| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luciana Ferreira | + 2640 word(s) | 2640 | 2020-11-05 07:46:59 | | | |
| 2 | Camila Xu | Meta information modification | 2640 | 2020-11-11 02:11:07 | | |
Video Upload Options
In thyroid cancer, calcification is mainly present in classical papillary thyroid carcinoma (PTC) and in medullary thyroid carcinoma (MTC), despite being described in benign lesions and in other subtypes of thyroid carcinomas. Thyroid calcifications are classified according to their diameter and location. At ultrasonography, microcalcifications appear as hyperechoic spots ≤ 1 mm in diameter and can be named as stromal calcification, bone formation, or psammoma bodies (PBs), whereas calcifications > 1 mm are macrocalcifications. The mechanism of their formation is still poorly understood. Microcalcifications are generally accepted as a reliable indicator of malignancy as they mostly represent PBs. In order to progress in terms of the understanding of the mechanisms behind calcification occurring in thyroid tumors in general, and in PTC in particular, we decided to use histopathology as the basis of the possible cellular and molecular mechanisms of calcification formation in thyroid cancer. We explored the involvement of molecules such as runt-related transcription factor-2 (Runx-2), osteonectin/secreted protein acidic and rich in cysteine (SPARC), alkaline phosphatase (ALP), bone sialoprotein (BSP), and osteopontin (OPN) in the formation of calcification. The present review offers a novel insight into the mechanisms underlying the development of calcification in thyroid cancer.
1. Definition
Thyroid nodules (TNs) are defined by the American Thyroid Association (ATA) as “discrete lesions within the thyroid gland, radiologically distinct from surrounding thyroid parenchyma” [1]. They are extremely common and frequently identified in patients, with no symptoms, by self-examination or in undergoing evaluation for other medical conditions [2]. TNs may be discovered by palpation during a general physical examination or by radiographic exams, such as carotid duplex ultrasound (US), magnetic resonance imaging (MRI), computed tomography (CT) scans, or 18-fluorodeoxyglucose uptake on positron emission tomography scan (18FDG-PET) scanning. When detected in the latter exams, TNs do not correspond to palpable lesions and are therefore called as “thyroid incidentalomas” [3].
Pathological calcifications can include dystrophic calcification, i.e., deposition of calcium at sites of cell injury and necrosis, and metastatic calcification, which refers to deposition of calcium in normal tissues caused by hypercalcemia (usually a consequence of parathyroid hormone excess); the latter will not be address in this review. The simplest way that thyroid nodular calcifications can be classified is according to their diameter and location, as microcalcifications and macrocalcifications.
2. Introduction
The method of identification determines the prevalence of TNs in the general population. When addressing palpation only, the prevalence ranges from 4 to 7% [4][5], whereas US detects nodules in 20–76% of the adult population [5][6], especially with the current use of high-resolution US techniques [2]. The incidence of malignancy detected in TNs is relatively low, ranging from 1.6 to 12% [7][8]. US is the primary tool for the diagnosis and the initial cancer risk stratification of TNs. Indeed, it guides decision making for fine-needle aspiration (FNA) biopsy, the subsequent clinical assessments at the time of long-term follow-up [9], and the eligibility for active surveillance of suspicious nodules [10]. US features evaluated in each nodule include echogenicity, composition (solid, cystic, mixed), margins, calcifications or other hyperechoic foci, shape, and relations with the thyroid capsule [11][12]. US patterns associated with malignancy comprehend hypoechogenicity; infiltrative, irregular, or lobulated margins; microcalcifications; taller-than-wide shape; and absence of a halo [13].
It was reported that 19.8–32.1% of TNs have some type of calcification [14][15] and that the prevalence of calcification in TNs is around 40% in malignant and 20% in benign nodules [16]. On the basis of Thyroid Image Reporting and Data System (TIRADS) scoring, microcalcifications are predictive of malignancy [16] and central macrocalcifications are usually predictive of benign pathology. Other diseases may be associated with calcifications, such as nodular goiter or Graves’ disease, and regardless of various studies on the topic, no clear association between calcifications and histopathologic classification has been demonstrated [17][18]. In contrast, microcalcifications in cervical lymph nodes are predictive of PTC metastasis [19].
3. Types of Calcification in Thyroid
Pathological calcifications can include dystrophic calcification, i.e., deposition of calcium at sites of cell injury and necrosis, and metastatic calcification, which refers to deposition of calcium in normal tissues caused by hypercalcemia (usually a consequence of parathyroid hormone excess); the latter will not be address in this review. The simplest way that thyroid nodular calcifications can be classified is according to their diameter and location. Under US, microcalcifications appear as hyperechoic (i.e., increased echogenicity relative to thyroid tissue) spots ≤ 1 mm in diameter with or without posterior acoustic shadows or as simple fine acoustic shadows [20]. They can be named as stromal calcification, bone formation, or psammoma bodies (PBs). Another type of small echogenic focus seen in the thyroid is inspissated colloid, which may cause a comet tail reverberation artifact in US [21][22].
Calcifications > 1 mm with posterior acoustic shadow are macrocalcifications, and although there are some different classifications for the types of macrocalcifications [23][24], the most commonly found terms are “egg-shell, annular or rim-like peripheral calcification” and “coarse dense calcifications” [16][25][26]. Regardless of size, all the aforementioned types of calcification represent forms of so-called dystrophic calcification (DC), since one is dealing with calcification occurring in degenerated or necrotic tissue.
The main types of calcifications are summarized in Table 1 and Figure 1. A more detailed description will be made for PBs, since they are more closely related with neoplastic transformation.
