
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mukesh Kumar | + 2693 word(s) | 2693 | 2020-10-09 10:06:55 | | | |
| 2 | Peter Tang | -233 word(s) | 2460 | 2020-11-10 03:31:42 | | |
Video Upload Options
• Contaminated waste water is one of the most serious risks for living organisms as well as to the environment.
• Nanotechnology offers best expectations over traditional technologies for wastewater treatment.
• Adsorption technology is the phenomenon of adhesion of solid substances onto the surface of adsorbent.
• Graphene-based nanoadsorbents exhibited a great potential towards effective removal of lead ions from aqueous solution.
• Graphene preparation, characterization, and applications of graphic-based composites for the removal of lead ions from aqueous solution have been discussed.
1. Introduction
Water decontamination is one of the most serious challenges among scientists globally due to the increasing population, pollution, and global warming [1][2][3][4][5]. Wastewater from developing industries, such as chemical manufacturing, metallurgical, battery manufacturing, papermaking, and mining industries produce a very large amount of various toxic pollutants in the form of heavy metal ions [6][7][8][9]. Excess heavy metal ions concentration in wastewater is a serious risk to public health as well as to other living organisms on Earth [10][11][12]. These toxic heavy metal pollutants are widely found in the Earth's crust which tends to bioaccumulate in living organisms; they are non-biodegradable, which can cause various diseases, genetic disorders, and lethal ecological effects [12][13][14][15]. Heavy metal ions in aqueous media pose several toxic threats to the human health as well as to the other living organisms even at low concentrations. As per the regulatory system of the WHO (World Health Organization), the permissible concentration of Pb(II) (Lead (II)) in wastewater is approximately 0.01mg/L [16][17][18]. Moreover, the excess exposure of Pb(II) can lead to irreversible brain damage, cardiovascular disease, cognitive impairment, encephalopathy disease symptoms and even death [19][20][21][22]. Pb(II) is also extremely toxic to the nervous system, kidneys, and may lead to a wide range of human health issues such as nausea, anemia, infertility, coma, convulsions, hemochromatosis, renal failure, cancer, adverse effects on the metabolism and intelligence, and dermatitis brass chills, and cramps in the calves [12][14][23][24][25][26][27].
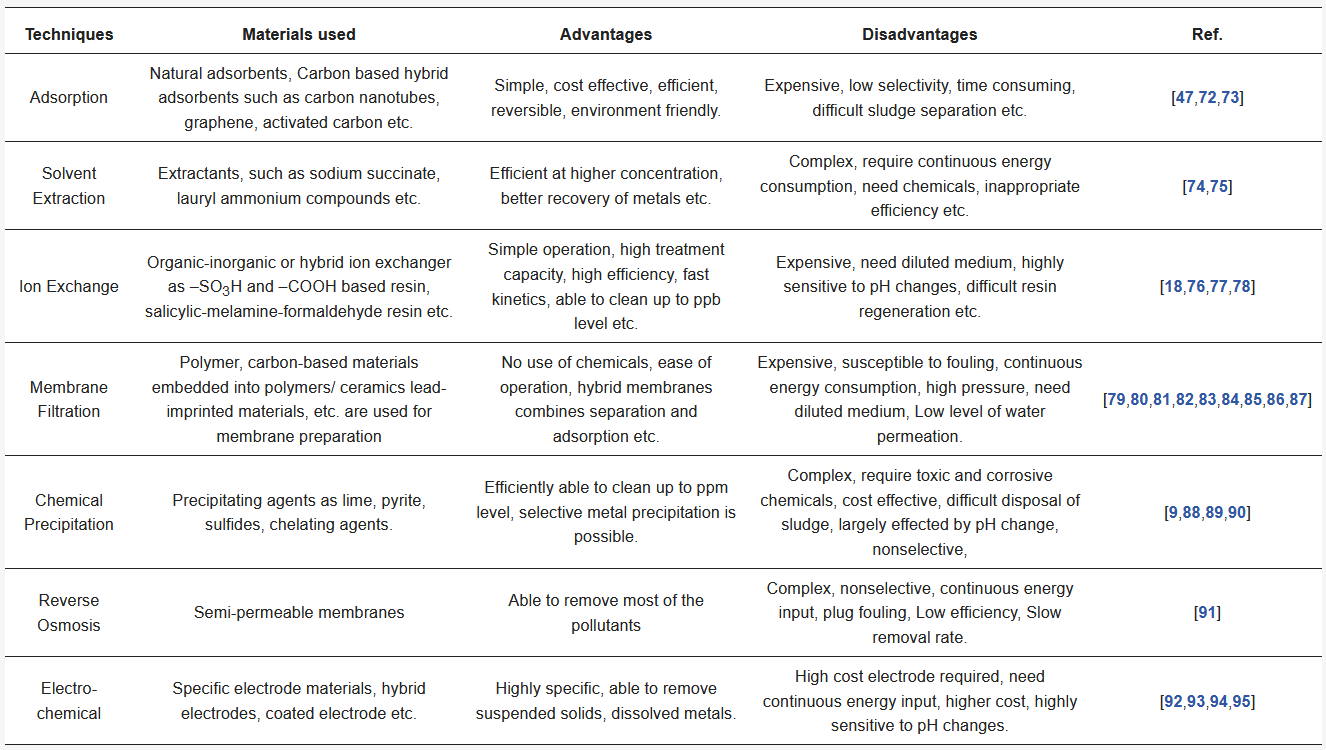
The current tight regulatory systems do not allow the release of heavy metal ions-contaminated wastewaters into the environment and require the removal of all toxic pollutants before discharging. Therefore, the development of efficient and economical novel materials and technologies for the effective removal of metal ion pollutants are required. Several traditional techniques have been used for the removal of heavy metal ions from aqueous medium, including reverse osmosis, precipitation, biosorption, ion-exchange, electrochemical processes, membrane filtration, irradiation, coagulation, and adsorption [28][29][30][31][32][33][34][35][36][37][38][39][40]. Table 1 lists the techniques used for Pb(II) ions removal from the wastewaters. The main disadvantages of conventional techniques include high cost, metallic sludge generation, incomplete removal, and disposal of secondary waste. Therefore, a cost effective, potential and convenient decontamination technology for wastewater treatment is highly desirable. The high efficiency, cost-effective, and simple operation makes adsorption technology one of the most promising technology for the effective removal of the heavy metal ions from the aqueous solution which adsorb metallic ions at solid-liquid interfaces [41][42][43][44][45][46][47][48][49]. On the other hand, adsorption technology has several challenges, such as selective recovery and reuse of valuable adsorbents and pollutants in the presence of humic substances [50][51]. Adsorbents developed with favorable structures, morphologies, superior adsorption capacities, and ease of separation are a major focus of the current research [26][52][53][54][55][56][57][58][59][60]. Recently, the development of nanotechnology has attracted considerable attention due to the remarkable potential of the nanoadsorbents for the selective and effective removal of ionic pollutants from aqueous solution when compared with the traditional adsorbents [13][33][61][62][63][64][65][66]. The nanoadsorbents play a major role in the separation of heavy metal ions because they are effective sorbents due to their unique structural properties and specific adsorption tendency. Nanoadsorbents have several unique advantages over conventional adsorbents due to their large specific surface area to volume ratio, tunable pore size, regeneration, reusability etc. [33][56][67][68][69][70][71].
