Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Maria Łętocha | -- | 2168 | 2022-10-18 13:42:29 | | | |
| 2 | Anna Maria Łętocha | + 60 word(s) | 2228 | 2022-10-19 13:42:06 | | | | |
| 3 | Anna Maria Łętocha | -3 word(s) | 2225 | 2022-10-20 10:03:16 | | | | |
| 4 | Dean Liu | Meta information modification | 2225 | 2022-10-21 09:00:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Łętocha, A.; Miastkowska, M.; Sikora, E. Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/29952 (accessed on 07 February 2026).
Łętocha A, Miastkowska M, Sikora E. Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/29952. Accessed February 07, 2026.
Łętocha, Anna, Małgorzata Miastkowska, Elżbieta Sikora. "Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications" Encyclopedia, https://encyclopedia.pub/entry/29952 (accessed February 07, 2026).
Łętocha, A., Miastkowska, M., & Sikora, E. (2022, October 18). Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. In Encyclopedia. https://encyclopedia.pub/entry/29952
Łętocha, Anna, et al. "Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications." Encyclopedia. Web. 18 October, 2022.
Copy Citation
Alginates are the most widely used natural polymers in the pharmaceutical, food and cosmetic industries. Usually, they are applied as a thickening, gel-forming and stabilizing agent. Moreover, the alginate-based formulations such as matrices, membranes, nanospheres or microcapsules are often used as delivery systems. Alginate microparticles (AMP) are biocompatible, biodegradable and nontoxic carriers, applied to encapsulate hydrophilic active substances, including probiotics.
alginate
microparticles
active substances
probiotics
1. Introduction
“Microparticle” is the term used for spherical particles with diameters in the micrometer range, typically from 1 to 1000 µm. Polymeric microparticles are usually formed by a polymer matrix in which a smaller amount of an active compound could be immobilized [1]. Generally, taking into account the method of microparticle preparation, their morphology, and the distribution of the encapsulated actives, microparticles can be divided into two categories: “microspheres” and “microcapsules” [1][2][3][4].
Microspheres usually are characterized as matrix systems in which the active substance is homogeneously dispersed. In contrast, microcapsules are heterogenous particles where a membrane shell surrounds the core (solid or liquid) and forms a reservoir with an encapsulated active compound [1][5][6]. In some cases, to overcome problems with the mechanical stability of such, the carriers and low-actives-loading, polymer-coated microspheres are obtained [7][8].
Compared with other effective carriers of the active compounds, such as nanoparticles, the advantage of the microcarriers is that they do not penetrate into the interstitium, and thus act locally [5][9]. The choice of microcapsules over nanocapsules in the case of cosmetic products can be an ideal solution for epidermal action, where the encapsulated ingredient is designed to act on the top of the epidermis. Moreover, nanocapsules are not suitable, due to their small size, to encapsulate active ingredients with larger than nano sizes, including bacteria.
The microparticles can be prepared from a large variety of starting materials, both natural and synthetic origin, and with different preparation techniques. Most drug delivery systems are prepared using natural polymers, such as polysaccharides (e.g., starch, dextran, alginate, hyaluronic acid and chitosan) or proteins (e.g., collagen, gelatin and albumin) [10][11]. Among synthetic polymers applied to obtain the carriers, there are poly(lactide-co-glycolide) (PLGA), (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), poly(sebacic anhydride) and poly(ε-caprolactone) [2]. Moreover, thermosensitive polymers, e.g., poly(N-isopropyacrylamide) (PNIPAAm) [12], and pH-sensitive polymers (Eudragit L100 and Eudragit S100) are used [13][14].
Among the natural raw materials used to obtain microparticles, alginates are the most popular and deserve special attention. In the food industry, alginate-based formulations are used as texture modifiers [15][16][17] or to improve the stability and long-term efficacy of active compounds [18][19][20]. In the case of the cosmetic and pharmaceutic industries, alginate systems are used to improve stability and to protect the encapsulated compounds against external conditions, e.g., UV light, temperature [21][22][23], or gastric environments in the case of oral application [24][25][26][27]. The alginate microparticles (AMP) are biocompatible, biodegradable and nontoxic delivery systems allowed to encapsulate different active substances, including probiotics [28][29][30].
