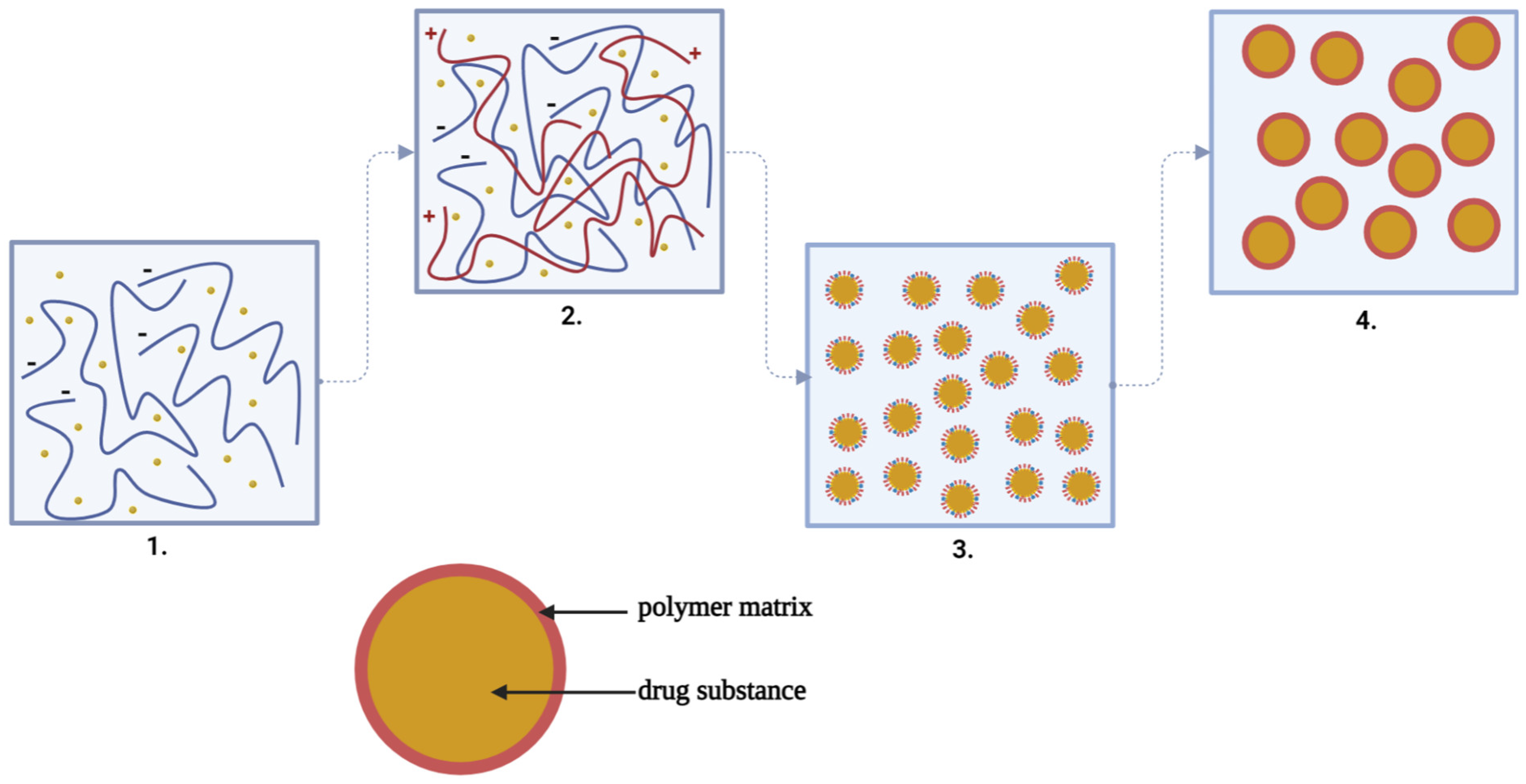
Figure 4. The steps of complex coacervation: 1. Suspension of the drug substance in the solution of the anionic polymer (−). 2. Adding the anionic polymer solution with drug substance to the droplets in the solution of the cationic polymer (+). 3. The adsorption of the coacervate at the surface of the particles of the active substance forming a continuous gelatinous envelope around it. 4. The polymeric matrix solidification/hardening at high temperature (drying) and the separation of the microcapsules by centrifugation or filtration takes place [53][54] (scheme made with Biorender program).