Table 1. Techniques characteristics used for Pb(II) removal [72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95]
Carbon (carbon nanotubes (CNTs), fullerenes, graphene, and graphene derivatives) based adsorbent materials are being used for the effective removal of the heavy metal ions because of their extraordinary high surface area to volume ratio and light weight as compared to other materials [66][96][97]. Currently, adsorbents based on the carbon nanotubes [98][99], activated carbon [100][101][102][103][104][105], graphene/ graphene oxide (GO) [60][67][106][107][108][109][110][111][112][113][114][115][116][117], graphene magnetite's [118][119][120][121][122][123] polymeric adsorbents [124][125][126][127][128][129][130][131][132] and other type of adsorbents [133][134][135][136][137] have attracted substantial attention for the removal of heavy metal ions from the wastewaters. Carbon nanotubes have significant adsorption capacities for the effective removal of heavy metal ions from aqueous solutions as compared to non-functionalized and activated carbon-based adsorbents [99][138][139][140][141][142]. Although, carbon nanotubes-based materials are the promising adsorbents but their high cost and lower availability, limit the large-scale applications as adsorbents. In addition to this, water permeation through CNTs membranes is a major concern which is still unclear even after both the experimentally and molecular dynamic studies. Therefore, economic, readily available, and effective adsorbents are the prime concern of the 21st century for the effective removal of heavy metal ions from wastewaters. Although, the presence of humic substances has strong complexation binding ability with metal ions because of their abundant oxygen-containing functional groups, yet graphene-based adsorbents have been proven the best substituents for the removal of heavy metal ions from aqueous medium [66][67][143]. Therefore, the adsorption behavior of heavy metal ions onto the surface of graphene-based nanoadsorbents is the chief concern of this study.
Graphene, a flat, single-atom thick sheet of sp2-hybridized carbon atoms with a two-dimensional honeycomb densely packed lattice arrangement, is an essential non-classical carbon adsorbent [144]. Graphene has a variety of real-time applications because of its extraordinary excellent thermal, mechanical and electrical properties [145]. Graphene is considered a promising material for the comprehensive adsorption of wide variety of pollutants from aqueous systems owing to its theoretically large specific surface area to volume ratio (≈2675m2/g), high Young's Modulus (1.06 × 103 Gegapascals) and high thermal conductivity (3 × 103 Watt per meter kelvin) [113][146]. The wet chemical redox process is one of the most effective method for the production of graphene from graphite, where GO is an intermediate with a high density of negatively-charged reactive functional groups, viz. hydroxyl, carboxylic, and epoxy groups [147][148][149]. The presence of several functional groups makes GO soluble in polar and non-polar solvents as the oxygen functionality makes GO hydrophilic and the graphene domain makes GO hydrophobic. All the functional groups present on the edges and on the basal plane of GO play an important role in the heavy metal ions removal process [150]. Therefore, GO could be the best potential scavenger for the effective removal of cationic pollutants through electrostatic interactions between the positively-charged pollutants and negatively-charged functional groups of GO [27][151]. The relative high surface area, surface functionality, and better conductivity of graphene sheets play a key role in the better adsorption of several heavy metal ions through the preconcentration of aqueous medium [67][144]. Nair et al. reported that a few layered thick GO sheet membrane can completely block the passage of pollutants in the form of liquids and gases in a dry state while facilitating the permeation of water vapors [152]. On the other hand, aggregation, which is a major disadvantage of graphene layers, could be prevented partially through its composite formation [41][153]. In addition, composite formation may impart enhanced efficiency for the removal of heavy metal ions from wastewater due to the synergistic effects of the materials used. Electrosorption, a simple, novel, ecofriendly and recently attractive heavy metal ions adsorption technique which does not requires toxic or non-toxic chemicals and involves ideal nanostructured materials with a high surface area (such as activated carbon, carbon nanotubes, and carbon aerogels) onto which an external electric field is applied to remove metal ions from aqueous solutions [93][154][155][156][157]. Hence, this review includes various surface modification approaches and post synthesis, assembly steps, which will enable the exploitation of GO as a novel adsorbent material for cost effective water purification and the removal of heavy metals through graphene and its composites, which may be helpful in the purification of potable and safe drinking water as well as the removal of heavy metal ions from wastewaters and to clean up the environmental problems.
2. Removal of Lead
Pb(II) is one of the most useful heavy metal with wide spectrum applications worldwide. On the other hand, the presence of Pb(II) in aqueous system is a major threat to mankind as well as to the ecosystem owing to its high toxicity. Therefore, removal of Pb(II) ions from the wastewater aqueous system is quite essential to diminish the toxic threats. An adsorption process is largely dependent on the surface area and pore size of the adsorbents, whereas, the adsorption of metal ions is based on chemical adsorption onto specific adsorption sites, i.e., adsorption capacity of an adsorbent increases with the increase of its functional groups [158][159]. Graphene is thanked to have several active functional groups such as carboxylic, hydroxyl, and epoxy functional groups acting as better adsorption sites [160][161][162][163]. Hence, graphene and graphene derivatives due to their easy preparation, surface modifications, bulk availability and high adsorption capacities have been extensively studied for the removal of Pb(II) ions from aqueous system.
