Globally, sugarcane (
Saccharum spp.) is one of the most pertinent agricultural cash crops that is widely cultivated in countries having subtropical and tropical climates such as United States, India, Pakistan, China, Brazil, Australia, Cuba, and Philippines
[1][2][3]. A number of industrial high-end products including sugar (75% of sucrose’s global demand is met from sugarcane while remaining comes from sugar-beet crop), biofuel, different types of waxes, and a diverse variety of bio-fibers are obtained from sugarcane worldwide
[4]. There are two wild species (
S. robustum and
S. spontaneum) and ur mainly cultivated species (
S. edule,
S. barberi,
S. sinense, and
S. officinarum) of the Saccharum genus
[5].
Succharum spontaneum has recently emerged as an important genetic resource for utilization in various breeding programs of sugarcane
[6][7]. Most of the new sugarcane varieties have been developed from the interspecific hybridization of
Saccharum officinarum and
Saccharum spontaneum. The resulting varieties tend to be polyploids and aneuploids with chromosome counts ranging from 80 to 120
[8][9][10].
Historically, sugarcane crop improvement has remained centered toward sucrose content enhancement; however, recently fiber, lignin content, and biomass have become important components of modern breeding strategies
[11][12][13][14]. It has been established that success of sugarcane breeding programs in order to acquire the desired traits will depend on the genetic diversity in the active germplasm banks
[11][12][13][14]. For cultivar development, the regular provision of germplasm collections with accessions to diverse genetic backgrounds and quantifying genetic variability in these collections have remained essential tasks for improved management and conservation
[15][16]. Recently developed biotechnological tools and approaches have emerged as potent strategies to improving traditional breeding programs, notably for understanding gene structures, genomic locations, and plant transformations. Numerous molecular studies have investigated the sugarcane genome constitution and structure and have revealed the genome size of sugarcane to be over 10 Gbp, with genes that exist in up to 10–12 allelic forms
[17][18][19]. The estimated monoploid genome size ranges from 800–900 Mb, depending on the ploidy level of crop’s variety
[20].
2. Fundamentals of Gene Editing Technology
The use of gene-editing technology has provided great opportunities to researchers in the field of molecular biology by facilitating the modification and targeting of specific genes accurately. Through gene editing technologies, plant breeders have been able to develop new cultivars with desired traits. The gene editing processes can successfully knockout or insert any gene for inducing a particular function or trait
[21]. Previously, numerous transgenic systems for eukaryotic genome manipulation have been developed including clustered regularly interspaced short palindromic repeats (CRISPR), transcriptional activator-like effector nucleases (TALENs)
[22][23][24], and zinc finger nucleases (ZFNs)
[25][26]. Among gene editing techniques, CRISPR/Cas9 system provides a low-cost, simple, and easy-to-use method for gene editing. In CRISPR/Cas9 tools, it is possible to induce site-specific double-strand breaks (DSBs) in related genes to obtain mutations. This has assisted to overcome the limitation of ZFNs and TALENs which have complex technical design processes to achieve specific objectives making them highly time-consuming and onerous in functioning
[27]. However, CRISPR/Cas9 system assist in preparing double strand breaks that can be repaired by the cellular DNA repair mechanisms, namely the non-homologous end junction (NHEJ) or homologous directed repair (HDR) pathways
[28].
Figure 1 illustrates few basic and advanced gene editing approaches that are being employed to develop crops varieties having desired agro-botanical traits and physiological characteristics.
Figure 1. The basic genome editing techniques for developing new cultivars having desired traits.
2.1. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) System
Genome-editing via CRISPR is a recent technological advancement to develop new crop varieties having desired phenotypic and physiological traits along with exploring numerous biological phenomenon in crop plants. Besides having different site-directed nucleases for genome editing, the CRISPR/Cas-based genome editing approach offers a variety of advantages such as simplicity, ease of access, low cost, and flexibility
[24][29]. There are different types of CRISPR/Cas systems, and each type is distinguished by a different structure of the effector module, including unique characteristic proteins
[30]. The CRISPR/Cas9 is the first system for eukaryotic genome editing and is currently the most widely used genome editing system.
