
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sirius Huang | -- | 1397 | 2022-10-18 01:39:24 |
Video Upload Options
Non-invasive micro-test technology (NMT) is a scientific research technology used for measuring physiological events of intact biological samples. NMT is used for research in many biological areas such as gene function, plant physiology, biomedical research, and environmental science. Most living things experience a constant exchange of ions and molecules with their surroundings as a result of biological processes. NMT uses specialized flux sensors, derived from microelectrodes, to measure this dynamic ion/molecule activity called flux around an intact sample. These fluxes reveal information about physiological phenomena. Each NMT flux sensor is selective or specific for a particular ion/molecule of choice. Some of the more commonly published ion/molecule flux sensors are those that are commercially available, such as Ca2+, H+, K+, Na+, Cl−, Mg2+, Cd2+, NH4+, NO3−, Pb2+, Cu2+, O2, H2O2, and IAA (indole-3-acetic acid). Some other flux sensors include glutamate, glucose, Zn2+, Hg2+, and more that have been designed by individual laboratories. NMT measures how much, how fast, and in what direction the chosen ions/molecules are moving. This is defined as diffusion flux, which is the amount of substance per unit area per unit time. The principle of how NMT measures flux was described in the 1990s by a few different laboratories; Lionel Jaffe at the Marine Biological Laboratory described the Vibrating Probe technique, and Ian Newman at the University of Tasmania described the MIFE™ technique. There are also technologies SERIS and SIET that use this principle.
1. Basic Procedure
1.1. Live Sample Preparation
Different samples need different amounts of preparation work. For example, small organisms, condensed organelles, and cultured cells, tissues, or organs can be measured with little alteration, but anything below the skin or surface of large organisms must be exposed in order to measure. Generally, once the sample is prepared so that the desired measurement site is revealed, NMT causes no further damage or interference with the test; because of this, NMT is most commonly used for measuring live, intact samples. The live sample must be secured in a Petri dish so it doesn’t move or shift. A single cell can be adhered by means such as a polylysine-coated slide; this also works for other small samples like condensed organelles.[1] Large tissue samples like roots may be weighted down with filter paper and resin tiles. Plenty of other large samples can be measured as well, such as organs or whole small organisms like zebrafish.[2]
1.2. Liquid Media Composition
The sample must be surrounded by liquid media, which will be different depending on the sample type, purpose of the test, and ions/molecules that will be measured. This media is a useful way to manipulate the sample’s environment by adding things like drugs, stressors, or other biotic/abiotic stimuli.[1]
This step can be the most challenging simply because it allows many possibilities for test manipulation. To get started in designing a specific new test, there is plenty of literature documenting successful composition of liquid media.[3][4]
1.3. Recording
The prepared sample is placed under a microscope with a flux sensor which is controlled by a computer. The operator uses arrow keys to move the flux sensor to the desired point and distance from the sample, aided by a microscope camera.[5]
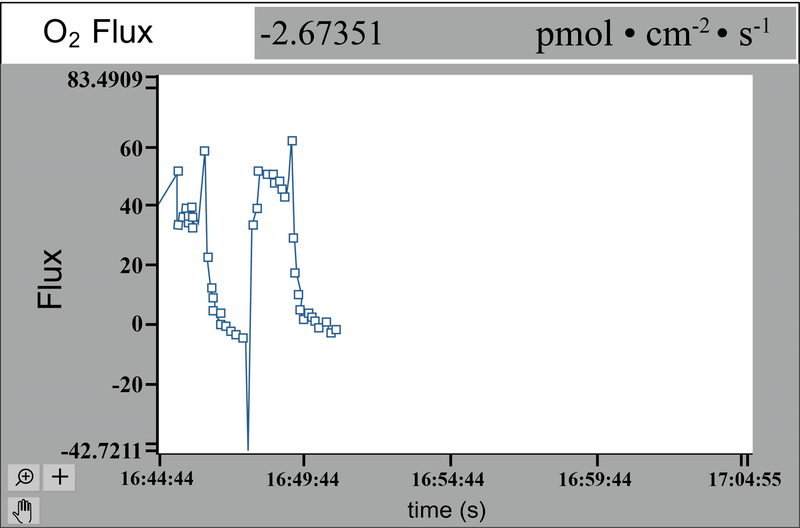
The NMT flux sensor measures the flux and the data are plotted on-screen during the test. These fluxes are most often measured in the unit 10−12 moles • cm−2 • s−1, or sometimes as small as 10−15 moles • cm−2 • s−1, allowing flux to be measured from something as small as a single cell.[5][6] During the test, further changes can be introduced like a stressor or other abiotic stimulus, and the flux patterns will change on-screen to show the physiological changes. For example, cold stress can be studied by adding ice-cold test buffer to the solution during testing.[7]
2. Variations

2.1. 1-Dimensional
This is the most common NMT test in which the flux sensor is moved in one direction in reference to the sample, generally perpendicular to the sample boundaries.