2.1. Removal of lead using functionalized GO/ RGO/ GO-aerogel
An adduct of GO and EDTA, was prepared using silylation by reacting N-(trimethoxysilylpropyl) ethyledinediamine triacetic acid (EDTA-silane) with the -OH functionality of the GO layers [164]. Hummer's and Offeman's modified double stage oxidation process was used to produce GO. In the first preoxidized step, natural graphite was treated with potassium persulfate and phosphorous pentaoxide in sulfuric acid followed by the oxidation with potassium permanganate, conc. sulfuric acid and hydrogen peroxide. GO was filtered and washed with 0.1 M HCl and deionized water. Resulting GO was then reacted through silylation process with EDTA-silane and was filtered followed by repeatedly washing with methanol and water. For comparison, a reduced GO adduct, EDTA-RGO black was prepared by direct thermal reduction treatment at 300 ◦C for 30 min. Adsorption isotherms analysis was performed in a plastic vial using 10 mg or 25 mg adsorbent to a 100 or 200 mL solution of Pb(II) ions at room temperature. It is evident that the adsorption of positively-charged metal ions depend on the charge present on the surface of the adsorbents as functionalities [165][166]. Zeta potential of the adsorbents at different pH was determined using Nano-ZS, Zeta Sizer and correlated with the adsorption of Pb(II) ions. At a particular pH, more negative zeta potential indicated the highest carboxylic and hydroxyl functionalities on the surface of EDTA-GO and GO adsorbents than activated carbon and EDTA-RGO was found to be consistent with Boehm's titration results [167][168][169][170]. The enhanced adsorption for Pb(II) ions from the aqueous solution was observed with an EDTA-GO adduct (Figure 1) when compared with GO and EDTA-RGO adduct. The maximum adsorption capacities of the EDTA-GO adduct and GO for Pb(II) were found to be 479±46 and 328±39mg/g, respectively, which were greater than those of oxidized carbon nanotubes and activated carbon-based adsorbents [164]. The superior performance of EDTA-GO was correlated with the chelating characteristics of EDTA with functionalized graphene sheets. Mainly two adsorption process were considered to be responsible for the improved efficiency of removal of Pb(II) ions with the EDTA-GO adduct, i.e., the ion-exchange reaction process between Pb(II) and carboxylic and hydroxyl functional groups responsible for chelate complex formation between Pb(II) and EDTA onto the GO surface, as shown below .
The second adsorption mechanism involves the stable complex formation of EDTA with Pb(II) ions for the complete removal of Pb(II) ions from aqueous solution. After adsorption, Pb(II) equilibrium concentration reached lower than food and drug administration drinking water concentration i.e < 10ppm. Higher adsorption capacity was attributed with the higher stability constant (Log K≈18.0) of Pb(II)-EDTA complex. Langmuir and Freundlich adsorption isotherms exhibited good agreement with the experimental data having a correlation coefficient (R2) 0.975 and 0.933 at pH 6.8 respectively. The adsorption phenomenon rate for Pb(II) ions onto the surface of EDTA-GO was found to be pH dependent. It was noted that as pH of the aqueous medium increased from 5.0 to 8.0, adsorption capacity of EDTA-GO also increased due to chelate formation of Pb(II) with the hydroxyl, carboxylic functional groups of GO and EDTA. Low adsorption at high acidic medium was attributed due to neutralization of COO- and O- surface charges and relative competition between proton and Pb(II) ions. Lower zeta potential until pH 8.0 indicated that there were enough negative charge density to provide the strong electrostatic attraction between adsorbent's functional group and Pb(II) ions. However, as the pH of the medium increased from 8.0, zeta potential rise indicated that negative charge density decreased and Pb(II) ions hydrolzse to give Pb(OH)+, Pb(OH)2, [Pb(OH)3]-, [Pb3(OH)4]2+ [Pb2(OH)2]2+ [Pb6(OH)8]4+ and [Pb4(OH)4]4+ [171][172][173]. As it is desirable to have appropriate both the adsorption and desorption capacities to be an economical and ideal adsorbent, desorption experiments at different pH were carried out using Pb(II) pretreated EDTA-GO to determine desorption capacity. Desorption rate of Pb(II) was found to be maximum in acidic medium as consistent with zeta potential indicated that at lower pH, neutralization of functional groups leads to the competition between proton and Pb(II) ions adsorption [174]. Atomic absorption spectroscopy results were found to be in good agreement indicated that Pb(II) ions could be repeatedly desorbed from EDTA-GO adsorbent even after 10 cycles.
3. Conclusions and future perspective
Graphene based nanoadsorbents have been proven to play the vital role in the selective adsorption of heavy metal ions, such as Pb(II), from wastewater. The selective recovery and reuse of valuable adsorbents and pollutants are the important challenges for the adsorption technology using nanoadsorbents with favorable structures, morphology, superior adsorption capacities, and ease of separation. Graphene is always thanked for its unique property of functionalization, used to alter its properties and, consequently, its applications. This review exploited recently-developed graphene-based novel nanoadsorbents for the effective and selective removal of Pb(II) ions from wastewaters. In addition to the various own functionalities, GO was further surface modified with the negatively-charged functional groups to enhance the selective and effective Pb(II) ion adsorption from wastewaters. Due to wide varieties of surface functional groups, high surface area, and a preponderance of exposed edges planes, GO exhibited remarkable superior adsorption of Pb(II) ions. In addition, GO composites with ion scavengers (for example, EDTA), metal nanoparticles, magnetites, polymers, and aerogels have been reviewed for the adsorption of Pb(II) ions under the influence of temperature, pH of the medium, adsorbate, and sorbent loadings, etc., and exhibited superior adsorption than pristine GO for Pb(II) ion adsorption from an aqueous solution. One of the major advantages revealed by the graphene-based nanoadsorbents is the recovery of adsorbents and adsorbates even after a series of life cycles. Although simple and effective routes for GO-based nanoadsorbents with high surface area and pore size for superior adsorption of heavy metal ions are still in demand, graphene-based nanoadsorbents have proven their potential adsorbability for the expediency of mankind and the environment.
References
- Rao, Z.; Feng, K.; Tang, B.; Wu, P. Surface decoration of amino-functionalized metal-organic framework/graphene oxide composite onto polydopamine-coated membrane substrate for highly efficient heavy metal removal. ACS Appl. Mater. Interfaces 2017, 9, 2594–2605.
- Nriagu, J.O.; Pacyna, J.M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 1988, 333, 134–139.
- Afkhami, A.; Saber-Tehrani, M.; Bagheri, H. Simultaneous removal of heavy metal ions in wastewater samples using nano-alumina modified with 2,4-dinitrophenylhydrazine. J. Hazard. Mater. 2010, 181, 836–844.
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol. 2007, 98, 2243–2257.
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310.
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561.
- Cesaro, A.; Naddeo, V.; Belgiorno, V. Wastewater treatment by combination of advanced oxidation processes and conventional biological systems. J. Bioremed. Biodeg. 2013, 4, 8.
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418.
- Chen, C.; Wang, X. Adsorption of Ni(II) from aqueous solution using oxidized multiwall carbon nanotubes. Ind. Eng. Chem. Res. 2006, 45, 9144–9149.
- Cervantes, C.; Jesus, C.G.; Silvia, D.; Felix, G.C.; Herminia, L.T.; Carlos, T.G.J.; Rafael, M.S. Interactions of chromium with microorganism and plants. FEMS Microbiol. Rev. 2001, 25, 335–347.
- Teow, Y.; Asharani, P.V.; Hande, M.P.; Valiyaveettil, S. Health impact and safety of engineered nanomaterials. Chem. Commun. 2011, 47, 7025–7038.