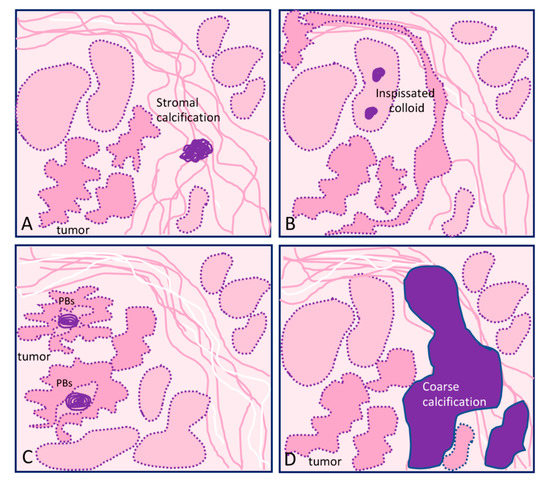
Figure 1. Graphical representation of the different types of calcification in thyroid tissue sections: (A) focus of stromal calcification (in purple color) in the tumor stroma, (B) inspissated colloid calcified, (C) psammoma bodies (PBs) (in purple color) located in the papillary thyroid carcinoma present inside lymphatic vessels or in the stalk of the papillae, and (D) coarse macrocalcification (in purple color). Shapes in pink correspond to non-tumor thyroid; shapes in deeper pink correspond to tumor thyroid.
Table 1. Main types of calcification found in thyroid lesions.
| Localization | Type of Lesion | Description | |
|---|---|---|---|
| Microcalcification ≤1 mm | |||
| Psammoma bodies (PBs) | Inside lymph vessels or in the papillae axis | True PBs Classical PTC |
50–70 μm round-shaped, concentrically laminated, calcified concretions with a glassy appearance (Figure 1C). |
| Inspissated colloid calcified | Inside follicles | False PBs Benign nodules |
Thick colloid (colloid crystals) can present microcalcifications over inspissated colloid and lead to focal hyperechogenic foci; can potentially be confused with PBs (Figure 1B). |
| Stromal microcalcification | Around follicles | False PBs | Spherical crystalline bodies with a diameter of 0.1–2.5 μm. Usually too small to be detected by light microscopy and apparently arise within basal laminae as a result of concentric deposition of calcium salts or calcifications of membrane-bound vesicles. Calcified collagen fibrils can rarely be observed [27][28] (Figure 1A). |
| Bone calcification | Connective tissue | False PBs | Bone formation is considered when there is both bone matrix and osteocytes in the connective tissue of a thyroid nodule, regardless of being neoplastic or not [29][30] |
| Macrocalcification >1 mm | |||
| Eggshell, annular, or rim-like calcifications | Benign and malignant lesions | Annular or rim-like peripheral calcification, defined in US as curvilinear hyperechoic structures parallel to the margin of the nodule. | |
| Coarse calcifications | Stroma | Benign and malignant lesions | An irregularly shaped focus of calcification (can comprise micro- and macrocalcifications) (Figure 1D). |
3.1. Microcalcifications: Psammoma Bodies (PBs)
A common finding in thyroid are the calcifications known as PBs, sometimes designated as calcospherites. Most PBs are 50–70 μm round-shaped calcified concretions (Figure 1C). These structures present a glassy appearance, are concentrically laminated, and stain dark blue to black in Giemsa and purple in hematoxylin and eosin (HE) staining (Figure 2).
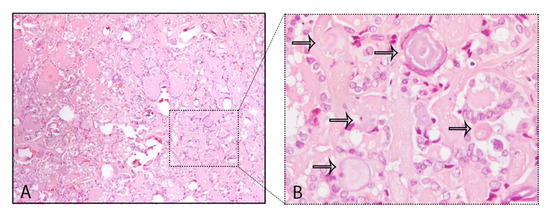
Figure 2. Psammoma bodies (PBs) in a papillary thyroid carcinoma: (A) visible PBs with purple color in hematoxylin and eosin (HE) staining, 10×; (B) magnified inset with PBs marked with the black arrows, 40×.
The main problem in histopathology, although not frequent, is to distinguish PBs from stromal calcification. PBs can usually be distinguished from granular calcium deposits associated with degeneration and from condensed colloid on the basis of typical concentric lamination, while the lack of birefringence distinguishes them from oxalate crystals often present in benign thyroid lesions [28]. The mechanism of PB formation in thyroid tumors remains controversial. Johannessen and Sobrinho-Simões [27] reported that they probably represent the end stage of two biologic events: (i) thickening of the basal lamina of the vascular stalk of the neoplastic papillae, followed by vascular thrombosis, calcification, and (possibly endothelial) cell necrosis, and (ii) intralymphatic necrosis and calcification of tumor thrombi in the thyroid adjacent to the main tumor or in the opposite thyroid lobe; these authors stressed that PB is an umbrella term covering several different entities that share light microscopic features that can be separated on electron microscopy. Johannessen and Sobrinho-Simões concluded that true "psammoma bodies" are formed by calcification of intravascular tumor thrombi or of infarcted tips of malignant papillae [27]. Cotran et al. [30] pointed that in the process of dystrophic calcification, single necrotic cells constitute seed crystals that become incrusted with the mineral deposits and the progressive acquisition of outer layers may create its lamellated configurations, giving rise to PBs. According to Majno et al. [31], dystrophic calcification has two major phases—initiation (nucleation) and propagation—which can occur intracellularly or extracellularly, whereas initiation of intracellular calcification occurs in the mitochondria of dead or dying cells. The initiators of extracellular calcification include phospholipids found in the membrane-bound vesicles, which are about 200 nm in diameter. Other authors [32] analyzed components of PBs in meningiomas and considered capillaries and degenerative cells as initiators of the formation of such calcareous bodies. Another possible mechanism refers to a humoral immune reaction—Tabuchi et al. [33] identified through immunohistochemistry the presence of immunoglobulin G (IgG)s in blood vessel whorls and in PBs in meningiomas, but there are no further studies corroborating these data. Several studies also reveal that the derivation and localization of PBs (within papillary cores, tumor stroma, or lymphatic vessels) is crucial in terms of context (site, morphology, and several important components for their definition) [27][34].
