
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Beatrix Zheng | -- | 1104 | 2022-10-18 01:42:22 |
Video Upload Options
HSD2 neurons are a small group of neurons in the brainstem which are uniquely sensitive to the mineralocorticosteroid hormone aldosterone, through expression of HSD11B2. They are located within the caudal medulla oblongata, in the nucleus of the solitary tract (NTS). HSD2 neurons are activated during a prolonged deficit in body sodium or fluid volume, as occurs after dietary sodium deprivation or during frank hypovolemia. They are also activated by supraphysiologic stimulation of the mineralocorticoid receptor. They are inactivated when salt is ingested. To date, HSD2 neurons have been identified and studied only in rats and mice.
1. Basic Characteristics
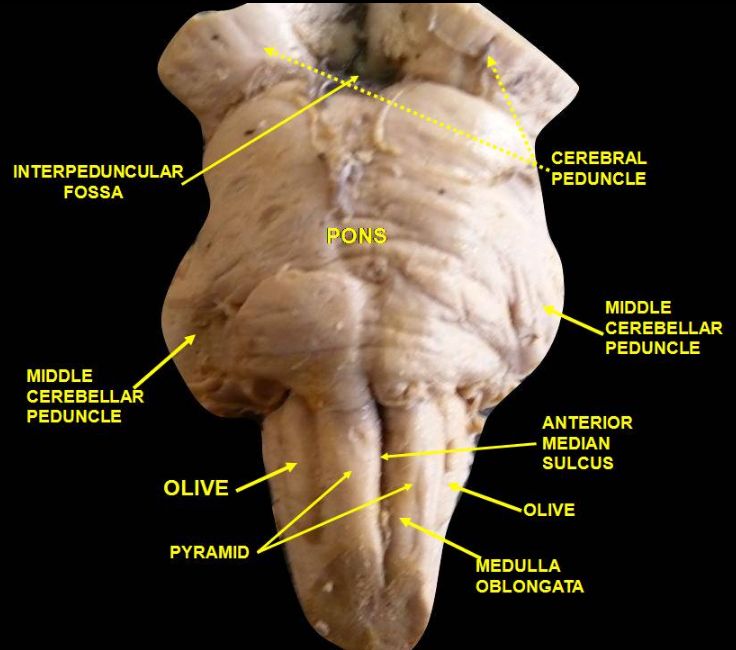
The term "HSD2 neurons" is used in the scientific literature to refer to a subpopulation of neurons in the NTS which express both the mineralocorticoid receptor (MR) [1] and 11-beta-hydroxysteroid dehydrogenase type 2 (HSD2).[1][2] HSD2 is an enzyme that metabolizes cortisol and other glucocorticosteroids, which typically prevent aldosterone from binding to the mineralocorticoid receptor. This pre-receptor mechanism for modifying hormone binding is necessary for cellular sensitivity to aldosterone because, under physiologic conditions, cortisol circulates at 100-1000 times higher concentrations than aldosterone. As both cortisol and aldosterone bind the mineralocorticoid receptor with equal affinity, cortisol effectively crowds out aldosterone in cells without abundant HSD2. In cells with HSD2, however, aldosterone has increased access to the MR, such that increases and decreases in the circulating concentration of this hormone will produce a change in receptor activity. In HSD2 neurons (and all other cells that express both HSD2 and MR), aldosterone binds to MR and translocates it from the cytoplasm to the nucleus, causing transcriptional changes. Unlike aldosterone-sensitive cells in epithelial tissues (e.g. in the kidney), the physiologic effects of aldosterone-MR activation in HSD2 neurons are unknown. It has been suggested, but not proven, that aldosterone promotes the firing activity of these neurons.[3] Aldosterone is not necessary for HSD2 neuron activation because this can be evoked by sodium deprivation even in rats without adrenal glands,[4] which are the exclusive source of circulating aldosterone.
HSD2 neurons express the transcription factor Phox2b.[5] This means that HSD2 neurons probably release the excitatory transmitter glutamate onto their synaptic target neurons, as all Phox2b-expressing neurons in the NTS express the vesicular glutamate transporter VGlut2.[6] HSD2 neurons do not produce a wide array of other proteins that typify most other subtypes of NTS neurons, including tyrosine hydroxylase, choline acetyltransferase, nitric oxide synthase, cholecystokinin, neurotensin, neuropeptide FF, substance P, somatostatin, inhibin-β, glucagon-like peptide-1, corticotropin-releasing hormone, dynorphin, calretinin, and calbindin. A small number of HSD2 neurons (less than 2%) may express the neuropeptide galanin.[1] Their lack of expression of the aforementioned markers suggests that HSD2 neurons form a unique subpopulation within the NTS. To date, there is no information available about the electrophysiologic characteristics of these neurons.