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182.
- Gopalakrishnan, A.; Krishnan, R.; Thangavel, S.; Venugopal, G.; Kim, S.-J. Removal of heavy metal ions from pharma-effluents using graphene-oxide nanosorbents and study of their adsorption kinetics. J. Ind. Eng. Chem. 2015, 30, 14–19.
- Draszawka-Bołzan, B. Effect of heavy metals on living organisms. World Sc.News 2014, 5, 26–34.
- Tirtom, V.N.; Dinçer, A.; Becerik, S.; Aydemir, T.; Çelik, A. Removal of lead(II) ions from aqueous solution by using crosslinked chitosan-clay beads. Desalin. Water Treat. 2012, 39, 76–82.
- Gollavelli, G.; Chang, C.-C.; Ling, Y.-C. Facile synthesis of smart magnetic graphene for safe drinking water: heavy metal removal and disinfection control. ACS Sustain. Chem. Eng. 2013, 1, 462–472.
- World Health Organization. Guidelines for Drinking Water, Quality; World Health Organization: Geneva, Switzerland, 1984.
- Arbabi, M.; Hemati, S.; Amiri, M. Removal of lead ions from industrial wastewater: A review of removal methods. Int. J. Epidemiol. Res. 2015, 2, 105–109.
- Zhu, H.; Xu, Y.; Liu, A.; Kong, N.; Shan, F.; Yang, W.; Barrow, C.J.; Liu, J. Graphene nanodots-encaged porous gold electrode fabricated via ion beam sputtering deposition for electrochemical analysis of heavy metal ions. Sens. Actuar. B 2015, 206, 592–600.
- Rashed, M. Lead removal from contaminated water using mineral adsorbents. Environmentalist 2001, 21, 187–195.
- Kaushal, A.; Singh, S.K. Adsorption phenomenon and its application in removal of lead from waste water: A review. Int. J. Hydrog. 2017, 1, 1–8.
- Santhosh, C.; Nivetha, R.; Kollu, P.; Srivastava, V.; Sillanpää, M.; Grace, A.N.; Bhatnagar, A. Removal of cationic and anionic heavy metals from water by 1D and 2D-carbon structures decorated with magnetic nanoparticles. Sci. Rep. 2017, 7, 14107.
- Wang, G.; Zhang, S.; Yao, P.; Chen, Y.; Xu, X.; Li, T.; Gong, G. Removal of Pb(II) from aqueous solutions by Phytolacca americana L. biomass as a low cost biosorbent. Arabian J. Chem. 2018, 11, 99–110.
- Luzardo, F.H.M.; Velasco, F.G.; Correia, I.K.S.; Silva, P.M.S.; Salay, L.C. Removal of lead ions from water using a resin of mimosa tannin and carbon nanotubes. Environ. Technol. Innov. 2017, 7, 219–228.
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58.
- Zhao, G.; Ren, X.; Gao, X.; Tan, X.; Li, J.; Chen, C.; Huang, Y.; Wang, X. Removal of Pb(II) ions from aqueous solutions on few-layered graphene oxide nanosheets. Dalton Trans. 2011, 40, 10945–10952.
- Rosillo-Lopez, M.; Salzmann, C.G. Highly efficient heavy-metal extraction from water with carboxylated graphene nanoflakes. RSC Adv. 2018, 8, 11043–11050.
- Zhang, Y.; Zhang, S.; Chung, T.-S. Nanometric graphene oxide framework membranes with enhanced heavy metal removal via nanofiltration. Environ. Sci. Technol. 2015, 49, 10235–10242.
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19.
- Shimizu, Y.; Okuno, Y.I.; Uryu, K.; Ohtsubo, S.; Watanabe, A. Filtration characteristics of hollow fiber microfltration membranes used in membrane bioreactor for domestic wastewater treatment. Water Res. 1996, 30, 2385–2392.
- Sulyman, M.; Namiesnik, J.; Gierak, A. Low-cost adsorbents derived from agricultural by-products/wastes for enhancing contaminant uptakes from wastewater: A review. Pol. J. Environ. Stud. 2017, 26, 479–510.
- Gupta, V.K.; Nayak, A.; Agarwal, S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environ. Eng. Res. 2015, 20, 1–18.
- Wang, X.; Guo, Y.; Yang, L.; Han, M.; Zhao, J.; Cheng, X. Nanomaterials as sorbents to remove heavy metal ions in wastewater treatment. J. Environ. Anal. Toxicol. 2012, 2, 1–5.
- Bhatluri, K.K.; Manna, M.S.; Ghoshal, A.K.; Saha, P. Supported liquid membrane based removal of lead(II) and cadmium(II) from mixed feed: Conversion to solid waste by precipitation. J. Hazard. Mater. 2015, 299, 504–512.
- Dabrowski, A.; Hubicki, Z.; Podkoscielny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106.
- Charerntanyarak, L. Heavy metals removal by chemical coagulation and precipitation. Water Sci. Technol. 1999, 39, 135–138.
- Kumar, B.; Smita, K.; Flores, L.C. Plant mediated detoxification of mercury and lead. Arabian J. Chem. 2017, 10, S2335–S2342.
- Kumari, P. A low cost material, banana peel for the removal of lead (II) from aqueous solutions. Int. Res. J. Eng. Technol. 2017, 4, 1404–1406.
- Liu, P.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Separation and recovery of heavy metal ions and salt ions from wastewater by 3D graphene-based asymmetric electrodes via capacitive deionization. J. Mater. Chem. A 2017, 5, 14748–14757.
- Guo, R.; Wang, R.; Yin, J.; Jiao, T.; Huang, H.; Zhao, X.; Zhang, L.; Li, Q.; Zhou, J.; Peng, Q. Fabrication and highly efficient dye removal characterization of beta-cyclodextrin-based composite polymer fibers by electrospinning. Nanomaterials 2019, 9, 127.
- Yusuf, M.; Elfghi, F.M.; Zaidi, S.A.; Abdullaha, E.C.; Khan, M.A. Applications of graphene and its derivatives as an adsorbent for heavy metal and dye removal: a systematic and comprehensive overview. RSC Adv. 2015, 5, 50392–50420.
- Fan, Q.; Li, Z.; Zhao, H.; Jia, Z.; Xu, J.; Wu, W. Adsorption of Pb(II) on palygorskite from aqueous solution: Effects of pH, ionic strength and temperature. Appl. Clay Sci. 2009, 45, 111–116.
- Zhang, F.; Wang, B.; He, S.; Man, R. Preparation of graphene-oxide/polyamidoamine dendrimers and their adsorption properties toward some heavy metal ions. J. Chem. Eng. Data 2014, 59, 1719–1726.