Besides thyroid, where PBs are found mainly in papillary thyroid carcinomas (PTCs) [24][35], meningiomas (45%) [36] and serous cystadenocarcinomas of ovary [37] also present PBs. PBs were reported rarely in other neoplasms, such as in insulinomas [38], lactotrope adenoma of the pituitary [39], serous papillary adenoma of borderline malignancy of the broad ligament, uterine serous carcinoma [40], endocervical adenocarcinoma [41], cholangiocellular carcinoma [42], chromophobe renal cell carcinoma [43], and in psammomacarcinoma of the peritoneum [44].
PBs are diagnostic only if clearly distinguished from coarse calcification and from inspissated colloid (Table 1) [45][46]. For that matter, coarse calcification is an irregularly shaped focus whereas inspissated colloid can present noncalcified colloid together with calcified bodies. Inspissated colloid can be found in a number of malignant or benign tumors, namely, Hürthle cell tumors, as well as non-neoplastic conditions including colloid goiter and Hashimoto’s thyroiditis [46][47][48][49]. Some tips can help in the distinction between PBs, namely, the location and context, the diagnosis of PTC, and the existence of inspissated colloid non-calcified together with inspissated colloid calcifications.
3.2. Macrocalcifications
Macrocalcifications may result from two pathologic processes. On one hand, degeneration of follicular cell lesions that leads to cystic formation due to infarction, hemorrhage, subsequent fibrosis, and occasionally calcification, with the latter being the final stage of scarring from a histopathological standpoint [50]. Macrocalcifications may be irregular in shape, and have been classified in different types according to the observations of the authors [16][26][51]. The two most commonly found classifications for macrocalcifications are described below and in Table 1.
3.2.1. Eggshell, Annular, or Rim-Like Calcifications
This type of calcification in the TNs is commonly associated with benign nodules when it is complete. In contrast, uneven thickness or discontinuity of the calcification, it is suspicious for thyroid malignancy [52][53]. Focal interruption of an eggshell calcification can be explained by tumor infiltration through the broken calcification rim. Indeed, the presence of tissue outside the calcification should suggest malignancy and lead to a US-guided FNA [16].
3.2.2. Coarse Macrocalcifications
Coarse macrocalcifications can also be referred to as dystrophic calcification and can be found in benign and malignant conditions of the thyroid including colloid goiters and anaplastic carcinomas (Figure 1D). On color or power Doppler US, a spoke-wheel vascular pattern centered on a coarse calcification is a strong argument in favor of benign follicular hyperplasia or thyroid adenoma [26]. Although a peripheral distribution can be seen in malignancy, the study results are conflicting and it is controversial if peripheral/rim coarse calcification has an increased malignancy risk [51].
The lack of a standard terminology and of a subclassification of the calcifications regarding morphologic features contributes to the absence of consensus for the importance of sonographically detectable calcifications. Several US categorization systems for echogenic foci in TN were developed, with some studies showing that echogenic foci previously termed as “microcalcifications” were present in benign nodules besides malignant TNs [21][22]. Tahvildari et al. [54] stressed that many authors referred to as “microcalcifications” that which do not exclusively represent PBs but rather other entities, including stromal calcifications and sticky or inspissated colloid. On the basis of the aforementioned data, the American College of Radiology Thyroid Imaging, Reporting and Data System (ACR TIRADS) proposed a terminology to describe echogenic foci in TN and has removed the word “microcalcification” from its lexicon and replaced it with more precise descriptors [17].
4. Calcification in Different Types of Thyroid Cancer
Among different types of thyroid carcinoma, calcification is mainly found in classic PTC and in MTC, although it has also been described in other thyroid carcinomas [54] (Table 2).
PBs within the thyroid gland are typically associated with classical PTCs, being found in up to 65% of cases [28][55][56][57]. In PTCs, microcalcifications representing true PBs are developed within the cores of papillae and/or in the tumor stroma and/or inside lymphatic vessels but often do not present calcification of neoplastic follicular colloid substance [68]. This explains why the follicular variant of PTC (FVPTC) has a very low frequency of microcalcifications (PBs) [58]. When FVPTC is infiltrative, the frequency of calcification can be higher but is rare in the encapsulated FVPTC [56][57]. Diffuse sclerosing variant of papillary thyroid carcinoma (DSVPTC) is an uncommon variant of PTC, characterized by diffuse involvement of one or both lobes of the thyroid gland [59]. This subtype also presents numerous PBs, so much that Koshikawa et al. [60] included “abundant psammoma bodies” in the list of cytological findings for DSVPTC. In MTC, calcification is a very common US finding and the reported incidences can vary from 20 to 54% [61][62].
Table 2. Main types of calcification present in thyroid lesions.