In simple coacervation, phase separation occurs due to incompatibility thermodynamics or repulsion between molecules, and in complex coacervation, the separation of phases occurs in aqueous solution due to the strong affinity of the oppositely charged species [60]. Microparticles obtained by complex coacervation compared to those obtained by simple coacervation are insoluble in water, have excellent controlled release properties and are resistant to heat [61].
The advantage of complex coacervation is that in the preparation, a wide range of natural, synthetic or semisynthetic polymeric substances can be used as microencapsular materials, such as chitosan, alginate, carboxymethylcellulose, gelatin or polyethylene glycol, etc., with different electrical charges [52][53].
Currently, complex coacervation is one of the most effective methods of microencapsulation in both the food and pharmaceutical fields [54]. In the pharmaceutical field, a wide range of pharmaceutical forms can be prepared by complex coacervation, such as microcapsules [62], microspheres [63], hydrogels [64], nanoparticles, etc., because it proved to be an advantageous method, as seen in Table 1. Biswas S. et al., encapsulated a measles antigen in nanoparticles by ionotropic gelling (mixing) of chitosan with sodium tripolyphosphate and covering the nanoparticles obtained with a layer of alginate under light stirring at room temperature. The vaccine formulation method has been shown to be advantageous, the formulation being able to protect the antigen at oral administration from enzymatic and gastric degradation [65]. Mixing with tripolyphosphate and alginate coating of chitosan microspheres also gave good results in the encapsulation of heparin in microparticles for oral administration. Thus, the microparticles showed a particle size of 335 nm, which is optimal for oral administration, and in vitro studies suggested that over 75% of the drug substance successfully crossed the intestinal epithelium [66].
Table 1. Micro-/nanoparticles prepared by complex coacervation.
| Obtained PF | Polymers Used | DS | Advantages of the Method of Complex Coacervation | Ref. |
|---|---|---|---|---|
| MPs | Ch, CMC | Indomethacin | Modified-release PF with few adverse effects were obtained | [62] |
| Ms | Ch, Gelatin B | Tramadol | Reducing the frequency of dosages | [63] |
| NPs | Ch, Na-Alg | Insulin | The possibility of directing the manifestation of the effect to a specific target such as the colon | [67] |
| Mc | Ch, Na-Alg | Amoxicillin | Increased patient compliance | [68] |
| NPs | Ch, Na-Alg | Nifedipine | Obtaining PF with a size appropriate to absorption at GI level | [69] |
| Ms | Na-Alg, Ch | Selenium | Allows one to obtain fast-release PF in phosphate buffer solution (pH = 7.4) | [70] |
| Ms | Ch, Na-Alg | Quercetin | Allows the encapsulation in the PF of some hydrophobic DS | [11] |
| Ms | Ch, Gelatin B | Ketorolac tromethamine | The low degree of crystallinity is an advantage for controlled release | [71] |
| Ms | Na-Alg, Ch | Isoniazid | The type of polymers included in the matrix can extend the duration of release of the DS | [72] |
| Ms | Na-Alg, Gelatin B | Buryti oil | By using this encapsulation method, certain DS of polyphenolic type or volatile oils are protected from attacks of environmental factors | [73] |
| Ms | Na-Alg, Ch | Prednisolone | Rough PF can be obtained, with a similar appearance, wrinkled/smooth at the surface, with a compact structure and large number of folds, stable from temperature, and can be used at normal physiological temperature as delivery systems of the drug | [74] |
| Ms | Na-Alg, Ch | Prednisolone | Avoids the use of toxic reticular chemical agents | [75] |
| Mc | Na-Alg, Gelatin A | Astaxanthin oleoresin | Allows the obtaining of Ms with a high degree of entrapping and release of the embedded ingredients | [76] |