- Yu, R.; Shi, Y.; Yang, D.; Liu, Y.; Qu, J.; Yu, Z.-Z. Graphene oxide/chitosan aerogel microspheres with honeycomb-Cobweb and radially oriented microchannel structures for broad spectrum and rapid adsorption of water contaminants. ACS Appl. Mater. Interfaces 2017, 9, 21809–21819.
- Ali, I.; Gupta, V.K. Advances in water treatment by adsorption technology. Nat. Protoc. 2006, 1, 2661–2667.
- Dabrowski, A. Adsorption-from theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224.
- Shen, Y.; Chen, B. Sulfonated graphene nanosheets as a superb adsorbent for various environmental pollutants in water. Environ. Sci. Technol. 2015, 49, 7364–7372.
- La, D.D.; Thi, H.P.N.; Nguyen, T.A.; Bhosale, S.V. Effective removal of Pb(II) using a graphene@ternary oxides composite as an adsorbent in aqueous media. New J. Chem. 2017, 41, 14627–14634.
- Khajeh, M.; Laurent, S.; Dastafkan, K. Nanoadsorbents: classification, preparation, and applications (with emphasis on aqueous media). Chem. Rev. 2013, 113, 7728–7768.
- Yang, S.; Hu, J.; Chen, C.; Shao, D.; Wang, X. Mutual effects of Pb(II) and humic acid adsorption on multiwalled carbon nanotubes/polyacrylamide composites from aqueous solutions. Environ. Sci. Technol. 2011, 45, 3621–3627.
- Bulbula, S.T.; Dong, Y.; Lu, Y.; Yang, X.-Y. Graphene oxide coating enhances adsorption of lead ions on mesoporous SiO2 spheres. Chem. Lett. 2018, 47, 210–212.
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091.
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review. Environ. Sci. Technol. 2016, 50, 7290–7304.
- Yu, S.; Wang, X.; Pang, H.; Zhang, R.; Song, W.; Fu, D.; Hayat, T.; Wang, X. Boron nitride-based materials for the removal of pollutants from aqueous solutions: A review. Chem. Eng. J. 2018, 333, 343–360.
- Sharma, V.K.; McDonald, T.J.; Jim, H.; Garg, V.K. Magnetic graphene-carbon nanotube iron nanocomposites as adsorbents and antibacterial agents for water purification. Adv. Colloid Interface Sci. 2015, 225, 229–240.
- Kyzas, G.Z.; Matis, K.A. Nanoadsorbents for pollutants removal: A review. J. Mol. Liq. 2015, 203, 159–168.
- Bhatia, M.; Babu, R.S.; Sonawane, S.H.; Gogate, P.R.; Girdhar, A.; Reddy, E.R.; Pola, M. Application of nanoadsorbents for removal of lead from water. Int. J. Environ. Sci. Technol. 2017, 14, 1135–1154.
- Zhao, L.; Deng, J.; Sun, P.; Liu, J.; Yang, Y. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ. 2018, 627, 1253–1263.
- Sophia, C.A.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17.
- Nupearachchi, C.N.; Mahatantila, K.; Vithanage, M. Application of graphene for decontamination of water; Implications for sorptive removal. Groundwater Sustain. Dev. 2017, 5, 206–215.
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662.
- Bora, T.; Dutta, J. Applications of nanotechnology in wastewater treatment-A review. J. Nanosci. Nanotechnol. 2014, 14, 613–626.
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946.
- Cai, Z.; Dwivedi, A.D.; Lee, W.-N.; Zhao, X.; Liu, W.; Sillanpõõ, M.; Zhao, D.; Huang, C.-H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: development and future trends. Environ. Sci. Nano 2018, 5, 27–47.
- Jiang, M.; Qi, Y.; Liu, H.; Chen, Y. The role of nanomaterials and nanotechnologies in wastewater treatment: a bibliometric analysis. Nanoscale Res. Lett. 2018, 13, 233.
- Das, R.; Vecitis, C.D.; Schulze, A.; Cao, B.; Ismail, A.F.; Lu, X.; Chen, J.; Ramakrishna, S. Recent advances in nanomaterials for water protection and monitoring. Chem. Soc. Rev. 2017, 46, 6946–7020.
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 2011, 45, 10454–10462.
- Zhang, Y.; Wu, B.; Xu, H.; Liu, H.; Wang, M.; He, Y.; Pan, B. Nanomaterials-enabled water and wastewater treatment. NanoImpact 2016, 3–4, 22–39.
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137.
- Hu, L.; Li, Y.; Zhang, X.; Wang, Y.; Cui, L.; Wei, Q.; Ma, H.; Yan, L.; Du, B. Fabrication of magnetic watersoluble hyperbranched polyol functionalized graphene oxide for high-efficiency water remediation. Sci. Rep. 2016, 6, 28924.
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712.
- Yang, J.; Wu, J.-X.; Lü, Q.-F.; Lin, T.-T. Facile preparation of lignosulfonate-graphene oxide-polyaniline ternary nanocomposite as an effective adsorbent for Pb(II) ions. ACS Sustain. Chem. Eng. 2014, 2, 1203–1211.
- Zhao, T.; Yao, Y.; Wang, M.; Chen, R.; Yu, Y.; Wu, F.; Zhang, C. Preparation of MnO2-modified graphite sorbents from spent Li-ion batteries for the treatment of water contaminated by lead, cadmium, and silver. ACS Appl. Mater. Interfaces 2017, 9, 25369–25376.
- Shilimkar, T.N.; Anuse, M.A. Rapid extraction oflead(II) from succinate media with n-octylaniline in toluene. Sep. Purif. Technol. 2002, 26, 185–193.
- Wojciechowska, A.; Wieszczycka, K.; Wojciechowska, I.; Olszanowski, A. Lead (II) extraction from aqueous solutions by pyridine extractants. Sep. Purif. Technol. 2017, 177, 239–248.
- Chanthapon, N.; Sarkar, S.; Kidkhunthod, P.; Padungthon, S. Lead removal by a reusable gel cation exchange resin containing nano-scale zero valent iron. Chem. Eng. J. 2018, 331, 545–555.
- Rahman, N.; Haseen, U.; Rashid, M. Synthesis and characterization of polyacrylamide zirconium (IV) iodate ion-exchanger: its application for selective removal of lead (II) from wastewater. Arabian J. Chem. 2017, 10, S1765–S1773.
- Pehlivan, E.; Altun, T. Ion-exchange of Pb2+, Cu2+, Zn2+, Cd2+, and Ni2+ ions from aqueous solution by Lewatit CNP 80. J. Hazard. Mater. 2007, 140, 299–307.