2.2. 2-Dimensional
There is not much documented application of 2-dimensional measurement in NMT; the possibility was demonstrated with the Vibrating Probe technique by Degenhardt et al in 1998.[8] They moved the flux sensor both perpendicular and then parallel to a plant root, then summed up the flux vectors to generate the 2-dimensional flux direction. In this kind of manner, NMT can measure 2D fluxes as well, using the same software that measures 3D fluxes.[9]
2.3. 3-Dimensional
One of the pioneers of 3D ion/molecule flux mapping is Joseph Kunkel.[10] To generate a 3-dimensional view of fluxes, the flux sensor must take measurements in the X, Y, and Z directions at each point around a sample. In 2006, a view of H+ and O2 3D flux vectors around a pollen tube was produced using Mageflux software developed by Yue Xu.[11]
2.4. Simultaneous
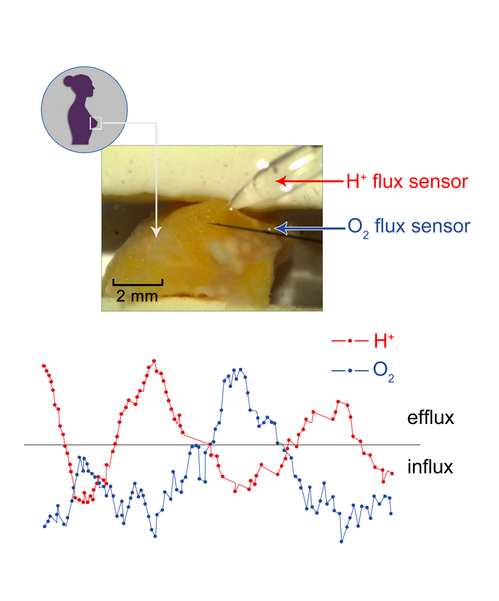
NMT flux sensors can be set up to measure two or more different ion/molecule fluxes at the same time, allowing the user to see the flux changes simultaneously, and to see the relationship between them.[12] Combining two particular flux measurements simultaneously can be a strong indicator of physiological phenomena.[11] For example, measuring both H+ and O2 simultaneously from a tumor sample (see figure to the right) can provide significant information about cancer metabolism that is far more useful than measuring only one at a time.