2. Input and Output Connections
The efferent projections (axonal output) of HSD2 neurons have been investigated to a significant degree using conventional neuroanatomical tracers. Their primary output targets are the pre-locus coeruleus (pre-LC), the innermost portion of the external lateral parabrachial subnucleus (PBel), and the anterior, ventrolateral bed nucleus of the stria terminalis (BSTvl).[7] The next-order input and output connections of these target regions have been investigated in detail as well.[8][9][10] Additional information about the efferent projections of HSD2 neurons can be found in ref.[7]
Regarding the afferent (input) connections to HSD2 neurons, available information is less complete. Experiments with conventional tracers and immunofluorescence staining have demonstrated peripheral viscerosensory input from the vagus nerve,[11] input from nearby neurons in the NTS and area postrema,[12][13] and descending input from the medial central nucleus of the amygdala (CeA) [14] and paraventricular hypothalamic nucleus (PVN).[15] It is likely that other sources of input exist, but a comprehensive study of HSD2 neuron afferent connections has not been conducted.
3. HSD2 Neuron Activity
An immediate-early gene, c-fos, has been used to study the activation and inactivation of HSD2 neurons extensively in vivo. The presence of nuclear c-Fos implies recent, elevated neuronal activity, and c-Fos disappears after neurons become quiescent. Very few HSD2 neurons exhibit any c-Fos in a normal animal. If, however, sodium is removed from the diet for several days to a week, most HSD2 neurons become c-Fos-positive.[16] Then, if salty food is eaten or a concentrated saline solution is imbibed, their c-Fos disappears.[4] Several other experimental conditions that reduce extracellular fluid volume—including PEG-hypovolemia, diuresis, and adrenalectomy—also activate HSD2 neurons,[4] although none do so to as great an extent as simply removing sodium from the diet.[17]
All of these conditions, with the exception of adrenalectomy, cause a large elevation of circulating aldosterone. Correspondingly, repeated administration of the mineralocorticosteroid hormone deoxycorticosterone acetate (DOCA) produces a moderate increase in HSD2 neuron activity (c-Fos) without any sodium or volume deficit.[18] However, even after adrenalectomy, HSD2 neurons become activated by sodium deprivation, proving that MR activation is not necessary for their activity. Thus, aldosterone may be sufficient, but is not necessary for their activation, meaning that these neurons integrate additional neural or hormonal input signals.
All of the aforementioned manipulations which activate HSD2 neurons also produce sodium appetite in rats. If sodium-deprived rats are allowed access to salt, they imbibe a large quantity of it, and soon afterwards their HSD2 neurons are inactivated (they exhibit little or no c-Fos within 1–2 hours).[4][16] This phenomenon of salt-intake-induced inactivation also occurs after sodium appetite and HSD2 neuron activation are produced by DOCA, which does not produce any sodium or volume deficit.[18] Thus, HSD2 neuron inactivation by salt intake does not reflect simply the repletion of a physiologic deficit, and may instead reflect active inhibition triggered by salt ingestion. The exact mechanism for this inhibition remains unknown.
An interesting and unique feature of HSD2 neuron activity is that they are not activated by several stimuli that produce pronounced c-Fos activation in most other neurons in the NTS. These stimuli include severe dehydration induced by hypertonic saline administration,[19] salt ingestion (above), and changes in blood pressure. Thus, HSD2 neurons are selectively activated by conditions which do not significantly affect surrounding NTS neurons,[4] and they are not stimulated (or are actively inhibited) by conditions that do prominently activate most other NTS neurons.[16][19]
4. HSD2 Neuron Functions
The close association between sodium deprivation and HSD2 neuron activation—and between salt ingestion and HSD2 neuron inactivation—led to the suggestion that these neurons are important for driving sodium appetite.[4] Other functional roles have been hypothesized. For discussion, see reviews in [17] and.[3] At present, however, no data exist to show whether these neurons are necessary or sufficient for any particular neurologic or physiologic function.