The objective of this article was to provide a review of the techniques used for AMP production. The most popular alginate encapsulation methods, such as the spray-drying technique, extrusion and emulsification are described. The technological parameters important in the microencapsulation process and their impact on the quality of the alginate carriers are discussed. Particular attention was paid to the microencapsulation of alginates based on the emulsification technique, as the process allows to obtain small microparticles, containing both hydrophilic and hydrophobic ingredients with high efficiency. Moreover, the examples of the actives enclosed in the AMP, and their application in the food, pharmaceutical and cosmetic industries, are presented. Most scientific articles focus on applications of AMP in the food and pharmaceutical industries [28][31][32][33]. Recently, the environmental application of alginate carriers has also appeared more often [34]. However, the cosmetics industry has not been the subject of wide scientific interest so far.
2. Alginate characteristics
Alginates are natural polysaccharides, polyanionic polymers, obtained from marine algae [35][36], usually from three species of marine brown algae, Phaeophyceae: Macrocystis pyrifera, Laminaria digitata and Laminaria saccharina. The structure of alginates depends on the source of the sea algae, i.e., its species, geographic origin, or seasonal varieties [37]. Alginates are unbranched copolymers of D-Mannuronic acid (M block) and L-Guluronic acid (G block) linked by β (1–4) glycosidic bonds [37][38][39][40][41][42][43]. These blocks are arranged in an irregular block pattern with different GG, MM and MG block proportions (Figure 1) [30][35][37][38][42]. Blocks M and G are placed in the different locations along the chain (e.g., MMMM, GGGG, MMGG, GMGM) and in various amounts [38].
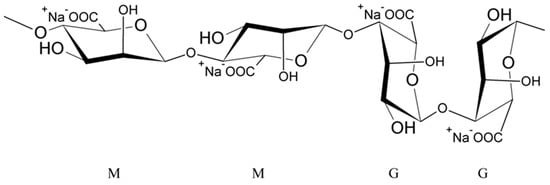
Figure 1. Structure of sodium alginate.
Cross-Linking of Alginates
Monovalent metal ions with alginates form soluble salts, while divalent and multivalent cations (except Mg2+) form gels or precipitate. The affinity of alginates to various cations and selective ion binding are the basis of the alginates’ ability to form hydrogels. Alginates containing a large number of guluronic acid blocks form gels with much greater strength compared with mannuronate-rich alginates because G blocks have a stronger affinity with divalent ions than M blocks [35]. The interaction of alginates with divalent cations, which are in particular calcium cations, leads to the formation of biodegradable gels [38]. Polymerization is based on the cross-linking of copolymers through ionic bonds between Ca2+ cations and alginate anions [38][44]. Each Na+ cation ionically binds to only one carboxyl group of the alginate chain, while the Ca2+ cation interacts with two carboxyl groups that come from different polymer chains. The exchange of Na+ ions into Ca2+ ions is relatively easy and takes place when an aqueous solution of sodium alginate is mixed with a solution containing calcium ions [41]. The mechanical properties of the formed gels are determined by both the concentration of divalent ions and the alginic acid salts in the reaction mixture [38][44]. The distinctive molecular structure resulting from these interactions is referred to as the “egg–box” model (Figure 2) [38][44][45][46]. It should be underlined that only the G blocks take part in the cross-linking process. The homopolymers of G blocks form ordered, three-dimensional regions wherein Ca2+ ions are embedded like eggs in a cardboard box [47].
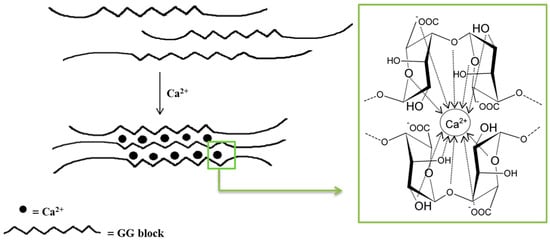
Figure 2. Scheme of an alginate gel formation by calcium cations (“egg–box” model).
The immediate cross-linking of alginates, due to the action of calcium ions, causes the formation of particles of different diameters and different porosity [48]. The gel strength increases with the increase in G blocks content. Moreover, the parameters of the cross-linking process are important. Temperatures ranging from 60 to 80 °C are needed to dissolve alginates in the water. Moreover, it is known that alginate gels are insoluble in acidic environments [49]. Additionally, the technique used to obtain alginate particles influences the capsule size, which can range in size from nanoparticles to macroparticles. Macroparticles are particles larger than 1000 microns in size and are easily visible [50][51]. They are applied especially in the field of dietary supplements [52] or drugs [53][54][55][56]. The size of microparticles ranges from 100 nanometres to 1000 μm [57][58][59], and particles with a diameter below 100 nanometres are classified as nanoparticles [60].