| Mc | Na-Alg, Ch | Triamcinolone | The use of Ch with high molecular weight together with Na-Alg has been observed to lead to Ms of lower sizes, mucoadhesive with better release rates | [77] |
| Mc | Na-Alg, Ch | Nitrofurantoin | Limitation of the occurrence of GI side effects manifested by nausea and vomiting given by certain DS (nitrofurantoin) following oral administration | [78] |
| MPs | Na-Alg, Gelatin B | Ginger volatile oil | Allows one to obtain PF with high stability to light, heat and oxygen | [79] |
| MPs | Gelatin, gum arabic | Lutein | Obtained particle have good stability at light, heat and oxygen | [80] |
| Mc | Gelatin, Na-Alg | Eugenol | If one of the polymers of the matrix is Na-Alg, it can potentiate the antioxidant effect of MPs | [81] |
| Mc | Gelatin B, corn oil, acacia BP 1993, bloom strength 225 | Vit.A palmitate | Allow the incorporation of large amounts of lipophilic drugs | [82] |
| Mc | Ch, karaya gum, paraffin oil, formaldehyde | Diclofenac sodium | It favors the sustained release of the active ingredient from the particulate system | [83] |
| Mc | Na-Alg, HACC | Tea tree | Obtained PF with spherical shape and antimicrobial effect | [84] |
| Nc | Acacia, gelatin | Capsaicin | Obtained spherical and stabile particulate system | [85] |
| Mps | Ch, Na-Alg, CMC | Tanic acid | Could be used in formulations for dental abscess and superficial tissue treating wounds | [86][87] |
| Mps | I-carrageenan, Ch, gellan | Curcumin | These PF can be destined for oral administration with the colon as the therapeutic target for the controlled drug release | [88][89] |
| Ms | Na-Alg | Stellaria media | Such microspheres can be destined for oral administration. | [90] |
References
- Fertah, M.; Belfkira, A.; Dahmane, E.-m.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714.
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12.
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2009.
- Sachan, N.K.; Pushkar, S.; Jha, A.; Bhattcharya, A. Sodium alginate: The wonder polymer for controlled drug delivery. J. Pharm. Res. 2009, 2, 1191–1199.
- Sellimi, S.; Younes, I.; Ben Ayed, H.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367.
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43.
- Fernando, I.P.S.; Lee, W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2010, 391, 123823.
- Fiset, J.-F.; Blais, J.-F.; Riveros, P.A. Review on the Removal of Metal Ions from Effluents Using Seaweeds, Alginate Derivatives and Other Sorbents. Rev. Sci. L’eau 2008, 21, 283–308.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126.
- Engineer, C.; Parikh, J.; Raval, A. Review on hydrolytic degradation behavior of biodegradable polymers from controlled drug delivery system. Trends Biomater. Artif. Organs 2011, 25, 79–85.
- Frenț, O.D.; Duteanu, N.; Teusdea, A.C.; Ciocan, S.; Vicaș, L.; Jurca, T.; Muresan, M.; Pallag, A.; Ianasi, P.; Marian, E. Preparation and Characterization of Chitosan-Alginate Microspheres Loaded with Quercetin. Polymers 2022, 14, 490.
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of alginate microspheres for drug delivery: A review. Int. J. Biol. Macromol. 2020, 153, 1035–1046.
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434.
- Buranachai, T.; Praphairaksit, N.; Muangsin, N. Chitosan/Polyethylene Glycol Beads Crosslinked with Tripolyphosphate and Glutaraldehyde for Gastrointestinal Drug Delivery. AAPS PharmSciTech 2010, 11, 1128–1137.
- Ghimire, K.N.; Inoue, K.; Ohto, K.; Hayashida, T. Adsorption study of metal ions onto crosslinked seaweed Laminaria japonica. Bioresour. Technol. 2008, 99, 32–37.
- Paudyal, H.; Pangeni, B.; Inoue, K.; Kawakita, H.; Ohto, K.; Ghimire, K.N.; Alam, S. Preparation of novel alginate based anion exchanger from Ulva japonica and its application for the removal of trace concentrations of fluoride from water. Bioresour. Technol. 2013, 148, 221–227.