3. Applications
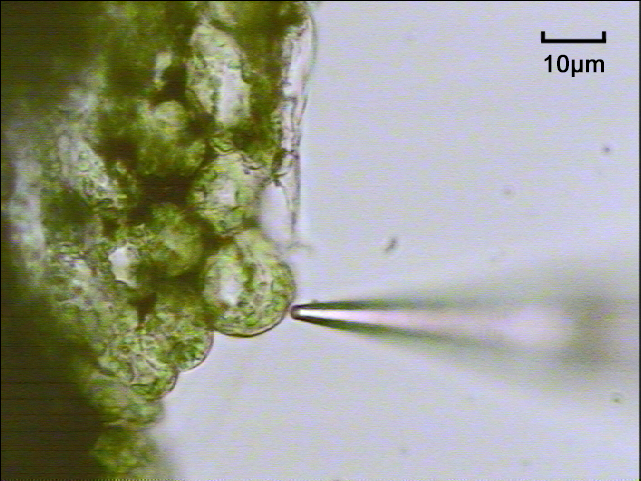
3.1. Genetics
It is widely accepted that both intracellular and extracellular ionic and molecular activities are vital to many physiological processes, also making them useful indicators of gene functions. By measuring dynamic ion/molecule fluxes, NMT has helped research on genes related to factors such as cold stress,[7] salt tolerance,[13] cadmium uptake,[14] and nutrient uptake in plants.[9] In biomedical genetic research, NMT has measured samples such as liver cells to investigate gene expression through fluxes.[15]
3.2. Plant Biology
NMT has been widely applied in plant biology in fields such as abiotic/biotic stress,[5][13] plant nutrition,[9][16] plant growth and development,[17] plant/microbe interaction,[18] plant defense,[19] photosynthesis,[20] signal transduction research,[21] and more. Roots are commonly measured, in addition to many other plant samples such as leaf tissue, root hairs, guard cells, salt gland cells, mesophyll cells, and condensed organelles like chloroplasts and vacuoles. NMT can help identify plants that are more resistant to stressors like salt, temperature, drought, and disease.[5][13] It’s also a useful tool for studying plant nutrition absorption and regulation mechanisms in ways such as monitoring rates of nutrient uptake at the root surface.[16]
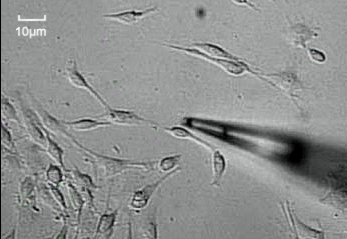
3.3. Biomedical
NMT is applied in biomedical research in various fields such as neuroscience,[22] tumor research,[23] drug screening,[24] metabolism,[11] and bone research.[25] NMT is a useful tool for evaluating the effects of treatments for diseases like diabetes and cancer in tissue samples, as it can measure the flux changes in response to treatments.[22][26] Measured samples include tumor tissue, neurons, brain tissue, liver cells, bone, muscle, and many more. Metabolism rates can be measured by NMT using O2 and H+ fluxes.[11] In bone research, the flux of Ca2+ measured by NMT helps research on bone healing and growth.[26]
3.4. Environmental
Various areas of environmental research in which NMT has been applied include water pollution detection,[4] biological early warning,[27] water quality assessment,[28] and heavy metal detection.[29] With global heavy metal pollution of crops on the rise, more NMT heavy metal flux sensors such as Cd2+, Cu2+, and Pb2+ have been used in research to identify plants that can tackle this problem with phytoremediation.[30] For water quality and pollution research, water plants and algae have been measured, as well as biofilms and fish embryos.[31]
References
- Sun, J.; Zhang, X.; Deng, S.; Zhang, C.; Wang, M.; Ding, M.; Zhao, R.; Shen, X. et al. (2012). "Extracellular ATP Signaling Is Mediated by H2O2 and Cytosolic Ca2+ in the Salt Response of Populus euphratica Cells.". PLOS ONE 7 (12): e53136. doi:10.1371/journal.pone.0053136. PMID 23285259. Bibcode: 2012PLoSO...753136S. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3532164
- Sun, T.; Xu, Y.; Li, P.; Yu, S.G.; Yin, L.P. (2007). "Non-invasive scanning ion-selective electrode technique and its applications to the research of higher plants.". Progress in Natural Science 17 (6): 625–629. doi:10.1080/10002007088537450. https://dx.doi.org/10.1080%2F10002007088537450
- Kunkel, J.G; Lin, L.Y.; Xu, Y.; Prado, A.M.M; Feijó, J.A.; Hwang, P.P.; Hepler, P.K. (2001). "The strategic use of Good buffers to measure proton gradients around growing pollen tubes.". Cell biology of plant and fungal tip growth.. IOS Press. pp. 81–94. ISBN 1-58603-083-3. https://books.google.com/books?id=wbYRAkaZfqMC&pg=PA81.