|
Calcification (micro/macro) |
Tumor Subtype |
|
classical/conventional PTC |
|
|
infiltrative follicular variant of PTC |
|
|
Microcalcifications [65] |
diffuse sclerosing variant of PTC |
|
Lymph node involvement with nodal microcalcifications [66][67] |
macrofollicular variant of PTC |
|
Micro- and macrocalcifications [68] |
Hürthle cell carcinoma |
|
Microcalcifications [66] |
hobnail variant of PTC |
|
Microcalcifications [69] Macrocalcifications [70] |
hobnail variant of PTC |
|
Hürthle cell tumors |
|
|
Micro and macrocalcifications [23] |
MTC |
5. Conclusion/Future Prospects
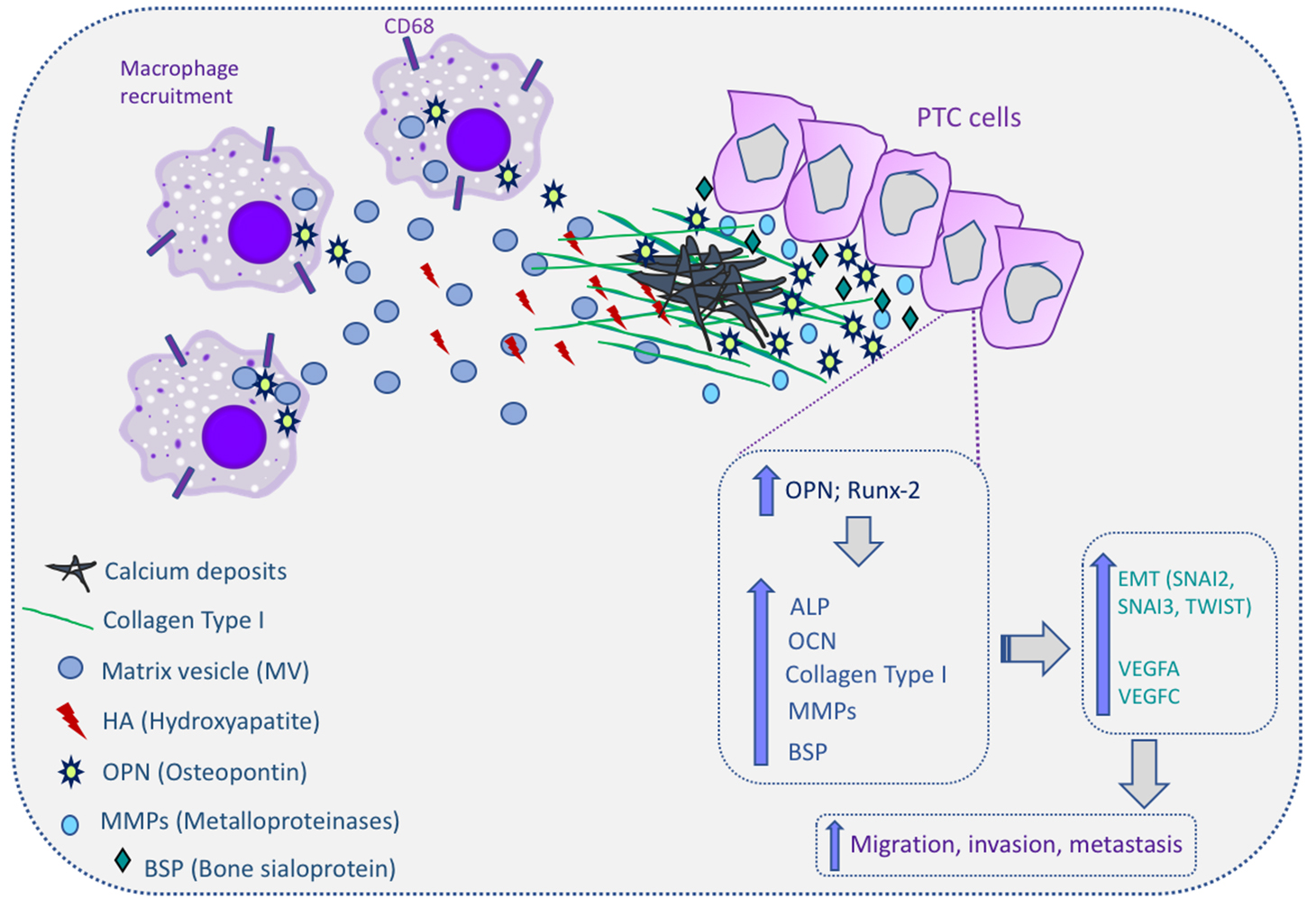
Thyroid cancer, in particular PTC, presents distinct types of calcification processes, notably the psammoma bodies. The role and significance of these calcifications for thyroid cancer diagnosis and prognosis has been explored in several studies but without conclusive indications. Accepting the adjuvant role of microcalcifications in the imagological and cytological diagnosis of PTC, its significance in tumor aggressiveness, including metastatic behavior of PTC, remains controversial. Overall, the process by which calcification occurs in thyroid cancer remains poorly understood. However, all aforementioned data relating several dysregulated molecules with the calcification process in thyroid provides some clues and improves the understanding of the molecular mechanisms of microcalcification in thyroid cancer as well as in the tumorigenesis. The proposed molecular mechanism for calcification in papillary thyroid cancer are summarized in Figure 3.
Figure 3. Molecular mechanism of calcification in papillary thyroid cancer (PTC) cells. Macrophages can be recruited to the PTC microenvironment and release matrix vesicles (MVs) to the extracellular matrix (ECM). MVs contain hydroxyapatite (HA), which initiates the calcification process. Osteopontin (OPN) and runt-related transcription factor-2 (Runx-2) are overexpressed in PTC cells and this increases the expression of alkaline phosphatase (ALP), osteocalcin (OCN), collagen type I, metalloproteinases (MMPs), and bone sialoprotein (BSP). All these molecules are involved in the induction of calcium deposits in the ECM, culminating in the calcification process in thyroid tissues, and also induce the expression of epithelial–mesenchymal transition genes (snail family transcriptional repressor (SNAI)2, SNAI3, and twist-related protein 1 (TWIST1)) and angiogenic factors (vascular endothelial growth factor (VEGF)A and VEGFC). Up arrows mean increase; The cells expressing CD68 correspond to macrophages.
References
- David S Cooper; Gerard M. Doherty; Bryan R. Haugen; Richard T. Kloos; Stephanie L. Lee; Susan J. Mandel; Ernest L. Mazzaferri; Bryan McIver; Furio Pacini; Martin Schlumberger; et al.Steven I. ShermanDavid L. StewardR Michael Tuttle Revised American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2009, 19, 1167-1214, 10.1089/thy.2009.0110.