- Zahoor, A.; Sharma, S.; Khuller, G.K. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int. J. Antimicrob. Agents 2005, 26, 298–303.
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Physical and mechanical properties of alginate based composite gels. Trends Food Sci. Technol. 2020, 106, 150–159.
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095.
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodríguez-Nogales, J.M. Research progress in coating techniques of alginate gel polymer for cell encapsulation. Carbohydr. Polym. 2017, 170, 1–14.
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389.
- Chaturvedi, K.; Ganguly, K.; More, U.A.; Reddy, K.R.; Dugge, T.; Naik, B.; Aminabhavi, T.M.; Noolvi, M.N. Sodium alginate in drug delivery and biomedical areas. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 59–100.
- Kong, H.J.; Smith, M.K.; Mooney, D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029.
- Batista, P.S.P.; de Morais, A.M.M.B.; Pintado, M.M.E.; de Morais, R.M.S.C. Alginate: Pharmaceutical and Medical Applications. In Extracellular Sugar-Based Biopolymers Matrices; Springer: Berlin/Heidelberg, Germany, 2019; pp. 649–691.
- Guo, X.; Wang, Y.; Qin, Y.M.; Shen, P.L.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628.
- Huq, T.; Fraschini, C.; Khan, A.; Riedl, B.; Bouchard, J.; Lacroix, M. Alginate based nanocomposite for microencapsulation of probiotic: Effect of cellulose nanocrystal (CNC) and lecithin. Carbohydr. Polym. 2017, 168, 61–69.
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14.
- Agulhon, P.; Markova, V.; Robitzer, M.; Quignard, F.; Mineva, T. Structure of Alginate Gels: Interaction of Diuronate Units with Divalent Cations from Density Functional Calculations. Biomacromolecules 2012, 13, 1899–1907.
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocoll. 2021, 118, 106782.
- Ahmed, S.; Ikram, S. Chitosan & its derivatives: A review in recent innovations. Int. J. Pharm. Sci. Res. 2015, 6, 14.
- Ćirić, A.; Krajišnik, D.; Čalija, B.; Đekić, L. Biocompatible non-covalent complexes of chitosan and different polymers: Characteristics and application in drug delivery. Arh. Farm. 2020, 70, 173–197.
- Markovic, D.; Zarubica, A.; Stojkovic, N.; Vasic, M.; Cakic, M.; Nikolic, G.; Dragana, M.; Aleksandra, Z.; Nikola, S.; Marija, V.; et al. Alginates and similar exopolysaccharides in biomedical application and pharmacy: Controled delivery of drugs. Adv. Technol. 2016, 5, 39–52.
- Matricardi, P.; Di Meo, C.; Coviello, T.; Alhaique, F. Recent advances and perspectives on coated alginate microspheres for modified drug delivery. Expert Opin. Drug Deliv. 2008, 5, 417–425.
- Panos, I.; Acosta, N.; Heras, A. New Drug Delivery Systems Based on Chitosan. Curr. Drug Discov. Technol. 2008, 5, 333–341.
- Patel, M.A.; AbouGhaly, M.H.; Schryer-Praga, J.V.; Chadwick, K. The effect of ionotropic gelation residence time on alginate cross-linking and properties. Carbohydr. Polym. 2017, 155, 362–371.
- Mohamadnia, Z.; Zohuriaan-Mehr, M.J.; Kabiri, K.; Jamshidi, A.; Mobedi, H. Ionically cross-linked carrageenan-alginate hydrogel beads. J. Biomater. Sci. Polym. Ed. 2008, 19, 47–59.
- Chang, C.; Duan, B.; Zhang, L. Fabrication and characterization of novel macroporous cellulose–alginate hydrogels. Polymer 2009, 50, 5467–5473.
- Rezvanian, M.; Ahmad, N.; Amin, M.C.I.M.; Ng, S.-F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140.