- Ghaemi, N.; Zereshki, S.; Heidari, S. Removal of lead ions from water using PES-based nanocomposite membrane incorporated with polyaniline modified GO nanoparticles: Performance optimization by central composite design. Process Saf. Environ. Prot. 2017, 111, 475–490.
- Hajdu, I.; Bodnár, M.; Csikós, Z.; Wei, S.; Daróczi, L.; Kovács, B.; Győri, Z.; Tamás, J.; Borbély, J. Combined nano-membrane technology for removal of lead ions. J. Membr. Sci. 2012, 409–410, 44–53.
- Mehdipour, S.; Vatanpour, V.; Kariminia, H.-R. Influence of ion interaction on lead removal by a polyamide nanofiltration membrane. Desalination 2015, 362, 84–92.
- Sun, J.; Wu, L.; Li, Y. Removal of lead ions from polyether sulfone/Pb(II)-imprinted multi-walled carbon nanotubes mixed matrix membrane. J. Taiwan Inst. Chem. Eng. 2017, 78, 219–229.
- Gholami, A.; Moghadassi, A.R.; Hosseinia, S.M.; Shabani, S.; Gholami, F. Preparation and characterization of polyvinyl chloride based nanocomposite nanofiltration-membrane modified by iron oxide nanoparticles for lead removal from water. J. Ind. Eng. Chem. 2014, 20, 1517–1522.
- Xu, C.; Cui, A.; Xu, Y.; Fu, X. Graphene oxide-TiO2 composite filtration membranes and their potential application for water purification. Carbon 2013, 62, 465–471.
- Mattia, D.; Lee, K.P.; Calabrò, F. Water permeation in carbon nanotube membranes. Curr. Opin. Chem. Eng. 2014, 4, 32–37.
- Calabrò, F. Modeling the effects of material chemistry on water flow enhancement in nanotube membranes. MRS Bull. 2017, 42, 289–293.
- Zhaoa, D.; Zhaoa, J.; Ji, Y.; Liu, G.; Liu, S.; Jin, W. Facilitated water-selective permeation via PEGylation of graphene oxide membrane. J. Membr. Sci. 2018, 567, 311–320.
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical precipitation of heavy metals from acid mine drainage. Water Res. 2002, 36, 4757–4764.
- Huisman, J.L.; Schouten, G.; Schultz, C. Biologically produced sulphide for purification of process streams, effluent treatm ent and recovery of metals in the metal and mining industry. Hydrometallurgy 2006, 83, 106–113.
- Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Simultaneous removal of Pb(II) and Cr(III) by magnetite nanoparticles using various synthesis conditions. J. Ind. Eng. Chem. 2014, 20, 3543–3549.
- Prihasto, N.; Liu, Q.F.; Kim, S.-H. Pre-treatment strategies for seawater desalination by reverse osmosis system. Desalination 2009, 249, 308–316.
- Vidales, M.J.M.d.; Millán, M.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Irradiated-assisted electrochemical processes for the removal of persistent pollutants from real wastewater. Sep. Purif. Technol. 2017, 175, 428–434.
- Liu, Y.; Yan, J.; Yuan, D.; Li, Q.; Wu, X. The study of lead removal from aqueous solution using an electrochemical method with a stainless steel net electrode coated with single wall carbon nanotubes. Chem. Eng. J. 2013, 218, 81–88.
- Hussin, F.; Abnisa, F.; Issabayeva, G.; Aroua, M.K. Removal of lead by solar-photovoltaic electrocoagulation using novel perforated zinc electrode. J. Clean. Prod. 2017, 147, 206–216.
- Pang, F.M.; Kumar, P.; Teng, T.T.; Omar, A.K.M.; Wasewar, K.L. Removal of lead, zinc and iron by coagulation-flocculation. J. Taiwan Inst. Chem. Eng. 2011, 42, 809–815.
- Sweetman, M.J.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.E.; Hayball, J.D. Activated carbon, carbon nanotubes and graphene: materials and composites for advanced water purification. C J. Carbon Res. 2017, 3, 18.
- Zhao, J.; Wang, Z.; White, J.C.; Xing, B. Graphene in the aquatic environment: Adsorption, dispersion, toxicity and transformation. Environ. Sci. Technol. 2014, 48, 9995–10009.
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410.
- Tehrani, M.S.; Azar, P.A.; Namin, P.E.; Dehaghi, S.M. Removal of lead ions from wastewater using functionalized multiwalled carbon nanotubes with tris(2-aminoethyl)amine. J. Environ. Protect. 2013, 4, 529–536.
- Taylor, R.M.; Kuennen, R.W. Removing lead in drinking water with activated carbon. Environ. Prog. 1994, 13, 65–71.
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead(II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220.
- Zaini, M.A.; Amano, Y.; Machida, M. Adsorption of heavy metals onto activated carbons derived from polyacrylonitrile fiber. J. Hazard. Mater. 2010, 180, 552–560.
- Machida, M.; Fotoohi, B.; Amamo, Y.; Ohba, T.; Kanoh, H.; Mercier, L. Cadmium(II) adsorption using functional mesoporous silica and activated carbon. J. Hazard. Mater. 2012, 221–222, 220–227.
- Machida, M.; Fotoohi, B.; Amamo, Y.; Mercier, L. Cadmium(II) and lead(II) adsorption onto hetero-atom functional mesoporous silica and activated carbon. Appl. Surf. Sci. 2012, 258, 7389–7394.
- Mohan, D.; Singh, K.P.; Singh, V.K. Wastewater treatment using low cost activated carbons derived from agricultural byproducts-A case study. J. Hazard. Mater. 2008, 152, 1045–1053.
- Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825.
- Shen, Y.; Fang, Q.; Chen, B. Environmental applications of three-dimensional graphene-based macrostructures: Adsorption, transformation, and detection. Environ. Sci. Technol. 2015, 49, 67–84.
- Seenivasan, R.; Chang, W.-J.; Gunasekaran, S. Highly sensitive detection and removal of lead ions in water using cysteine-functionalized graphene oxide/polypyrrole nanocomposite film electrode. ACS Appl. Mater. Interfaces 2015, 7, 15935–15943.
- Falahati, F.; Baghdadi, M.; Aminzadeh, B. Treatment of dairy wastewater by graphene oxide nanoadsorbent and sludge separation, using in situ sludge magnetic impregnation (ISSMI). Pollution 2018, 4, 29–41.
- Kyzas, G.Z.; Deliyanni, E.A.; Bikiaris, D.N.; Mitropoulos, A.C. Graphene composites as dye adsorbents: Review. Chem. Eng. Res. Des. 2018, 129, 75–88.
- Lua, N.; He, G.; Liua, J.; Liu, G.; Li, J. Combustion synthesis of graphene for water treatment. Ceram. Intern. 2018, 44, 2463–2469.