There are two components in the CRISPR/Cas9 system including a single guide RNA (sgRNA) and a Cas9 nuclease. The Cas9 is guided to cut the target sequence by sgRNA, which binds to the target sequence. By detecting the 3’-NGG motif (also called original spacer adjacent motif (PAM)) in the target sequence, sgRNA is able to identify the target sequence. The DNA sequences that complement the first 20 bases of sgRNA may be used as targets, but DNA can only be cleaved if PAM exists at the 3’ end of the DNA target. The motif sequence of PAM varies depending on the bacteria from which the CRISPR system was derived and the Cas protein variations used. The PAM sequence of spcas9 is 5’-NGG, and the PAM sequences for different bacteria and Cas9 variants are presented in
Table 1. Transcription of sgRNA is typically driven by the U6 promoter, while Cas9 genes are typically driven by CaMV (cauliflower mosaic virus) 35S promoter or the ubiquitin promoter
[31]. The Cas9 genes are usually codon-optimized to enhance the expression of the target plant species
[32]. The nuclear localization signal (NLS) is also often fused to the Cas9 gene in order to direct it toward the nucleus with utmost accuracy
[31]. The CRISPR/Cas9 system has been successfully used in genome editing of many plant species for producing mutants having requisite traits
[33] (
Figure 2 and
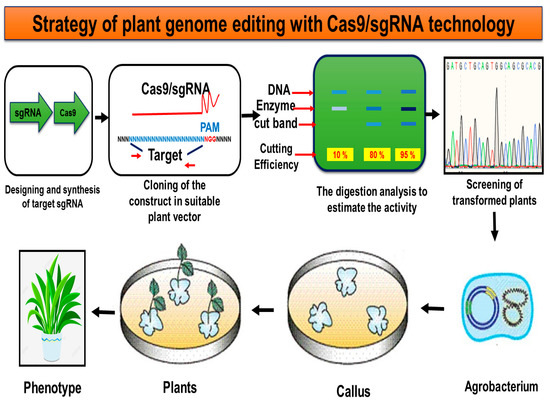
Table 1).
Figure 2. The basic steps of genome editing in plants using CRISPR/Cas9/sgRNA technology beginning with the selection of target genes.
Table 1. Few prominent CRISPR/Cas9 online resources indicating fundamental information and application protocols.
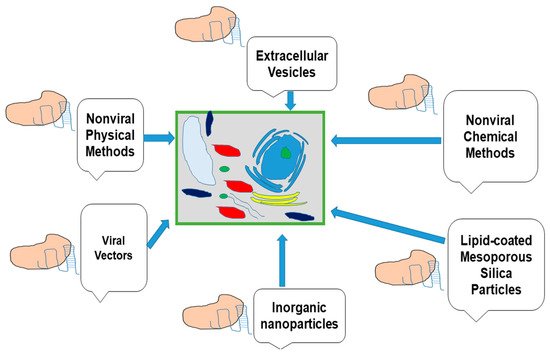
2.2. Delivery of CRISPR Components
The CRISPR components can be delivered into the plant genome such as DNA, mRNA (in vitro transcript), and proteins
[34]. The various types of delivery technologies include infection through penetration by Agrobacterium
[35][36], gene knockouts or particle bombardment
[37], electroporation
[38], and virus-based delivery systems
[39]. The mRNA can also be used to deliver genome editing reagents as this type of transient delivery is effective in producing the stable transgenic events along with reducing the threat of deviation from targets
[40]. Besides above delivery systems, a pre-assembled Cas9 gRNA ribonucleoprotein (RNP) delivery system has also been reported (
Figure 3 and
Figure 4) involving the direct delivery of RNP complexes which eliminates the possibility of foreign DNA being introduced into the host genome
[41].