- Chopra, M.; Bernela, M.; Kaur, P.; Manuja, A.; Kumar, B.; Thakur, R. Alginate/gum acacia bipolymeric nanohydrogels—Promising carrier for Zinc oxide nanoparticles. Int. J. Biol. Macromol. 2015, 72, 827–833.
- De Santis, S.; Diociaiuti, M.; Cametti, C.; Masci, G. Hyaluronic acid and alginate covalent nanogels by template cross-linking in polyion complex micelle nanoreactors. Carbohydr. Polym. 2014, 101, 96–103.
- Hall, K.; Asfura, K.G.; Stabler, C. Microencapsulation of islets within alginate/poly(ethylene glycol) gels cross-linked via Staudinger ligation. Acta Biomater. 2011, 7, 614–624.
- Yang, C.H.; Wang, M.X.; Haider, H.; Yang, J.; Sun, J.-Y.; Chen, Y.M.; Zhou, J.; Suo, Z. Strengthening Alginate/Polyacrylamide Hydrogels Using Various Multivalent Cations. ACS Appl. Mater. Interfaces 2013, 5, 10418–10422.
- Zhou, Q.; Kang, H.; Bielec, M.; Wu, X.; Cheng, Q.; Wei, W.; Dai, H. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr. Polym. 2018, 197, 292–304.
- Hariyadi, D.M.; Hendradi, E.; Purwanti, T.; Fadil, F.D.G.P.; Ramadani, C.N. Effect of cross linking agent and polymer on the characteristics of ovalbumin loaded alginate microspheres. Int. J. Pharm. Pharm. Sci. 2014, 6, 469–474.
- Yan, S.; Wang, T.; Feng, L.; Zhu, J.; Zhang, K.; Chen, X.; Cui, L.; Yin, J. Injectable In Situ Self-Cross-Linking Hydrogels Based on Poly(l-glutamic acid) and Alginate for Cartilage Tissue Engineering. Biomacromolecules 2014, 15, 4495–4508.
- da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohydr. Polym. 2009, 77, 736–742.
- Moxon, S.R.; Corbett, N.J.; Fisher, K.; Potjewyd, G.; Domingos, M.; Hooper, N.M. Blended alginate/collagen hydrogels promote neurogenesis and neuronal maturation. Mater. Sci. Eng. C 2019, 104, 109904.
- Pires, A.L.R.; Motta, L.D.A.; Dias, A.M.; de Sousa, H.C.; Moraes, M.; Braga, M.E. Towards wound dressings with improved properties: Effects of poly(dimethylsiloxane) on chitosan-alginate films loaded with thymol and beta-carotene. Mater. Sci. Eng. C 2018, 93, 595–605.
- Babu, V.R.; Sairam, M.; Hosamani, K.M.; Aminabhavi, T.M. Preparation of sodium alginate–methylcellulose blend microspheres for controlled release of nifedipine. Carbohydr. Polym. 2007, 69, 241–250.
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28.
- Black, K.A.; Priftis, D.; Perry, S.L.; Yip, J.; Byun, W.Y.; Tirrell, M. Protein Encapsulation via Polypeptide Complex Coacervation. ACS Macro Lett. 2014, 3, 1088–1091.
- Frenț, O.D.; Vicaș, L.; Jurca, T.; Ciocan, S.; Duteanu, N.; Pallag, A.; Muresan, M.; Marian, E.; Negrea, A.; Micle, O. A Review: Uses of Chitosan in Pharmaceutical Forms. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–37.
- Popovici, I.; Lupuleasa, D. The influence of different polymers on the pharmaco-technological characteristics of propiconazole nitrate bioadhesive oromucosal tablets. Tehn. Farm. 2017, 3, 689–728.
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286.
- Huang, G.-Q.; Sun, Y.-T.; Xiao, J.-X.; Yang, J. Complex coacervation of soybean protein isolate and chitosan. Food Chem. 2012, 135, 534–539.
- Neamtu, B.; Tita, O.; Neamtu, M.; Tita, M.; Hila, M.; Maniu, I. Identification of Probiotic Strains from Human Milk in Breastfed Infants with Respiratory Infections. Acta Univ. Cibiniensis. Ser. E Food Technol. 2014, 18, 73–84.