3. Alginate Encapsulation Techniques
- (a)
-
Physical methods such as spray drying, extrusion, lyophilization, supercritical fluid precipitation and solvent evaporation;
- (b)
-
Physico-chemical methods including coacervation, liposomes and ionic gelation;
- (c)
-
Chemical methods such as interfacial polymerization and molecular inclusion complexation.
In the case of alginate capsules preparation, the most frequently used methods are the spray-drying technique and either extrusion or emulsification/gelation [61].
3.1. Spray-Drying Technique
The spray-drying technique is the oldest technique used in alginate capsules. The concept of this method was first described in Samuel Percy’s patent in 1872 [64]. The process was introduced commercially in the 1920s, but spray drying was fully applied on a large scale in the early 1980s [65][66]. In this method, initially, a solution containing the active ingredient should be prepared with the dissolved polymer matrix. The solution is then pressurized and sprayed to form a “mist” in the drying chamber. Hot gas (air or nitrogen) is also blown into the drying chamber. The hot gas makes it possible to evaporate the solvent. The capsules are then transported to a cyclone separator for recovery [3]. In this technique, the control of the product’s feed, gas flow and temperature is important [67][68]. Figure 3 shows a schematic representation of the spray-drying procedure [3][63].
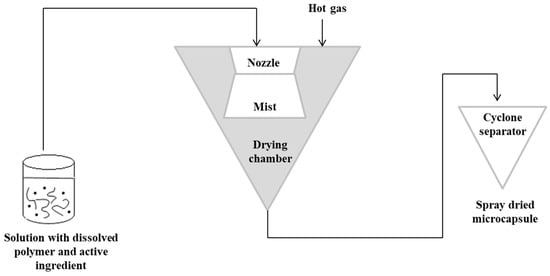
Figure 3. Scheme of the spray-drying encapsulation technique.
The spray-drying technique is applicable to encapsulate different kind of actives. Among substances encapsulated in alginate capsules, there were lipids [69] and hydrocarbons such as cellulase [70], carvacrol [71], or insulin [72]. Unfortunately, the main disadvantage of this method is the impossibility to encapsulate substances sensitive to elevated temperature and pressure.
3.2. Extrusion Technique
Another technique that has been used for a long time for producing microparticles is the extrusion technique [73]. It consists of the preparation of a hydrocolloid solution (an aqueous dispersion of a natural or synthetic polymer), e.g., sodium alginate. Then, the material for encapsulation is added to the solution, and the suspension is pressed into droplets into the gelling or hardener solution, e.g., calcium chloride [3][30][39][49][67][74][75][76][77]. Depending on the scale (laboratory or pilot scale), a syringe needle or an extruder is used for the process, respectively [39]. The size and shape of the obtained beads depend on factors such as the diameter of the needle and the distance of the needle from the hardener solution. This technique is the most popular in laboratory scale because of simplicity and low cost of production [78]. However, the main limitation in the case of its application in the large scale is the fact that microparticles are formed slowly [30].
3.3. Emulsification Technique
An alternative method commonly used to obtain alginate capsules is emulsification. The emulsification technique is more expensive than the extrusion method because a large amount of oil is needed to prepare the emulsion [79]. In this technique, the discontinuous phase (mixture of hydrocolloid and encapsulated material) is added to a large volume of continuous oil phase oil [3][30][39][46][49][67]. In the case of food applications, vegetable oils are used as a continuous phase, most often canola, sunflower or corn oils [78][80]. Additionally, to stabilize the droplets of the internal phase, an emulsifier should be added to the mixture formed. The obtained water-in-oil emulsion is continuously homogenized by stirring [3][30][39][49][67]. The speed of the emulsion mixing is a critical step because it affects both the shape and size of the capsules formed [81]. Very large capsules (around 1000 μm in diameter or larger) can result in poorly coated structures and a coarse texture [82], which in turn affects the dispersion quality of the capsules in the final product [79]. The final emulsion containing alginate droplets is broken by adding calcium chloride solution and centrifuged, thereby separating the oil and water phases to obtain microspheres [83][84]. Figure 4 shows a diagram of the encapsulation process using extrusion and emulsification techniques [46][67].