- S. Guth; U. Theune; J. Aberle; A. Galach; C. M. Bamberger; Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. European Journal of Clinical Investigation 2009, 39, 699-706, 10.1111/j.1365-2362.2009.02162.x.
- Ellen Marqusee; Carol B. Benson; Mary C. Frates; Peter M. Doubilet; P. Reed Larsen; Edmund S. Cibas; Susan J. Mandel; Usefulness of Ultrasonography in the Management of Nodular Thyroid Disease. Annals of Internal Medicine 2000, 133, 696-700, 10.7326/0003-4819-133-9-200011070-00011.
- Peter A. Singer; David S. Cooper; Gilbert H. Daniels; Paul W. Ladenson; Francis S. Greenspan; Elliot G. Levy; Lewis E. Braverman; Orlo H. Clark; I. Ross McDougall; Kenneth V. Ain; et al.Steven G. Dorfman Treatment Guidelines for Patients With Thyroid Nodules and Well-Differentiated Thyroid Cancer. Archives of Internal Medicine 1996, 156, 2165-2172, 10.1001/archinte.1996.00440180017002.
- Jane F. Desforges; Ernest L. Mazzaferri; Management of a Solitary Thyroid Nodule. New England Journal of Medicine 1993, 328, 553-559, 10.1056/nejm199302253280807.
- Gerry H. Tan; Thyroid Incidentalomas: Management Approaches to Nonpalpable Nodules Discovered Incidentally on Thyroid Imaging. Annals of Internal Medicine 1997, 126, 226-231, 10.7326/0003-4819-126-3-199702010-00009.
- Rebecca Smith-Bindman; Paulette Lebda; Vickie A. Feldstein; Dorra Sellami; Ruth B. Goldstein; Natasha Brasic; Chengshi Jin; John Kornak; Risk of Thyroid Cancer Based on Thyroid Ultrasound Imaging Characteristics. JAMA Internal Medicine 2013, 173, 1788-1796, 10.1001/jamainternmed.2013.9245.
- Il Seong Nam-Goong; Ha Young Kim; Gyungyub Gong; Ho Kyu Lee; Young Kee Shong; Won Bae Kim; Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clinical Endocrinology 2003, 60, 21-28, 10.1046/j.1365-2265.2003.01912.x.
- Cosimo Durante; Giorgio Grani; Livia Lamartina; Sebastiano Filetti; Susan J. Mandel; David S. Cooper; The Diagnosis and Management of Thyroid Nodules. JAMA 2018, 319, 914-924, 10.1001/jama.2018.0898.
- Juan P. Brito; Yasuhiro Ito; Akira Miyauchi; R Michael Tuttle; A Clinical Framework to Facilitate Risk Stratification When Considering an Active Surveillance Alternative to Immediate Biopsy and Surgery in Papillary Microcarcinoma. Thyroid 2015, 26, 144-149, 10.1089/thy.2015.0178.
- Juan P. Brito; Michael R. Gionfriddo; Alaa Al Nofal; Kasey R. Boehmer; Aaron L. Leppin; Carl Reading; Matthew Callstrom; Tarig A. Elraiyah; Larry J. Prokop; Marius N. Stan; et al.M. Hassan MuradJohn C. MorrisVictor M. Montori The Accuracy of Thyroid Nodule Ultrasound to Predict Thyroid Cancer: Systematic Review and Meta-Analysis. The Journal of Clinical Endocrinology & Metabolism 2014, 99, 1253-1263, 10.1210/jc.2013-2928.
- Paolo Campanella; Francesca Ianni; Carlo Antonio Rota; Salvatore Maria Corsello; Alfredo Pontecorvi; DIAGNOSIS IN ENDOCRINOLOGY: Quantification of cancer risk of each clinical and ultrasonographic suspicious feature of thyroid nodules: a systematic review and meta-analysis. European Journal of Endocrinology 2014, 170, R203-R211, 10.1530/eje-13-0995.
- Dario Tumino; Giorgio Grani; Marta Di Stefano; Maria Di Mauro; Maria Scutari; Teresa Rago; Laura Fugazzola; Maria Grazia Castagna; Fabio Maino; Nodular Thyroid Disease in the Era of Precision Medicine. Frontiers in Endocrinology 2020, 10, 907, 10.3389/fendo.2019.00907.
- Zhaohui Lu; Yiming Mu; Haiqing Zhu; Yukun Luo; Qinglong Kong; Jingtao Dou; Juming Lu; Clinical Value of Using Ultrasound to Assess Calcification Patterns in Thyroid Nodules. World Journal of Surgery 2010, 35, 122-127, 10.1007/s00268-010-0827-3.
- G. Chen; X.Q. Zhu; X. Zou; J. Yao; J.X. Liang; H.B. Huang; L.T. Li; L.X. Lin; Retrospective Analysis of Thyroid Nodules by Clinical and Pathological Characteristics, and Ultrasonographically Detected Calcification Correlated to Thyroid Carcinoma in South China. European Surgical Research 2009, 42, 137-142, 10.1159/000196506.
- Jee-Yeong Jeong; Young Sik Choi; Hye Jung Kwon; Jun Seop Lee; Jae Joon Heo; You Jin Han; Yo-Han Park; Jeong Hoon Kim; Relationship between patterns of calcification in thyroid nodules and histopathologic findings.. Endocrine Journal 2012, 60, 155-160, 10.1507/endocrj.ej12-0294.