- Yang, C.; Fang, S.; Chen, D.; Wang, J.; Liu, F.; Xia, C. (2015). "The possible role of bacterial signal molecules N-acyl homoserine lactones in the formation of diatom-biofilm (Cylindrotheca sp.)". Marine Pollution Bulletin 107 (1): 118–124. doi:10.1016/j.marpolbul.2016.04.010. PMID 27090887. http://ir.yic.ac.cn/handle/133337/17092.
- Jin, Z.; Wang, Z.; Ma, Q.; Sun, L.; Zhang, L.; Liu, Z.; Liu, D.; Hao, X. et al. (2017). "Hydrogen sulfide mediates ion fluxes inducing stomatal closure in response to drought stress in Arabidopsis thaliana". Plant & Soil 419 (1–2): 141–152. doi:10.1007/s11104-017-3335-5. https://dx.doi.org/10.1007%2Fs11104-017-3335-5
- Liu, C.Y.; Zhang, F.; Zhang, D.J.; Srivastava, A.K.; Wu, Q.S.; Zou, Y.N. (2018). "Mycorrhiza stimulates root-hair growth and IAA synthesis and transport in trifoliate orange under drought stress". Scientific Reports 8 (1): 1978. doi:10.1038/s41598-018-20456-4. PMID 29386587. Bibcode: 2018NatSR...8.1978L. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=5792640
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zheng, D.; Pan, Y.; Lin, X. et al. (2015). "COLD1 Confers Chilling Tolerance in Rice". Cell 160 (6): 1209–1221. doi:10.1016/j.cell.2015.01.046. PMID 25728666. https://dx.doi.org/10.1016%2Fj.cell.2015.01.046
- Degenhardt, J.; Larsen, P.B.; Howell, S.H.; Kochian, L.V. (1998). "Aluminum Resistance in the Arabidopsis Mutantalr-104 Is Caused by an Aluminum-Induced Increase in Rhizosphere pH". Plant Physiology 117 (1): 19–27. doi:10.1104/pp.117.1.19. PMID 9576770. PMC 35003. http://www.plantphysiol.org/content/117/1/19.full.
- Dai, J.L.; Duan, L.S.; Dong, H.Z. (2015). "Comparative Effect of Nitrogen Forms on Nitrogen Uptake and Cotton Growth Under Salinity Stress". Journal of Plant Nutrition 38 (10): 1530–1543. doi:10.1080/01904167.2014.983126. https://dx.doi.org/10.1080%2F01904167.2014.983126
- Kunkel, J.G.; Cordeiro, S.; Xu, Y.; Shipley, A.M.; Feijó, J.A. (2006). "Chapter 5: Use of Non-Invasive Ion-Selective Microelectrode Techniques for the Study of Plant Development.". in Volkov, A.G.. Plant Electrophysiology. Springer, Berlin, Heidelberg.. pp. 109–137. doi:10.1007/978-3-540-37843-3_5. ISBN 978-3-540-37843-3. https://dx.doi.org/10.1007%2F978-3-540-37843-3_5
- Xu, Y.; Sun, T.; Yin, L.P. (2006). "Application of non-invasive microsensing system to simultaneously measure both H+ and O2 fluxes around the pollen tube". Journal of Integrative Plant Biology 48 (7): 823–831. doi:10.1111/j.1744-7909.2006.00281.x. https://dx.doi.org/10.1111%2Fj.1744-7909.2006.00281.x
- Garnett, T.P.; Shabala, S.N.; Smethurst, P.J.; Newman, I.A. (2003). "Kinetics of ammonium and nitrate uptake by eucalypt roots and associated proton fluxes measured using ion selective microelectrodes.". Functional Plant Biology 30 (11): 1165–1176. doi:10.1071/FP03087. PMID 32689098. https://dx.doi.org/10.1071%2FFP03087
- Li, S.; Liu, J.; An, Y.; Cao, Y.; Liu, Y.; Zhang, J.; Geng, J.; Hu, T. et al. (2019). "MsPIP2;2, a novel aquaporin gene from Medicago sativa, confers salt tolerance in transgenic Arabidopsis.". Environmental and Experimental Botany. 165: 39–52. doi:10.1016/j.envexpbot.2019.05.020. https://dx.doi.org/10.1016%2Fj.envexpbot.2019.05.020