References
- Geerling, JC; Kawata, M; Loewy, AD (Jan 20, 2006). "Aldosterone-sensitive neurons in the rat central nervous system.". The Journal of Comparative Neurology 494 (3): 515–27. doi:10.1002/cne.20808. PMID 16320254. https://dx.doi.org/10.1002%2Fcne.20808
- Roland, BL; Li, KX; Funder, JW (Oct 1995). "Hybridization histochemical localization of 11 beta-hydroxysteroid dehydrogenase type 2 in rat brain.". Endocrinology 136 (10): 4697–700. doi:10.1210/endo.136.10.7664691. PMID 7664691. https://dx.doi.org/10.1210%2Fendo.136.10.7664691
- Geerling, JC; Loewy, AD (Sep 2009). "Aldosterone in the brain.". American Journal of Physiology. Renal Physiology 297 (3): F559–76. doi:10.1152/ajprenal.90399.2008. PMID 19261742. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2739715
- Geerling, JC; Engeland, WC; Kawata, M; Loewy, AD (Jan 11, 2006). "Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite.". The Journal of Neuroscience 26 (2): 411–7. doi:10.1523/JNEUROSCI.3115-05.2006. PMID 16407537. https://dx.doi.org/10.1523%2FJNEUROSCI.3115-05.2006
- Geerling, JC; Chimenti, PC; Loewy, AD (Aug 21, 2008). "Phox2b expression in the aldosterone-sensitive HSD2 neurons of the NTS.". Brain Research 1226: 82–8. doi:10.1016/j.brainres.2008.05.072. PMID 18620340. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2582384
- Kang, BJ; Chang, DA; Mackay, DD; West, GH; Moreira, TS; Takakura, AC; Gwilt, JM; Guyenet, PG et al. (Aug 2007). "Central nervous system distribution of the transcription factor Phox2b in the adult rat.". J Comp Neurol 503 (5): 627–41. doi:10.1002/cne.21409. PMID 17559094. https://dx.doi.org/10.1002%2Fcne.21409
- Geerling, JC; Loewy, AD (Jul 10, 2006). "Aldosterone-sensitive neurons in the nucleus of the solitary tract: efferent projections.". The Journal of Comparative Neurology 497 (2): 223–50. doi:10.1002/cne.20993. PMID 16705681. https://dx.doi.org/10.1002%2Fcne.20993
- Shin, JW; Geerling, JC; Loewy, AD (Dec 10, 2008). "Inputs to the ventrolateral bed nucleus of the stria terminalis.". The Journal of Comparative Neurology 511 (5): 628–57. doi:10.1002/cne.21870. PMID 18853414. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2748802
- Shin, JW; Geerling, JC; Stein, MK; Miller, RL; Loewy, AD (Sep 2011). "FoxP2 brainstem neurons project to sodium appetite regulatory sites.". Journal of Chemical Neuroanatomy 42 (1): 1–23. doi:10.1016/j.jchemneu.2011.05.003. PMID 21605659. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3148274
- Dong, HW; Petrovich, GD; Watts, AG; Swanson, LW (Aug 6, 2001). "Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain.". The Journal of Comparative Neurology 436 (4): 430–55. doi:10.1002/cne.1079. PMID 11447588. https://dx.doi.org/10.1002%2Fcne.1079
- Shin, JW; Geerling, JC; Loewy, AD (Jan 16, 2009). "Vagal innervation of the aldosterone-sensitive HSD2 neurons in the NTS.". Brain Research 1249: 135–47. doi:10.1016/j.brainres.2008.10.058. PMID 19010311. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2666048
- Sequeira, SM; Geerling, JC; Loewy, AD (Sep 15, 2006). "Local inputs to aldosterone-sensitive neurons of the nucleus tractus solitarius.". Neuroscience 141 (4): 1995–2005. doi:10.1016/j.neuroscience.2006.05.059. PMID 16828976. https://dx.doi.org/10.1016%2Fj.neuroscience.2006.05.059
- Miller, RL; Stein, MK; Loewy, AD (Oct 13, 2011). "Serotonergic inputs to FoxP2 neurons of the pre-locus coeruleus and parabrachial nuclei that project to the ventral tegmental area.". Neuroscience 193: 229–40. doi:10.1016/j.neuroscience.2011.07.008. PMID 21784133. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3185334
- Geerling, JC; Loewy, AD (Aug 1, 2006). "Aldosterone-sensitive neurons in the nucleus of the solitary tract: bidirectional connections with the central nucleus of the amygdala.". The Journal of Comparative Neurology 497 (4): 646–57. doi:10.1002/cne.21019. PMID 16739197. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2748794
- Geerling, JC; Shin, JW; Chimenti, PC; Loewy, AD (May 1, 2010). "Paraventricular hypothalamic nucleus: axonal projections to the brainstem.". The Journal of Comparative Neurology 518 (9): 1460–99. doi:10.1002/cne.22283. PMID 20187136. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2868510
- Geerling, JC; Loewy, AD (Oct 1, 2007). "Sodium deprivation and salt intake activate separate neuronal subpopulations in the nucleus of the solitary tract and the parabrachial complex.". The Journal of Comparative Neurology 504 (4): 379–403. doi:10.1002/cne.21452. PMID 17663450. https://zenodo.org/record/1063606/files/article.pdf.
- Geerling, JC; Loewy, AD (Feb 2008). "Central regulation of sodium appetite.". Experimental Physiology 93 (2): 177–209. doi:10.1113/expphysiol.2007.039891. PMID 17981930. https://dx.doi.org/10.1113%2Fexpphysiol.2007.039891
- Geerling, JC; Loewy, AD (Oct 18, 2006). "Aldosterone-sensitive NTS neurons are inhibited by saline ingestion during chronic mineralocorticoid treatment.". Brain Research 1115 (1): 54–64. doi:10.1016/j.brainres.2006.07.091. PMID 16935272. https://dx.doi.org/10.1016%2Fj.brainres.2006.07.091
- Geerling, JC; Loewy, AD (Mar 2007). "Sodium depletion activates the aldosterone-sensitive neurons in the NTS independently of thirst.". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 292 (3): R1338–48. doi:10.1152/ajpregu.00391.2006. PMID 17068161. https://dx.doi.org/10.1152%2Fajpregu.00391.2006