- Edward G. Grant; Franklin N. Tessler; Jenny K. Hoang; Jill E. Langer; Michael D. Beland; Lincoln L. Berland; John J. Cronan; Terry S. Desser; Mary C. Frates; Ulrike M. Hamper; et al.William D. MiddletonCarl C. ReadingLeslie M. ScouttA. Thomas StavrosSharlene A. Teefey Thyroid Ultrasound Reporting Lexicon: White Paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. Journal of the American College of Radiology 2015, 12, 1272-1279, 10.1016/j.jacr.2015.07.011.
- Michio Shimizu; Mitsuyoshi Hirokawa; Takuo Kanahara; Toshiaki Manabe; Calcium Oxalate Crystals in Thyroid Fine Needle Aspiration Cytology. Acta Cytologica 1998, 43, 575-578, 10.1159/000331148.
- R. Wahl; Regine Fuchs; E. Kallee; Oxalate in the Human Thyroid Gland. Clinical Chemistry and Laboratory Medicine 1992, 31, 559-565, 10.1515/cclm.1993.31.9.559.
- Bk Aribas; Kemal Arda; Nazan Ciledag; Elif Aktas; Mehmet Faik Çetindag; Predictive factors for detecting malignancy in central and lateral cervical lymph nodes in papillary carcinoma of the thyroid. Asia-Pacific Journal of Clinical Oncology 2011, 7, 307-314, 10.1111/j.1743-7563.2011.01408.x.
- Michael D. Beland; Lawrence Kwon; Ronald A. DeLellis; John J. Cronan; Edward G. Grant; Nonshadowing Echogenic Foci in Thyroid Nodules. Journal of Ultrasound in Medicine 2011, 30, 753-760, 10.7863/jum.2011.30.6.753.
- Harshawn Malhi; Michael D. Beland; Steven Yong Cen; Evan Allgood; Kristopher Daley; Sue E. Martin; John Joseph Cronan; Edward G. Grant; Echogenic Foci in Thyroid Nodules: Significance of Posterior Acoustic Artifacts. American Journal of Roentgenology 2014, 203, 1310-1316, 10.2214/ajr.13.11934.
- Kaoru Kobayashi; Tomoko Fujimoto; Hisashi Ota; Mitsuyoshi Hirokawa; Tomonori Yabuta; Hiroo Masuoka; Mitsuhiro Fukushima; Takuya Higashiyama; Minoru Kihara; Yasuhiro Ito; et al.Akihiro MiyaAkira Miyauchi Calcifications in Thyroid Tumors on Ultrasonography: Calcification Types and Relationship with Histopathological Type. Ultrasound International Open 2018, 4, E45-E51, 10.1055/a-0591-6070.
- Lu Yin; Wei Zhang; Wen-Kun Bai; Bin Ning; Relationship Between Morphologic Characteristics of Ultrasonic Calcification in Thyroid Nodules and Thyroid Carcinoma. Ultrasound in Medicine & Biology 2019, 46, 20-25, 10.1016/j.ultrasmedbio.2019.09.005.
- Bu Kyung Kim; Eun Mi Lee; Jeong Hoon Kim; So Young Oak; Su Kyoung Kwon; Young Sik Choi; Young Ok Kim; Relationship between ultrasonographic and pathologic calcification patterns in papillary thyroid cancer. Medicine 2018, 97, e12675, 10.1097/md.0000000000012675.
- Alexis Lacout; Carole Chevenet; Juliette Thariat; Pierre-Yves Marcy; Thyroid calcifications: a pictorial essay. Journal of Clinical Ultrasound 2016, 44, 245-251, 10.1002/jcu.22345.
- J V Johannessen; M Sobrinho-Simões; The origin and significance of thyroid psammoma bodies.. Laboratory Investigation 1980, 43, 287-96.
- O’rell, S.R.; Philips, J.. Fine-Needle Biopsy and Cytological Diagnosis of Thyroid Lesions; Karger: Basel, 1997; pp. 14.
- Yanhua Bai; Gengyin Zhou; Misa Nakamura; Takashi Ozaki; Ichiro Mori; Emiko Taniguchi; Akira Miyauchi; Yasuhiro Ito; Kennichi Kakudo; Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma. Modern Pathology 2009, 22, 887-894, 10.1038/modpathol.2009.38.
- 30. Cotran, R.S.; Kumar, V.; Collins, T.; Robbins, S.L. . Pathologic Basis of Disease; W.B. Saunders Co.&Harcourt Asia Pte Ltd: Noida: India, 1999; pp. 44.
- 31. Majno, G.; Joris, I. . Cells, Tissues, and Disease: Principle of General Pathology; Blackwell Science: Cambridge, MA, USA, 1996; pp. 1.
- M Cerdá Nicolás; [Meningiomas: morphologic and ultrastructural characteristics of psammoma bodies].. Archivos de neurobiologia 1992, 55, 256-61.
- K. Tabuchi; Y. Kawakami; A. Nishimoto; Immunohistochemical demonstration of IgG in meningioma. Acta Neurochirurgica 1981, 55, 201-211, 10.1007/bf01808437.
- G. H. Klinck; Theodore Winship; Psammoma bodies and thyroid cancer. Cancer 1959, 12, 656-662, 10.1002/1097-0142(195907/08)12:4<656::aid-cncr2820120406>3.0.co;2-#.
- Ye-Feng Cai; Qing-Xuan Wang; Chun-Jue Ni; Gui-Long Guo; Quan Li; Ou-Chen Wang; Liang Wu; Hai-Yan Du; Jane You; Xiao-Hua Zhang; et al. The Clinical Relevance of Psammoma Body and Hashimoto Thyroiditis in Papillary Thyroid Carcinoma. Medicine 2015, 94, e1881, 10.1097/md.0000000000001881.
- Siderlei S. Carneiro; Bernd W. Scheithauer; Antonio G. Nascimento; Takanori Hirose; Dudley H. Davis; Solitary Fibrous Tumor of the Meninges: A Lesion Distinct From Fibrous Meningioma:A Clinicopathologic and Immunohistochemical Study. American Journal of Clinical Pathology 1996, 106, 217-224, 10.1093/ajcp/106.2.217.