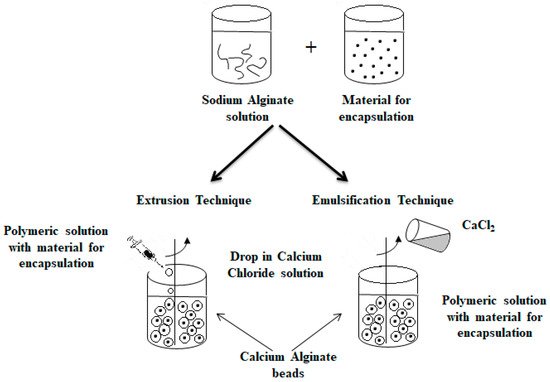
Figure 4. Scheme of the encapsulation process by extrusion (on the (left)) and by emulsification technique (on the (right)).
The main difference between the two techniques is the sequence of combining the alginate and calcium chloride solutions. The polymer solution containing the active ingredient is introduced into the calcium chloride solution in the case of the extrusion technique as opposed to the emulsification technique, where the CaCl2 solution is dripped into the polymer solution. Depending on the method used to obtain them, the calcium alginate beads are called balls (gelled droplets obtained by extrusion technique) or capsules, in the case of the products of the emulsification technique [85]. The main differences between these alginate beads are listed below [49][78][85][86]:
-
The balls have a porous network, while the capsules have a liquid core (water or oil);
-
The dimensions of the balls are much larger than in the case of the capsules;
-
The balls are uniform in size and shape in contrast to differing in size in capsules.
The differences in the morphology (structure) of both the microparticle types are perfectly illustrated in images obtained by electron microscopy TEM and SEM [87].
To sum up, some advantages and disadvantages of the above-presented methods of AMP preparation are summarized in Table 1.
Table 1. Advantages and disadvantages of alginate microencapsulation methods [3][79][84][88][89][90].
| Method | Advantages | Disadvantages |
|---|---|---|
| Extrusion |
|
|
| Emulsification |
|
|
| Spray-drying |
|
|
The data presented in Table 1 indicate that the currently used alginate encapsulation techniques are not universal. All methods have some pros and cons, but among the others, the emulsification is more universal. The method allows to obtain small microparticles, containing both hydrophilic and hydrophobic ingredients with high process efficiency. Moreover, the microencapsulation of alginates based on the emulsification technique could be modified by differing the polymer gelling process.
References
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H. Designing Polymeric Microparticles for Biomedical and Industrial Applications. Eur. Polym. J. 2013, 49, 2005–2021.
- Vilos, C.; Velasquez, L.A. Therapeutic Strategies Based on Polymeric Microparticles. J. Biomed. Biotechnol. 2012, 2012, 672760.
- Solanki, H.K.; Pawar, D.D.; Shah, D.A.; Prajapati, V.D.; Jani, G.K.; Mulla, A.M.; Thakar, P.M. Development of Microencapsulation Delivery System for Long-Term Preservation of Probiotics as Biotherapeutics Agent. BioMed Res. Int. 2013, 2013, 620719.
- Paques, J.P.; Van Der Linden, E.; Van Rijn, C.J.M.; Sagis, L.M.C. Preparation Methods of Alginate Nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171.
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20.
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug Delivery Systems: Advanced Technologies Potentially Applicable in Personalized Treatments. EPMA J. 2010, 1, 164–209.
- Matricardi, P.; Di Meo, C.; Coviello, T.; Alhaique, F. Recent Advances and Perspectives on Coated Alginate Microspheres for Modified Drug Delivery. Expert Opin. Drug Deliv. 2008, 5, 417–425.
- Teoh, P.L.; Mirhosseini, H.; Mustafa, S.; Hussin, A.S.M.; Manap, M.Y.A. Recent Approaches in the Development of Encapsulated Delivery Systems for Probiotics. Food Biotechnol. 2011, 25, 77–101.
- Wang, B.; Hu, L.; Siahaan, T.J. Drug Delivery: Principles and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016.
- Park, J.H.; Ye, M.; Park, K. Biodegradable Polymers for Microencapsulation of Drugs. Molecules 2005, 10, 146–161.