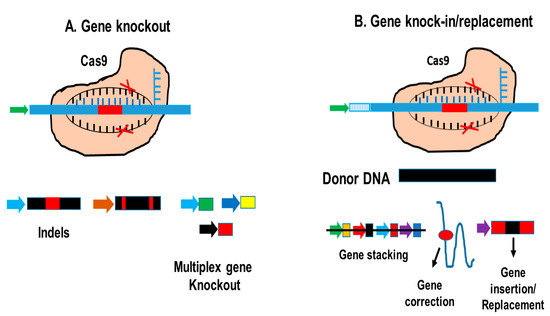
Figure 3. The CRISPR/Cas applications. (A) Using CRISPR/Cas, mutations can be introduced, which result in indels, gene deletions, and even multiple gene knockouts. (B) Gene insertion and replacement by homologous directed repair or no homologous end connections.
Figure 4. Various delivery technologies methods of CRISPR/Cas9 for plant genome editing.
First successfully delivered Cas9 gRNA RNPs were demonstrated in animal cells
[42]. These were subsequently used in plants to induce the formation of protoplasts (mediated by PEG) from somatic tissues of tobacco, Arabidopsis, rice, petunia, grapevine, and potato. Additionally in maize and wheat, Cas9 gRNA and RNPs have also been successfully introduced into embryonic cells using the gene-gun method
[43][44][45]. Recent research by Liu et al.
[46] describes applying a novel liposome-mediated transfection method to introduce pre-assembled Cas9 RNP gRNA into BY2 tobacco protoplasts for genome editing.
2.3. Emerging Perspectives of CRISPR System
The CRISPR/Cas9 system has been regarded as the simplest, unprecedently efficient, and highly specific that results in fewer off-targetting in comparison to conventional biotechnological approaches
[45][47]. Therefore, it has emerged as a promising tool for plant genome modification. This biotechnological system has been anticipated to strategic impact on plant biology’s basic and applied research for crops including sugarcane. This development should also impact crop breeding. Gene editing facilitates the accurate and predictable modification of good varieties or materials directly rather than the arduous backcross procedure used in traditional breeding methods. CRISPR/cas9 is expected to provide a more effective method for pyramid breeding since multiple traits can be modified simultaneously
[48]. Gene knockout by NHEJ is the most direct CRISPR/Cas9 technology application. To enhance crop yields and make the host more resistant to pathogens, negative regulators of grain development and disease resistance can be modified. Other gene modification methods such as gene expression regulation and epigenetic regulation may also be employed for crop improvement. Another advantage of CRISPR/Cas9 is introducing target genes into non-GM footprint crops through penetration, viral infection, or pre-assembled Cas9 protein sgRNA ribonucleoprotein transformation, thus circumventing traditional regulations of genetically modified organisms
[49].
With CRISPR/Cas9 technology, it is possible to delete whole chromosomes
[50] or specific genes
[51] depending on the target traits intended to be acquired in field crops including sugarcane. A 1.6 KB GUS gene was removed by Srivastava et al.
[51] via targeting both ends of a gene with Cas9 and two gRNAs. The transgenic plants tend to remove undesirable plant selection genes (such as the kanamycin resistance gene) while transgenic plants may retain few induced genes that has attracted criticism from regulators and consumers. However, the CRISPR technology holds advantage to replace and repair dysfunctional alleles
[52] or create a site for gene integration at specific locations that minimized off-targetting
[53].
2.4. CRISPR/Cas9 System in Polyploid Crops
Despite the fact that CRISPR/Cas9-mediated genome editing is widely used in plants, its efficiency continues to remain an unexplored subject. Especially in polyploid crops, it is necessary to knock out all copies of genes with the same function at the same time because of functional redundancy between parahomologous genes and homologous genes. Optimization of Cas9 codon, promoter, and target sequence composition (GC content) could impact the mutation efficiency of polyploid crops
[54]. The design of sgRNA in polyploid crops is more complex than in diploid species such as Arabidopsis and rice. Some advanced CRISPR/Cas9 tools have been developed for sgRNA design (CRISPR-p and CRISPR-p2.0); however, limitations still exist for genome editing of polyploid plants
[55]. To knock out both homologous and paralogous genes simultaneously, it is necessary to design sgRNA that targets all copies of each gene (parahomologous and homologous genes). The sgRNA can be designed manually after sequence analysis to target all genes or specific gene copies.