- Robboy, S.J.; Dugan, M.A.; Kurmann, R.J. . The Female Reproductive System; Lippincott-Raven: Philadelphia, PA, USA, 1999; pp. x.
- T F Warner; J J Baron; S R Mallin; J L Golding; Intestinal development in insulinoma containing Psammoma bodies. Recapitulation of ultrastructural features.. Archives of Pathology & Laboratory Medicine 1980, 104, 432-7.
- Lack, E.A.; Farber, J.L.; Rubin, E. . The Endocrine System; Lippincott, Eds.; -Raven: Philadelphia, PA, USA, 1999; pp. x2.
- R I Cameron; Extensive psammomatous calcification of the uterus and cervix associated with a uterine serous carcinoma. Journal of Clinical Pathology 2004, 57, 888-890, 10.1136/jcp.2004.017004.
- Vicki Seltzer; Mark Spitzer; Psammoma Bodies in Papillary Adenocarcinoma of the Endocervix. International Journal of Gynecological Pathology 1983, 2, 216-221, 10.1097/00004347-198302000-00013.
- S Yamada; Hayato Sanefuji; Hiroaki Morimoto; Yuji Harada; Shinichiro Mine; Isao Morimoto; Sumiya Eto; Parathyroid hormone-related peptide producing cholangiocellular carcinoma with a marked psammoma formation.. Journal of Gastroenterology and Hepatology 2000, 15, 1442-1446, 10.1046/j.1440-1746.2000.02222.x.
- R J Cohen; S Weinstein; T Robertson; L N Sellner; H J Dawkins; J E McNeal; Variant chromophobe renal cell carcinoma.. Archives of Pathology & Laboratory Medicine 2000, 124, x, 10.1043/0003-9985(2000)124<0904:VCRCC>2.0.CO;2.
- B. Piura; Alex Rabinovich; Ilana Yanai-Inbar; Psammomacarcinoma of the peritoneum.. European Journal of Obstetrics & Gynecology and Reproductive Biology 2001, 97, 231-234, 10.1016/s0301-2115(00)00508-x.
- M L Carcangiu; G Zampi; J Rosai; Papillary thyroid carcinoma: a study of its many morphologic expressions and clinical correlates.. Pathology annual 1984, 20, x.
- Russell M. Fiorella; William Isley; Leslie K. Miller; Peter J. Kragel; Multinodular goiter of the thyroid mimicking malignancy: Diagnostic pitfalls in fine-needle aspiration biopsy. Diagnostic Cytopathology 1993, 9, 351-357, 10.1002/dc.2840090321.
- D S Cooper; E Tiamson; P W Ladenson; Psammoma bodies in fine needle aspiration biopsies of benign thyroid nodules.. Thyroidology 1988, 1, 55-59.
- N Riazmontazer; G Bedayat; Psammoma bodies in fine needle aspirates from thyroids containing nontoxic hyperplastic nodular goiters.. Acta Cytologica 1991, 35, 563–566.
- J M Dugan; B F Atkinson; A Avitabile; M Schimmel; V A LiVolsi; Psammoma bodies in fine needle aspirate of the thyroid in lymphocytic thyroiditis.. Acta Cytologica 1987, 31, 330-334.
- Stuart Lindsay; THE PATHOLOGY OF THE NODULAR GOITER. California medicine 1949, 71, 207-208.
- Suzuka Taki; Shintaro Terahata; Ryohei Yamashita; Keiko Kinuya; Koji Nobata; Kiyoshi Kakuda; Yuko Kodama; Itaru Yamamoto; Thyroid calcifications. Clinical Imaging 2004, 28, 368-371, 10.1016/s0899-7071(03)00190-6.
- Yun Joo Park; Jeong-Ah Kim; Eun Ju Son; Ji Hyun Youk; Eun-Kyung Kim; Jin Young Kwak; Cheong Soo Park; Thyroid Nodules with Macrocalcification: Sonographic Findings Predictive of Malignancy. Yonsei Medical Journal 2013, 55, 339-344, 10.3349/ymj.2014.55.2.339.
- Chan H. Park; Franklin J. Rothermel; David M. Judge; Unusual Calcification in Mixed Papillary and Follicular Carcinoma of the Thyroid Gland. Radiology 1976, 119, 554-554, 10.1148/119.3.554.
- Ali M. Tahvildari; Lorraine Pan; Christina S. Kong; Terry Desser; Sonographic-Pathologic Correlation for Punctate Echogenic Reflectors in Papillary Thyroid Carcinoma. Journal of Ultrasound in Medicine 2016, 35, 1645-1652, 10.7863/ultra.15.09048.
- Ning Wang; Yuanhong Xu; Chunlin Ge; Renxuan Guo; Kejian Guo; Association of sonographically detected calcification with thyroid carcinoma. Head & Neck 2006, 28, 1077-1083, 10.1002/hed.20481.
- Seong Hoe Park; Eun Hee Suh; Je G. Chi; A Histopathologic Study on 1,095 Surgically Resected Thyroid Specimens. Japanese Journal of Clinical Oncology 1988, 18, 297–302, 10.1093/oxfordjournals.jjco.a039252.
- Rosai, J.; Carcangiu, M.; DeLellis, R.A. . Tumors of the Thyroid Gland; Armed Forces Institute of Pathology: Washington, DC, USA, 1992; pp. 1.