- Wang, J.; Liang, S.; Xiang, W.; Dai, H.; Duan, Y.; Kang, F.; Chai, T. (2019). "A repeat region from the Brassica juncea HMA4 gene BjHMA4R is specifically involved in Cd2+ binding in the cytosol under low heavy metal concentrations". BMC Plant Biology 19 (1): 89. doi:10.1186/s12870-019-1674-5. PMID 30819104. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=6394093
- Zhang, Z.Y.; Wang, W.J.; Pan, L.J.; Xu, Y.; Zhang, Z.M. (2009). "Measuring Ca2+ influxes of TRPC1-dependent Ca2+ channels in HL-7702 cells with Non-invasive Micro-test Technique.". World Journal of Gastroenterology 15 (33): 4150–4155. doi:10.3748/wjg.15.4150. PMID 19725149. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2738811
- Zhang, Y.; Zhou, Y.; Chen, S.; Liu, J.; Fan, K.; Li, Z.; Liu, Z.; Lin, W. (2019). "Gibberellins play dual roles in response to phosphate starvation of tomato seedlings, negatively in shoots but positively in roots.". Journal of Plant Physiology 234–235: 145–153. doi:10.1016/j.jplph.2019.02.007. PMID 30807885. https://dx.doi.org/10.1016%2Fj.jplph.2019.02.007
- Zhang, F.; Wang, P.; Zou, Y.N.; Wu, Q.S.; Kuča, K. (2019). "Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress". Archives of Agronomy and Soil Science 65 (9): 1316–1330. doi:10.1080/03650340.2018.1563780. https://dx.doi.org/10.1080%2F03650340.2018.1563780
- Wang, X.; Teng, Y.; Tu, C.; Luo, Y.; Greening, X.; Zhang, N.; Dai, S.; Ren, W. et al. (2018). "Coupling between Nitrogen Fixation and Tetrachlorobiphenyl Dechlorination in a Rhizobium−Legume Symbiosis.". Environmental Science & Technology 52 (4): 2217–2224. doi:10.1021/acs.est.7b05667. PMID 29363956. Bibcode: 2018EnST...52.2217W. https://dx.doi.org/10.1021%2Facs.est.7b05667
- Cui, Y.; Li, X.; Yu, M.; Li, R.; Fan, L.; Zhu, Y.; Lin, J. (2018). "Sterols regulate endocytic pathways during flg22-induced defense responses in Arabidopsis". Development 145 (19): dev165688. doi:10.1242/dev.165688. PMID 30228101. https://dx.doi.org/10.1242%2Fdev.165688
- Chen, P.; Yan, K.; Shao, H.; Zhao, S. (2013). "Physiological Mechanisms for High Salt Tolerance in Wild Soybean (Glycine soja) from Yellow River Delta, China: Photosynthesis, Osmotic Regulation, Ion Flux and antioxidant Capacity". PLOS ONE 8 (12): e83227. doi:10.1371/journal.pone.0083227. PMID 24349468. Bibcode: 2013PLoSO...883227C. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3861505
- Zhao, C.C.; Wang, Y.; Chan, K.X.; Marchant, D.B.; Franks, P.J.; Randall, D.; Tee, E.E.; Chen, G. et al. (2019). "Evolution of chloroplast retrograde signaling facilitates green plant adaptation to land". PNAS 116 (11): 5015–5020. doi:10.1073/pnas.1812092116. PMID 30804180. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=6421419
- Li, T.; Yuan, L.; Zhang, J.; Jiao, J.J.; Qi, J.S. (2017). "Real-time measurement of Ca2+ flux in hippocampal slice with non-invasive micro-test technique.". Acta Physiologica Sinica 69 (4): 467–476. doi:10.13294/j.aps.2017.0033. PMID 28825106. https://dx.doi.org/10.13294%2Fj.aps.2017.0033
- Hu, S.L.; Du, P.; Hu, R.; Li, F.; Feng, H. (2014). "Imbalance of Ca2+ and K+ fluxes in C6 glioma cells after PDT measured with scanning ion-selective electrode technique". Lasers in Medical Science 29 (3): 1261–1267. doi:10.1007/s10103-014-1518-3. PMID 24458531. https://dx.doi.org/10.1007%2Fs10103-014-1518-3
- Jin, S.; Yong, T.; Ping, Z. (2008). "Direct evidence for the correlation between extracellular H+ activities and drug-resistance of tumor cells by non-invasive micro-test technique". Acta Biophysica Sinica 24 (3): 191–197.