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural Polymers for the Microencapsulation of Cells. J. R. Soc. Interface 2014, 11, 20140817.
- Curcio, M.; Spizzirri, U.G.; Iemma, F.; Puoci, F.; Cirillo, G.; Parisi, O.I.; Picci, N. Grafted Thermo-Responsive Gelatin Microspheres as Delivery Systems in Triggered Drug Release. Eur. J. Pharm. Biopharm. 2010, 76, 48–55.
- Ammar, H.O.; Ghorab, M.; Kamel, R.; Salama, A.H. A Trial for the Design and Optimization of PH-Sensitive Microparticles for Intestinal Delivery of Cinnarizine. Drug Deliv. Transl. Res. 2016, 6, 195–209.
- Kumar, A.; Montemagno, C.; Choi, H.J. Smart Microparticles with a PH-Responsive Macropore for Targeted Oral Drug Delivery. Sci. Rep. 2017, 7, 3059.
- Tyle, P. Effect of Size, Shape and Hardness of Particles in Suspension on Oral Texture and Palatability. Acta Psychol. 1993, 84, 111–118.
- Leon, A.M.; Medina, W.T.; Park, D.J.; Aguilera, J.M. Mechanical Properties of Whey Protein/Na Alginate Gel Microparticles. J. Food Eng. 2016, 188, 1–7.
- Leon, A.M.; Medina, W.T.; Park, D.J.; Aguilera, J.M. Properties of Microparticles from a Whey Protein Isolate/Alginate Emulsion Gel. Food Sci. Technol. Int. 2018, 24, 414–423.
- Kim, E.S.; Lee, J.S.; Lee, H.G. Calcium-Alginate Microparticles for Sustained Release of Catechin Prepared via an Emulsion Gelation Technique. Food Sci. Biotechnol. 2016, 25, 1337–1343.
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; German, J.B.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Aliabadi, S.; Khaksar, R. Preparation and Characterization of Alginate and Alginate-Resistant Starch Microparticles Containing Nisin. Carbohydr. Polym. 2014, 103, 573–580.
- Chandrasekar, V.; Coupland, J.N.; Anantheswaran, R.C. Characterization of Nisin Containing Chitosan-Alginate Microparticles. Food Hydrocoll. 2017, 69, 301–307.
- Cheng, S.Y.; Yuen, M.C.W.; Kan, C.W.; Cheuk, K.K.L.; Chui, C.H.; Lam, K.H. Cosmetic Textiles with Biological Benefits: Gelatin Microcapsules Containing Vitamin C. Int. J. Mol. Med. 2009, 24, 411–419.
- Chelaru, C.; Ignat, M.; Albu, M.; Meghea, A. Polymeric Microcapsules for Cosmetic Applications, Based on Lemon Essential Oil (Citrus limon). UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2015, 77, 101–112.
- Yingngam, B.; Kacha, W.; Rungseevijitprapa, W.; Sudta, P.; Prasitpuriprecha, C.; Brantner, A. Response Surface Optimization of Spray-Dried Citronella Oil Microcapsules with Reduced Volatility and Irritation for Cosmetic Textile Uses. Powder Technol. 2019, 355, 372–385.
- Tran, V.T.; Benoît, J.P.; Venier-Julienne, M.C. Why and How to Prepare Biodegradable, Monodispersed, Polymeric Microparticles in the Field of Pharmacy? Int. J. Pharm. 2011, 407, 1–11.
- Coppi, G.; Iannuccelli, V.; Leo, E.; Bernabei, M.T.; Cameroni, R. Chitosan-Alginate Microparticles as a Protein Carrier. Drug Dev. Ind. Pharm. 2001, 27, 393–400.
- Coppi, G.; Iannuccelli, V.; Leo, E.; Bernabei, M.T.; Cameroni, R. Protein Immobilization in Crosslinked Alginate Microparticles. J. Microencapsul. 2002, 19, 37–44.
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M. Alginate Microparticles as Oral Colon Drug Delivery Device: A Review. Carbohydr. Polym. 2017, 168, 32–43.
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417.
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335.
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V. Technology and Potential Applications of Probiotic Encapsulation in Fermented Milk Products. J. Food Sci. Technol. 2015, 52, 4679–4696.