- Dae Sik Kim; Ji-Hoon Kim; Dong Gyu Na; Sung-Hye Park; Eunhee Kim; Kee-Hyun Chang; Chul-Ho Sohn; Young Ho Choi; Kim Dae Sik; Kim Ji-Hoon; et al.Na Dong GyuPark Sung-HyeKim EunheeChang Kee-HyunSohn Chul-HoChoi Young Ho Sonographic Features of Follicular Variant Papillary Thyroid Carcinomas in Comparison With Conventional Papillary Thyroid Carcinomas. Journal of Ultrasound in Medicine 2009, 28, 1685-1692, 10.7863/jum.2009.28.12.1685.
- DeLellis, R.A.; Lloyd, R.V.; Heitz, P.U.; Eng, C.. World Health Organization Classification of Tumours Pathology and Genetics Tumours of Endocrine Organs; IARC PRESS: Lyon, France, 2004; pp. x.
- Takashi Koshikawa; Nozomi Takagi; Mitsuyoshi Hirokawa; Cytological findings of the diffuse sclerosing variant of papillary thyroid carcinoma. The Journal of the Japanese Society of Clinical Cytology 2013, 53, 515-520, 10.5795/jjscc.53.515.
- Mei-Juan Liu; Zhong-Feng Liu; Yuan-Yuan Hou; Yan-Ming Men; Yu-Xi Zhang; Ling-Yun Gao; Hao Liu; Ultrasonographic characteristics of medullary thyroid carcinoma: a comparison with papillary thyroid carcinoma. Oncotarget 2017, 8, 27520-27528, 10.18632/oncotarget.15897.
- Pablo Valderrabano; Donald L. Klippenstein; John B. Tourtelot; Zhenjun Ma; Zachary J. Thompson; Howard S. Lilienfeld; Bryan McIver; New American Thyroid Association Sonographic Patterns for Thyroid Nodules Perform Well in Medullary Thyroid Carcinoma: Institutional Experience, Systematic Review, and Meta-Analysis. Thyroid 2016, 26, 1093-1100, 10.1089/thy.2016.0196.
- Hye Jin Baek; Dong Wook Kim; Gi Won Shin; Young Jin Heo; Jin Wook Baek; Yoo Jin Lee; Young Jun Cho; Ha Kyoung Park; Tae Kwun Ha; Do Hun Kim; et al.Soo Jin JungJi Sun ParkKi Jung Ahn Ultrasonographic Features of Papillary Thyroid Carcinomas According to Their Subtypes. Frontiers in Endocrinology 2018, 9, 223, 10.3389/fendo.2018.00223.
- Nicola M. Hughes; Andreea Nae; Josephine Barry; Brendan Fitzgerald; Linda Feeley; Patrick Sheahan; Sonographic differences between conventional and follicular variant papillary thyroid carcinoma. European Archives of Oto-Rhino-Laryngology 2017, 274, 2907-2913, 10.1007/s00405-017-4557-0.
- Suja Pillai; Vinod Gopalan; Robert A. Smith; Alfred King-Yin Lam; Diffuse sclerosing variant of papillary thyroid carcinoma—an update of its clinicopathological features and molecular biology. Critical Reviews in Oncology/Hematology 2015, 94, 64-73, 10.1016/j.critrevonc.2014.12.001.
- Jung Hee Shin; Ultrasonographic imaging of papillary thyroid carcinoma variants. Ultrasonography 2017, 36, 103-110, 10.14366/usg.16048.
- Ji Young Joung; Tae Hyuk Kim; Dae Joon Jeong; Sun-Mi Park; Yoon Young Cho; Hye Won Jang; Yoon Yang Jung; Young Lyun Oh; Hyun Sook Yim; Yoo-Li Kim; et al.Jae Hoon ChungChang-Seok KiSun Wook Kim Diffuse sclerosing variant of papillary thyroid carcinoma: major genetic alterations and prognostic implications. Histopathology 2016, 69, 45-53, 10.1111/his.12902.
- Nathalie Oliveira Santana; Ricardo Miguel Costa De Freitas; Vinicius Neves Marcos; Maria Cristina Chammas; Rosalinda Yossie Asato Camargo; Cláudia Kliemann Schmerling; Felipe Augusto Brasileiro Vanderlei; Ana Oliveira Hoff; Suemi Marui; Debora Lucia Seguro Danilovic; et al. Diagnostic performance of thyroid ultrasound in Hürthle cell carcinomas.. Archives of Endocrinology and Metabolism 2019, 63, 300-305, 10.20945/2359-3997000000131.
- Vincent Cracolici; Thomas Krausz; Nicole A. Cipriani; Ubiquitin Immunostaining in Thyroid Neoplasms Marks True Intranuclear Cytoplasmic Pseudoinclusions and May Help Differentiate Papillary Carcinoma from NIFTP. Head and Neck Pathology 2018, 12, 522-528, 10.1007/s12105-018-0905-7.
- Sung-Hye You; Kyu Eun Lee; Roh-Eul Yoo; Hye Jeong Choi; Kyeong Cheon Jung; Jung Chan Kwon; Koung Mi Kang; Tae Jin Yoon; Seung Hong Choi; Chul-Ho Sohn; et al.Ji-Hoon Kim Prevention of total thyroidectomy in noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) based on combined interpretation of ultrasonographic and cytopathologic results. Clinical Endocrinology 2017, 88, 114-122, 10.1111/cen.13473.
- Sang Kwon Lee; Byung Hak Rho; Seong-Ku Woo; Hürthle cell neoplasm: Correlation of gray-scale and power Doppler sonographic findings with gross pathology. Journal of Clinical Ultrasound 2009, 38, 169-176, 10.1002/jcu.20684.
- John C. Sillery; Carl C. Reading; J. William Charboneau; Tara L. Henrichsen; Ian D. Hay; Jayawant N. Mandrekar; Thyroid Follicular Carcinoma: Sonographic Features of 50 Cases. American Journal of Roentgenology 2009, 194, 44-54, 10.2214/ajr.09.3195.