- Zhang, Y.; Yan, X.; Yan, Y.; Chen, D.; Huang, L.; Zhang, J.; Ke, Y.; Tan, S. The utilization o f a three-dimensional reduced graphene oxide and montmorillonite composite aerogel as a multifunctional agent for wastewater treatment. RSC Adv. 2018, 8, 4239–4248.
- Moharram, M.A.K.; Tohami, K.; Hotaby, W.M.E.; Bakr, A.M. Graphene oxide porous crosslinked cellulose nanocomposite microspheres for lead removal: Kinetic study. React. Funct. Polym. 2016, 101, 9–19.
- Tabish, T.A.; Memon, F.A.; Gomez, D.E.; Horsell, D.W.; Zhang, S. A facile synthesis of porous graphene for efcient water and wastewater treatment. Sci. Rep. 2018, 8, 1817.
- You, Y.; Jin, X.H.; Wen, X.Y.; Sahajwalla, V.; Chen, V.; Bustamante, H.; Joshi, R.K. Application of graphene oxide membranes for removal of natural organic matter from water. Carbon 2018, 129, 415–419.
- Suárez-Iglesias, O.; Collado, S.; Oulego, P.; Díaz, M. Graphene-family nanomaterials in wastewater treatment plants. Chem. Eng. J. 2017, 313, 121–135.
- Wang, S.; Sun, H.; Ang, H.M.; Tadé, M.O. Adsorptive remediation of environmental pollutants using novel graphenebased nanomaterials. Chem. Eng. J. 2013, 226, 336–347.
- Deng, J.-H.; Zhang, X.-R.; Ñ, G.-M.Z.; Ñ, J.-L.G.; Niu, Q.-Y.; Liang, J. Simultaneous removal of Cd(II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem. Eng. J. 2013, 226, 189–200.
- Wu, Z.g.; Deng, W.; Zhou, W.; Luo, J. Novel magnetic polysaccharide/graphene oxide @Fe3O4 gel beads for adsorbing heavy metal ions. Carbohyd. Polym. 2019, 216, 119–128.
- Usman, T.M.; Xintai, S.; Mengqi, Z.; Yinnian, L.; Ronglan, W.; Dejun, C. Preparation of hydroxypropyl-cyclodextrin-graphene/Fe3O4 and its adsorption properties for heavy metals. Surf. Interfac. 2019, 16, 43–49.
- Huang, D.; Wu, J.; Wang, L.; Liu, X.; Meng, J.; Tang, X.; Tang, C.; Xu, J. Novel insight into adsorption and co-adsorption of heavy metal ions and an organic pollutant by magnetic graphene nanomaterials in water. Chem. Eng. J. 2019, 358, 1399–1409.
- Mehta, D.; Mazumdar, S.; Singh, S.K. Magnetic adsorbents for the treatment of water/wastewater-A review. J. Water Process Eng. 2015, 7, 244–265.
- Wang, Y.; Li, L.; Luo, C.; Wang, X.; Duan, H. Removal of Pb2+ from water environment using a novel magnetic chitosan/graphene oxide imprinted Pb2+. Int. J. Biolog. Macromol. 2016, 86, 505–511.
- Mahmud, H.N.M.E.; Huq, A.K.O.; Yahyaa, R.b. The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: a review. RSC Adv. 2016, 6, 14778–14791.
- Alawa, B.; Srivstava, J.K.; Srivastava, A.; Palsania, J. Adsorption of heavy metal Pb(II) from synthetic waste water by polypyrrole composites. Int. J. Chem. Stud. 2015, 3, 4–8.
- Mahmud, H.N.M.E.; Huq, A.K.O.; Yahya, R. Polymer-based adsorbent for heavy metals removal from aqueous solution. Mater. Sci. Eng. 2017, 206, 012100.
- Alaba, P.A.; Oladoja, N.A.; Sani, Y.M.; Ayodele, O.B.; Mohammed, I.Y.; Olupinla, S.F.; Daud, W.M.W. Insight into wastewater decontamination using polymeric adsorbents. J. Environ. Chem. Eng. 2018, 6, 1651–1672.
- El-Kafrawy, A.F.; El-Saeed, S.M.; Farag, R.K.; El-Saied, H.A.-A.; El-SayedAbdel-Raouf, M. Adsorbents based on natural polymers for removal of some heavy metals from aqueous solution. Egypt. J. Pet. 2017, 26, 23–32.
- Malik, H.; Qureshi, U.A.; Muqeet, M.; Mahar, R.B.; Ahmed, F.; Khatri, Z. Removal of lead from aqueous solution using polyacrylonitrile/magnetite nanofibers. Environ. Sci. Pollut. Res. 2018, 25, 3557–3564.
- Hamouz, O.C.S.A.; Akintola, O.S. Removal of lead and arsenic ions by a new series of aniline based polyamines. Process Saf. Environ. Prot. 2017, 106, 180–190.
- Zare, E.N.; Motahari, A.; Sillanpää, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ. Res. 2018, 162, 173–195.
- Sajid, M.; Nazal, M.K.; Baig, N.; Osman, A.M. Removal of heavy metals and organic pollutants from water using dendritic polymers based adsorbents: A critical review. Sep. Purif. Technol. 2018, 191, 400–423.
- Hu, R.; Wang, X.; Dai, S.; Shao, D.; Hayat, T.; Alsaedi, A. Application of graphitic carbon nitride for the removal of Pb(II) and aniline from aqueous solutions. Chem. Eng. J. 2015, 260, 469–477.
- Ding, C.; Cheng, W.; Wang, X.; Wu, Z.-Y.; Sun, Y.; Chen, C.; Wang, X.; Yu, S.-H. Competitive sorption of Pb(II), Cu(II) and Ni(II) on carbonaceous nanofibers: A spectroscopic and modeling approach. J. Hazard. Mater. 2016, 313, 253–261.
- Huang, S.; Song, S.; Zhang, R.; Wen, T.; Wang, X.; Yu, S.; Song, W.; Hayat, T.; Alsaedi, A.; Wang, X. Construction of layered double hydroxides/hollow carbon microsphere composites and Its applications for mutual removal of Pb(II) and humic acid from aqueous solutions. ACS Sustain. Chem. Eng. 2017, 5, 11268–11279.
- Du, Y.; Wang, J.; Zou, Y.; Yao, W.; Hou, J.; Xia, L.; Peng, A.; Alsaedi, A.; Hayat, T.; Wang, X. Synthesis of molybdenum disulfide/reduced graphene oxide composites for effective removal of Pb(II) from aqueous solutions. Sci. Bull. 2017, 62, 913–922.
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331.