- Yang, M.; Liang, Z.; Wang, L.; Qi, M.; Luo, Z.; Li, L. Microencapsulation Delivery System in Food Industry-Challenge and the Way Forward. Adv. Polym. Technol. 2020, 2020, 7531810.
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of Alginate Microspheres in Therapeutics Delivery and Cell Culture: Past, Present and Future. Int. J. Pharm. 2019, 569, 118627.
- Martau, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector-Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837.
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-Based Composites for Environmental Applications: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 318–356.
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630.
- Gomathi, T.; Susi, S.; Abirami, D.; Sudha, P.N. Size Optimization And Thermal Studies on Calcium Alginate Nanoparticles. IOSR J. Pharm. 2017, 48, 1–7.
- Van Vlierberghe, S.; Graulus, G.J.; Samal, S.K.; Van Nieuwenhove, I.; Dubruel, P. Porous Hydrogel Biomedical Foam Scaffolds for Tissue Repair. In Biomedical Foams for Tissue Engineering Applications; Woodhead Publishing Limited: Thorston, UK, 2014; pp. 335–390.
- Wyrębska, Ł.; Szuster, L.; Stawska, H. Synteza i Aplikacja Nowych Pochodnych Wybranych Polisacharydów Część I: Przegląd Literatury. Technol. i Jakość Wyr. 2014, 59, 3–16.
- Asgari, S.; Pourjavadi, A.; Licht, T.R.; Boisen, A.; Ajalloueian, F. Polymeric Carriers for Enhanced Delivery of Probiotics. Adv. Drug Deliv. Rev. 2020, 161–162, 1–21.
- El Din, K.S. Deposition and Structural Formation of 3D Alginate Tissue Scaffolds. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2005.
- Walczak, J.; Marchewka, J.; Laska, J. Hydrożele Alginianowe Sieciowane Jonowo i Kowalencyjnie. Eng. Biomater. 2015, 18, 17–23.
- Paques, J.P. Formation of Alginate Nanospheres. Ph.D. Thesis, Wageningen University, Wageningen, NL, USA, 2014.
- Kim, S.; Jeong, C.; Cho, S.; Kim, S.B. Effects of Thermal Treatment on the Physical Properties of Edible Calcium Alginate Gel Beads: Response Surface Methodological Approach. Foods 2019, 8, 578.
- Jarmoluk, A.; Wrzeszcz, K.; Zimoch, A.; Marycz, K. Sieciowanie Alginianu Sodu Do Hydrożeli Dla Inżynierii Tkankowej. Przem. Chem. 2013, 92, 1018–1022.
- Gombotz, W.R.; Wee, S.F. Protein Release from Alginate Matrices. Adv. Drug Deliv. Rev. 2012, 64, 194–205.
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of Probiotics for Gastrointestinal Delivery. J. Control. Release 2012, 162, 56–67.
- Emmerichs, N.; Wingender, J.; Flemming, H.C.; Mayer, C. Interaction between Alginates and Manganese Cations: Identification of Preferred Cation Binding Sites. Int. J. Biol. Macromol. 2004, 34, 73–79.
- Tu, J.; Bolla, S.; Barr, J.; Miedema, J.; Li, X.; Jasti, B. Alginate Microparticles Prepared by Spray-Coagulation Method: Preparation, Drug Loading and Release Characterization. Int. J. Pharm. 2005, 303, 171–181.
- Gbassi, G.K.; Vandamme, T. Probiotic Encapsulation Technology: From Microencapsulation to Release into the Gut. Pharmaceutics 2012, 4, 149–163.
- Keyan, K.; Ramachandran, T.; Shumugasundaram, O.L.; Balasubramaniam, M.; Ragavendra, T. Microencapsulation of PCMs in Textiles: A Review. J. Text. Apparel Technol. Manag. 2012, 7, 1–10.
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of Active Ingredients in Polysaccharide–Protein Complex Coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145.
- Avula, B.; Parveen, I.; Zhao, J.; Wang, M.; Techen, N.; Wang, Y.H.; Riaz, M.; Bae, J.Y.; Shami, A.A.; Chittiboyina, A.G.; et al. A Comprehensive Workflow for the Analysis of Bio-Macromolecular Supplements: Case Study of 20 Whey Protein Products. J. Diet. Suppl. 2022, 19, 515–533.
- Drobník, J. Biodegradable Soluble Macromolecules as Drug Carriers. Adv. Drug Deliv. Rev. 1989, 3, 229–245.