- Espinosa-Andrews, H.; Báez-González, J.G.; Cruz-Sosa, F.; Vernon-Carter, E.J. Gum arabic−chitosan complex coacervation. Biomacromolecules 2007, 8, 1313–1318.
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53.
- Sahil, K.; Akanksha, M.; Premjeet, S.; Bilandi, A.; Kapoor, B. Microsphere: A review. Int. J. Res. Pharm. Chem. 2011, 1, 1184–1198.
- Chang, L.-W. Sequence Control of Complex Coacervation. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2020.
- Dong, Z.-J.; Xia, S.-Q.; Hua, S.; Hayat, K.; Zhang, X.-M.; Xu, S.-Y. Optimization of cross-linking parameters during production of transglutaminase-hardened spherical multinuclear microcapsules by complex coacervation. Colloids Surf. B Biointerfaces 2008, 63, 41–47.
- Tiyaboonchai, W.; Ritthidej, G.C. Development of indomethacin sustained release microcapsules using chitosan-carboxymethyl-cellulose complex coacervation. Development 2003, 25, 246.
- Basu, S.K.; Kavitha, K.; Rupeshkumar, M. Evaluation of Ionotropic Cross-Linked Chitosan/Gelatin B Microspheres of Tramadol Hydrochloride. AAPS PharmSciTech 2011, 12, 28–34.
- El-Leithy, E.S.; Shaker, D.S.; Ghorab, M.K.; Abdel-Rashid, R.S. Evaluation of Mucoadhesive Hydrogels Loaded with Diclofenac Sodium–Chitosan Microspheres for Rectal Administration. AAPS PharmSciTech 2010, 11, 1695–1702.
- Biswas, S.; Chattopadhyay, M.; Sen, K.K.; Saha, M.K. Development and characterization of alginate coated low molecular weight chitosan nanoparticles as new carriers for oral vaccine delivery in mice. Carbohydr. Polym. 2015, 121, 403–410.
- Bagre, A.P.; Jain, K.; Jain, N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharm. 2013, 456, 31–40.
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.J.; Ferreira, D. Alginate/Chitosan Nanoparticles are Effective for Oral Insulin Delivery. Pharm. Res. 2007, 24, 2198–2206.
- Arora, S.; Budhiraja, R.D. Chitosan-alginate microcapsules of amoxicillin for gastric stability and mucoadhesion. J. Adv. Pharm. Technol. Res. 2012, 3, 68–74.
- Li, P.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Chitosan-Alginate Nanoparticles as a Novel Drug Delivery System for Nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228.
- Cavalu, S.; Prokisch, J.; Laslo, V.; Vicas, S. Preparation, structural characterisation and release study of novel hybrid microspheres entrapping nanoselenium, produced by green synthesis. IET Nanobiotechnol. 2017, 11, 426–432.
- Basu, S.K.; Kavitha, K.; Rupeshkumar, M. Evaluation of Ketorolac Tromethamine Microspheres by Chitosan/Gelatin B Complex Coacervation. Sci. Pharm. 2010, 78, 79–92.
- Lucinda-Silva, R.M.; Evangelista, R.C. Microspheres of alginate-chitosan containing isoniazid. J. Microencapsul. 2003, 20, 145–152.
- Lemos, Y.P.; Marfil, P.H.M.; Nicoletti, V.R. Particle size characteristics of buriti oil microcapsules produced by gelatin-sodium alginate complex coacervation: Effect of stirring speed. Int. J. Food Prop. 2017, 20, 1439–1447.
- Honary, S.; Maleki, M.; Karami, M. The effect of chitosan molecular weight on the properties of alginate/ chitosan microparticles containing prednisolone. Trop. J. Pharm. Res. 2009, 8, 53–61.
- Wittaya-Areekul, S.; Kruenate, J.; Prahsarn, C. Preparation and in vitro evaluation of mucoadhesive properties of alginate/chitosan microparticles containing prednisolone. Int. J. Pharm. 2006, 312, 113–118.