Interestingly, when there are not many conserved regions in homologous genes, it is necessary to divide these genes into multiple groups and design sgRNAs based on the conserved regions in each group. A new homologous gene from Streptococcus pyogenes spCas9 has been demonstrated to be effective for gene editing in plants, including
Streptococcus thermophilus (stCas9) and
Staphylococcus aureus (saCas9)
[56]. In Streptococcus pyogenes, the RNA-guided endonuclease Cas9 is too large to be used for genome targeting. SPACas9 from Staphylococcus aureus also targets the genome with high efficiency similar to spCas9; however, its size is shorter than spCas9, which makes it easier to use for genome targeting
[57]. The CRISPR/cpf1 is a new variant of CRISPR that has shown its effectiveness in editing plant genomes as CRISPR/Cas9
[58]. The CRISPR/CPF1 PAM is tttn that is suitable for targeting complex regions of the genome such as the promoter region. The CRISPR/cpf1 produces DSB with a 5’ sticky end that tends to promote NHEJ gene mutation during the repair process. In addition, several extended PAM sequences have been identified for Cas9 and cpf1 that imparts extension to the recognition sites for the PAM sequences. Moreover, the CRISPR/Cas9 introduces a base editing function by introducing cytidine deaminase into the genome
[48][49].
2.5. Factors Affecting the Activity of CRISPR/Cas9
Among many factors that affect the activity of CRISPR/Cas9, the presence of an effective vector constitute the most strategic factor which ensures successful delivery of cas9 protein and sgRNA to the nucleus in CRISPR/Cas9 gene modification. The Cas9 gene and sgRNA gene can be combined on a single plasmid or separate plasmids
[59]. The Cas9 has been driven by promoters commonly used in plant transformation, namely ubiquitin and the 35S promoter of cauliflower mosaic virus (CaMV 35S). It is common practice to attach the Cas9 gene to molecular tags in order to detect/purify proteins and nuclear localization signals (NLS) and to facilitate the entry of the Cas9 protein into the nucleus
[60]. Xing et al.
[61] have demonstrated the effectiveness of pcambia-based sgRNA module vectors in many plant species. The module vectors were constructed by assembling two or more sgRNA expression cassettes using the golden gate or Gibson assembly methods. Additionally, it has been demonstrated that the vector has a high mutation efficiency (60–95%) in transgenic lines for maize. Moreover, many biallelic mutations can also be efficiently passed on to the next generation
[62].
Besides the presence of effective factor, several other factors influence the mutation efficiency of CRISPR/Cas9, including the specificity of the gene target, the location of the PAM sequence, the nature of the sgRNA sequence, the promoter of the Cas9 gene and sgRNA, the tissue of interest, and the conversion technology used. It has been suggested that target genes must be selceted carefully because some genes are essential for cell growth and gene knockout can be fatal to plants. Crops usually contain multiple copies of a gene due to rearrangements, polyploidy, or replication. Therefore, the nonspecific nature of sgRNA may result in biallelic mutation or chimerism. Among the acetyllactate synthase gene family members in maize, there are Als1 and ALS2 on chromosomes 4 and 5, respectively. It was reported that non-gene specific sgRNAs resulted in biallelic mutations in two ALS genes, resulting in the recovery of unstable events. A sgRNA designed for ALS2 based on the polymorphism between Als1 and ALS2 could induce the necessary mutation as inferred by Svitashev et al.
[43]. A target site’s GC content can also affect the stability of DNA sgRNA hybridization. The GC content is high, which allows DNA–RNA hybridization to be stable, but a more stable hybridization also increases miss rates. Up to 35% of the GC content in the target region exhibits good Cas9 enzyme activity with little deviation from the target
[63]. A PAM sequence is typically NGG, although nag can also be used to reduce nucleases’ ability to bind to genomic DNA
[64]. The NRG’s binding efficiency is only one fifth that of NGG’s. Each base in the PAM sequence influences the binding efficiency of nuclease. The PAM’s first nucleotide is the least conservative, but the G in position two improves binding efficiency by 90%, therefore NRG’s stability is lower than that of NGG’s
[65]. The activity of sgRNA also depends on its length. Long sgRNAs of 19 nucleotides are more effective than truncated sgRNAs of 17–18 nucleotides and longer 22–23 nucleotides sgRNAs.