- Kiyohara, H.; Nonaka, K.; Sekiya, M.; Matsumoto, T.; Nagai, T.; Tabuchi, Y.; Yamada, H. Polysaccharide-Containing Macromolecules in a Kampo (Traditional Japanese Herbal) Medicine, Hochuekkito: Dual Active Ingredients for Modulation of Immune Functions on Intestinal Peyer’s Patches and Epithelial Cells. Evid.-Based Complement. Altern. Med. 2011, 2011, 492691.
- Sharma, N.; Sharma, A.; Bhatnagar, A.; Nishad, D.; Karwasra, R.; Khanna, K.; Sharma, D.; Kumar, N.; Jain, G.K. Novel Gum Acacia Based Macroparticles for Colon Delivery of Mesalazine: Development and Gammascintigraphy Study. J. Drug Deliv. Sci. Technol. 2019, 54, 101224.
- MacEwan, S.R.; Callahan, D.J.; Chilkoti, A. Stimulus-Responsive Macromolecules and Nanoparticles for Cancer Drug Delivery. Nanomedicine 2010, 5, 793–806.
- Kaurav, H.; Hari Kumar, S.L.; Kaur, A. Mucoadhesive Microspheres as Carriers in Drug Delivery: A Review. Int. J. Drug Dev. Res. 2012, 4, 21–34.
- Gurung, B.D.; Kakar, S. An Overview on Microspheres. Int. J. Health Clin. Res. 2020, 3, 11–24.
- Popović, D.A.; Milinčić, D.D.; Pešić, M.B.; Kalušević, A.M.; Tešić, Ž.L.; Nedović, V.A. Encapsulation Technologies for Polyphenol-Loaded Microparticles in Food Industry. In Green Food Processing Techniques; Academic Press: Cambridge, MA, USA, 2019; pp. 335–367.
- Tiloke, C.; Phulukdaree, A.; Chuturgoon, A.A. The Chemotherapeutic Potential of Gold Nanoparticles Against Human Carcinomas: A Review. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 3–31.
- Tyagi, V.V.; Kaushik, S.C.; Tyagi, S.K.; Akiyama, T. Development of Phase Change Materials Based Microencapsulated Technology for Buildings: A Review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391.
- Baranauskaite, J.; Ockun, M.A.; Uner, B.; Tas, C.; Ivanauskas, L. Effect of the Amount of Polysorbate 80 and Oregano Essential Oil on the Emulsion Stability and Characterization Properties of Sodium Alginate Microcapsules. Molecules 2021, 26, 6304.
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506.
- Percy, S.R. Improvement in Drying and Concentrating Liquid Substances by Atomizing. US Patent No. 125406, 1872.
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889.
- Keshani, S.; Daud, W.R.W.; Nourouzi, M.M.; Namvar, F.; Ghasemi, M. Spray Drying: An Overview on Wall Deposition, Process and Modeling. J. Food Eng. 2015, 146, 152–162.
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of Bacteria: A Review of Different Technologies and Their Impact on the Probiotic Effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25.
- Kailasapathy, K. Microencapsulation of Probiotic Bacteria: Technology and Potential Applications. Curr. Issues Intest. Microbiol. 2002, 3, 39–48.
- Strobel, S.A.; Scher, H.B.; Nitin, N.; Jeoh, T. In Situ Cross-Linking of Alginate during Spray-Drying to Microencapsulate Lipids in Powder. Food Hydrocoll. 2016, 58, 141–149.
- Santa-Maria, M.; Scher, H.; Jeoh, T. Microencapsulation of Bioactives in Cross-Linked Alginate Matrices by Spray Drying. J. Microencapsul. 2012, 29, 286–295.
- Sun, X.; Cameron, R.G.; Bai, J. Effect of Spray-Drying Temperature on Physicochemical, Antioxidant and Antimicrobial Properties of Pectin/Sodium Alginate Microencapsulated Carvacrol. Food Hydrocoll. 2020, 100, 105420.
- Bowey, K.; Swift, B.E.; Flynn, L.E.; Neufeld, R.J. Characterization of Biologically Active Insulin-Loaded Alginate Microparticles Prepared by Spray Drying. Drug Dev. Ind. Pharm. 2013, 39, 457–465.