- Li, R.; Chen, R.; Liu, W.; Qin, C.; Han, J. Preparation of enteric-coated microcapsules of astaxanthin oleoresin by complex coacervation. Pharm. Dev. Technol. 2018, 23, 674–681.
- Lucinda-Silva, R.M.; Salgado, H.; Evangelista, R.C. Alginate–chitosan systems: In vitro controlled release of triamcinolone and in vivo gastrointestinal transit. Carbohydr. Polym. 2010, 81, 260–268.
- Hari, P.R.; Chandy, T.; Sharma, C.P. Chitosan/calcium alginate microcapsules for intestinal delivery of nitrofurantoin. J. Microencapsul. 1996, 13, 319–329.
- Wang, L.X.; Yang, S.W.; Cao, J.L.; Zhao, S.H.; Wang, W.W. Microencapsulation of Ginger Volatile Oil Based on Gelatin/Sodium Alginate Polyelectrolyte Complex. Chem. Pharm. Bull. 2016, 64, 21–26.
- Qv, X.-Y.; Zeng, Z.-P.; Jiang, J.-G. Preparation of lutein microencapsulation by complex coacervation method and its physicochemical properties and stability. Food Hydrocoll. 2011, 25, 1596–1603.
- Shinde, U.; Nagarsenker, M. Microencapsulation of eugenol by gelatin-sodium alginate complex coacervation. Indian J. Pharm. Sci. 2011, 73, 311.
- Junyaprasert, V.B.; Mitrevej, A.; Sinchaipanid, N.; Boonme, P.; Wurster, D.E. Effect of Process Variables on the Microencapsulation of Vitamin A Palmitate by Gelatin-Acacia Coacervation. Drug Dev. Ind. Pharm. 2001, 27, 561–566.
- Babu, G.M.M.; Himasankar, K.; Narayan, C.P.; Murthy, K.R. Controlled Release of Dicolfenac Sodium by Gum Karaya-Chitosan Complex Coacervate: In Vivo Evaluation. Indian J. Pharm. Sci. 2001, 63, 408.
- Chen, M.; Hu, Y.; Zhou, J.; Xie, Y.; Wu, H.; Yuan, T.; Yang, Z. Facile fabrication of tea tree oil-loaded antibacterial microcapsules by complex coacervation of sodium alginate/quaternary ammonium salt of chitosan. RSC Adv. 2016, 6, 13032–13039.
- Jincheng, W.; Xiaoyu, Z.; Sihao, C. Preparation and Properties of Nanocapsulated Capsaicin by Complex Coacervation Method. Chem. Eng. Commun. 2010, 197, 919–933.
- Aelenei, N.; Popa, M.I.; Novac, O.; Lisa, G.; Balaita, L. Tannic acid incorporation in chitosan-based microparticles and in vitro controlled release. J. Mater. Sci. Mater. Med. 2009, 20, 1095–1102.
- Iurciuc-Tincu, C.-E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642.
- Kamal, M.A.H.M.; Ahmed, M.; Wahed, M.I.I.; Amran, M.S.; Shaheen, S.M.; Rashid, M.; Anwar-Ul-Islam, M. Development of indomethacin sustained release microcapsules using ethyl cellulose and hydroxy propyl methyl cellulose phthalate by O/W emulsification. Dhaka Univ. J. Pharm. Sci. 2008, 7, 83–88.
- Cavalu, S.; Bisboaca, S.; Mates, I.M.; Pasca, P.M.; Laslo, V.; Costea, T.; Fritea, L.; Vicas, S. Novel Formulation Based on Chitosan-Arabic Gum Nanoparticles Entrapping Propolis Extract Production, physico-chemical and structural characterization. Rev. Chim. 2018, 69, 3756–3760.
- Miere, F.; Teusdea, A.C.; Laslo, V.; Fritea, L.; Moldovan, L.; Costea, T.; Uivaroșan, D.; Vicas, S.I.; Pallag, A. Natural Polymeric Beads for Encapsulation of Stellaria media Extract with Antioxidant Properties. Mater. Plast 2019, 56, 671–679.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
21.2K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
19 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