- Liu, W.; Dan, X.; Lu, W.W.; Zhao, X.; Ruan, C.; Wang, T.; Cui, X.; Zhai, X. et al. (2019). "Spatial Distribution of Biomaterial Microenvironment pH and Its Modulatory Effect on Osteoclasts at the Early Stage of Bone Defect Regeneration". ACS Appl Mater Interfaces 11 (9): 9557–9572. doi:10.1021/acsami.8b20580. PMID 30720276. https://dx.doi.org/10.1021%2Facsami.8b20580
- Li, F.; Porterfield, D.M.; Zheng, X.Y.; Wang, W.J.; Xu, Y.; Zhang, Z.M. (2012). "Abnormal mitochondrial function impairs calcium influx in diabetic mouse pancreatic beta cells". Chinese Medical Journal 125 (3): 502–510. doi:10.3760/cma.j.issn.0366-6999.2012.03.019. PMID 22490411. https://dx.doi.org/10.3760%2Fcma.j.issn.0366-6999.2012.03.019
- Sanchez, B.C.; Ochao-Acuña, H.; Porterfield, D.M.; Sepúlveda, M.S. (2008). "Oxygen Flux As an Indicator of Physiological Stress in Fathead Minnow (Pimephales promelas) Embryos: A Real-Time Biomonitoring System of Water Quality.". Environmental Science & Technology 42 (18): 7010–7017. doi:10.1021/es702879t. PMID 18853824. Bibcode: 2008EnST...42.7010S. https://dx.doi.org/10.1021%2Fes702879t
- Ma, J.; Zhou, B.; Duan, D.; Wei, Y.; Pan, K. (2018). "Silicon limitation reduced the adsorption of cadmium in marine diatoms". Aquatic Toxicology 202: 136–144. doi:10.1016/j.aquatox.2018.07.011. PMID 30031253. https://dx.doi.org/10.1016%2Fj.aquatox.2018.07.011
- Ma, J.; Cai, H.; He, C.; Zhang, W.; Wang, L. (2015). "A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells.". New Phytologist 206 (3): 1063–1074. doi:10.1111/nph.13276. PMID 25645894. https://dx.doi.org/10.1111%2Fnph.13276
- Li, L.Z.; Yu, S.Y.; Peijnenburg, W.J.G.M.; Luo, Y.M. (2017). "Determining the fluxes of ions (Pb2+, Cu2+ and Cd2+) at the root surface of wetland plants using the scanning ion-selective electrode technique.". Plant and Soil 414 (1–2): 1–12. doi:10.1007/s11104-016-3109-5. http://ir.yic.ac.cn/handle/133337/22050.
- Horng, J.L.; Chao, P.L.; Chen, P.Y.; Shih, T.H.; Lin, L.Y. (2015). "Aquaporin 1 Is Involved in Acid Secretion by Ionocytes of Zebrafish Embryos through Facilitating CO2 Transport". PLOS ONE 10 (8): e0136440. doi:10.1371/journal.pone.0136440. PMID 26287615. Bibcode: 2015PLoSO..1036440H. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4546062