- King, A.H. Encapsulation of Food Ingredients. A Review of Available Technology, Focusing on Hydrocolloids. ACS Symp. Ser. 1995, 590, 26–39.
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of Probiotic Cells for Food Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311.
- Gouin, S. Microencapsulation: Industrial Appraisal of Existing Technologies and Trends. Trends Food Sci. Technol. 2004, 15, 330–347.
- Kailasapathy, K. Encapsulation Technologies for Functional Foods and Nutraceutical Product Development. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2009, 4, 1–19.
- de Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for Preservation of Functionality and Targeted Delivery of Bioactive Food Components. Int. Dairy J. 2010, 20, 292–302.
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of Encapsulation Techniques of Probiotics for Yoghurt. Int. Dairy J. 2003, 13, 3–13.
- Mortazavian, A.; Razavi, S.H.; Ehsani, M.R.; Sohrabvandi, S. Principles and Methods of Microencapsulation of Probiotic Microorganisms. Iran. J. Biotechnol. 2007, 5, 1–18.
- Groboillot, A.F.; Champagne, C.P.; Darling, G.D.; Poncelet, D.; Neufeld, R.J. Membrane Formation by Interfacial Cross-Linking of Chitosan for Microencapsulation of Lactococcus Iactis. Biotechnol. Bioeng. 1993, 42, 1157–1163.
- Shima, M.; Morita, Y.; Yamashita, M.; Adachi, S. Protection of Lactobacillus Acidophilus from the Low PH of a Model Gastric Juice by Incorporation in a W/O/W Emulsion. Food Hydrocoll. 2006, 20, 1164–1169.
- Mortazavian, A.M.; Ehsani, M.R.; Azizi, A.; Razavi, S.H.; Mousavi, S.M.; Sohrabvandi, S.; Reinheimer, J.A. Viability of Calcium-Alginate-Microencapsulated Probiotic Bacteria in Iranian Yogurt Drink (Doogh) during Refrigerated Storage and under Simulated Gastrointestinal Conditions. Aust. J. Dairy Technol. 2008, 63, 25–30.
- Sultana, K.; Godward, G.; Reynolds, N.; Arumugaswamy, R.; Peiris, P.; Kailasapathy, K. Encapsulation of Probiotic Bacteria with Alginate-Starch and Evaluation of Survival in Simulated Gastrointestinal Conditions and in Yoghurt. Int. J. Food Microbiol. 2000, 62, 47–55.
- Ji, R.; Wu, J.; Zhang, J.; Wang, T.; Zhang, X.; Shao, L.; Chen, D.; Wang, J. Extending Viability of Bifidobacterium Longumin Chitosan-Coated Alginate Microcapsules Using Emulsification and Internal Gelation Encapsulation Technology. Front. Microbiol. 2019, 10, 1389.
- Kearney, L.; Upton, M.; McLoughlin, A. Enhancing the Viability of Lactobacillus Plantarum Inoculum by Immobilizing the Cells in Calcium-Alginate Beads Incorporating Cryoprotectants. Appl. Environ. Microbiol. 1990, 56, 3112–3116.
- Gentile, F.T.; Doherty, E.J.; Rein, D.H.; Shoichet, M.S.; Winn, S.R. Reactive Polymers Polymer Science for Macroencapsulation of Cells for Central Nervous System Transplantation. React. Polym. 1995, 25, 207–227.
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Muangsin, N.; Shah, A.A.; Prapasarakul, N. The Efficacy of Three Double-Microencapsulation Methods for Preservation of Probiotic Bacteria. Sci. Rep. 2021, 11, 13753.
- Przybysławska, M.; Winnicka, K. Technologie Otrzymywania Mikrokapsułek. Farm. Pol. 2012, 68, 283–289. Available online: https://www.ptfarm.pl/en/wydawnictwa/czasopisma/farmacja-polska/103/-/14209 (accessed on 22 August 2022).
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour Encapsulation and Controlled Release-A Review. Int. J. Food Sci. Technol. 2006, 41, 1–21.
- Dong, Q.Y.; Chen, M.Y.; Xin, Y.; Qin, X.Y.; Cheng, Z.; Shi, L.E.; Tang, Z.X. Alginate-Based and Protein-Based Materials for Probiotics Encapsulation: A Review. Int. J. Food Sci. Technol. 2013, 48, 1339–1351.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
4 times
(View History)
Update Date:
21 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