- Abbasa, A.; Al-Amera, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption. Sep. Purif. Technol. 2016, 157, 141–161.
- Tehrani, M.S.; Azar, P.A.; namin, P.E.; Dehaghi, S.M. Removal of lead ions from aqueous solution using multi-walled carbon nanotubes: The effect of functionalization. J. Appl. Environ. Biol. Sci. 2014, 4, 316–326.
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364.
- Farghali, A.A.; Tawab, H.A.A.; Moaty, S.A.A.; Khaled, R. Functionalization of acidified multi-walled carbon nanotubes for removal of heavy metals in aqueous solutions. J. Nanostr. Chem. 2017, 7, 101–111.
- Anitha, K.; Namsani, S.; Singh, J.K. Removal of heavy metal ions using a functionalized single-walled carbon nanotube: A molecular dynamics study. J. Phys. Chem. A 2015, 119, 8349–8358.
- Melo, B.A.G.d.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974.
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Graphene oxide and its application as an adsorbent for wastewater treatment. J. Chem. Technol. Biotechnol. 2014, 89, 196–205.
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 2008, 319, 1229–1232.
- Kumar, M.; Singh, K.; Dhawan, S.K.; Tharanikkarasu, K.; Chung, J.S.; Kong, B.-S.; Kim, E.J.; Hur, S.H. Synthesis and characterization of covalently-grafted graphene polyaniline nanocomposites and its use in a supercapacitor. Chem. Eng. J. 2013, 231, 397–405.
- Cai, W.W.; Piner, R.D.; Stadermann, F.J.; Park, S.; Shaibat, M.A.; Ishii, Y.; Yang, D.X.; Velamakanni, A.; An, S.J.; Stoller, M.; et al. Synthesis and solid-state NMR structural characterization of 13C-labeled graphite oxide. Science 2008, 321, 1815–1817.
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534.
- Sahu, A.; Choi, W.I.; Tae, G. A stimuli-sensitive injectable graphene oxide composite hydrogel. Chem. Commun. 2012, 48, 5820–5822.
- Bao, J.; Fu, Y.; Bao, Z. Thiol-functionalized magnetite/graphene oxide hybrid as a reusable adsorbent for Hg2+ removal. Nanoscale Res. Lett. 2013, 8, 486.
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Mudhoo, A.; Sillanpää, M. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res. 2013, 47, 4812–4832.
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 2012, 335, 442–444.
- Peng, W.; Li, H.; Liu, Y.; Song, S. A review on heavy metal ions adsorption from water by graphene oxide and its composites. J. Mol. Liq. 2017, 230, 496–504.
- Gao, Y.; Pan, L.; Li, H.; Zhang, Y.; Zhang, Z.; Chen, Y.; Sun, Z. Electrosorption behavior of cations with carbon nanotubes and carbon nanofibres composite film electrodes. Thin Solid Films 2009, 517, 1616–1619.
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead(II) from aqueous solution by adsorption on carbon aerogel Using a response surface methodological approach. Ind. Eng. Chem. Res. 2005, 44, 1987–1994.
- Bain, E.J.; Calo, J.M.; Spitz-Steinberg, R.; Kirchner, J.; Axén, J. Electrosorption/electrodesorption of arsenic on a granular activated carbon in the presence of other heavy metals. Energy Fuels 2010, 24, 3415–3421.
- Gabelich, C.J.; Tran, T.D.; Suffet, I.H.M. Electrosorption of inorganic salts from aqueous solution using carbon aerogels. Environ. Sci. Technol. 2002, 36, 3010–3019.
- Kuchta, B.; Firlej, L.; Maurin, G. Modeling of adsorption in nanopores. J. Mol. Model. 2005, 11, 293–300.
- Hyung, H.; Fortner, J.D.; Hughes, J.B.; Kim, J.-H. Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environ. Sci. Technol. 2007, 41, 179–184.
- Stafiej, A.; Pyrzynska, K. Adsorption of heavy metal ions with carbon nanotubes. Sep. Purif. Technol. 2007, 58, 49–52.
- Wang, H.; Zhou, A.; Peng, F.; Yu, H.; Yang, J. Mechanism study on adsorption of acidified multiwalled carbon nanotubes to Pb(II). J. Colloid Interface Sci. 2007, 316, 277–283.
- Schierz, A.; Zänker, H. Aqueous suspensions of carbon nanotubes: surface oxidation, colloidal stability and uranium sorption. Environ. Pollut. 2009, 157, 1088–1094.
- Huang, Z.-H.; Zheng, X.; Lv, W.; Wang, M.; Yang, Q.-H.; Kang, F. Adsorption of lead(II) ions from aqueous solution on low-temperature exfoliated graphene nanosheets. Langmuir 2011, 27, 7558–7562.
- Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Gorring, M.; Kasner, M.L.; Hou, S. Adsorption behavior of EDTA-graphene oxide for Pb(II) removal. ACS Appl. Mater. Interfaces 2012, 4, 1186–1193.
- Oyetade, O.A.; Nyamori, V.O.; Martincigh, B.S.; Jonnalagadda, S.B. Effectiveness of carbon nanotube-cobalt ferrite nanocomposites for the adsorption of rhodamine B from aqueous solutions. RSC Adv. 2015, 5, 22724–22739.
- Oyetade, O.A.; Nyamori, V.O.; Martincigh, B.S.; Jonnalagadda, S.B. Nitrogen-functionalised carbon nanotubes as a novel adsorbent for the removal of Cu(II) from aqueous solution. RSC Adv. 2016, 6, 2731–2745.
- Wang, H.J.; Zhou, A.L.; Peng, F.; Yu, H.; Chen, L.F. Adsorption characteristic of acidified carbon nanotubes for heavy metal Pb(II) in aqueous solution. Mater. Sci. Eng. A 2007, 466, 201–206.
- Norzilah, A.H.; Fakhru’l-Razi, A.; Choong, T.S.Y.; Chuah, A.L. Surface modification effects on CNTs adsorption of methylene blue and phenol. J. Nanomater. 2011, 2011, 1–18.
- Lu, C.; Chiu, H. Chemical modification of multiwalled carbon nanotubes for sorption of Zn2+ from aqueous solution. Chem. Eng. J. 2008, 139, 462–468.
- Li, Y.-H.; Wang, S.; Luan, Z.; Ding, J.; Xu, C.; Wu, D. Adsorption of cadmium(II) from aqueous solution by surface oxidized carbon nanotubes. Carbon 2003, 41, 1057–1062.
- Xu, D.; Tan, X.; Chen, C.; Wang, X. Removal of Pb(II) from aqueous solution by oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2008, 154, 407–416.
- Machida, M.; Mochimaru, T.; Tatsumoto, H. Lead(II) adsorption onto the graphene layer of carbonaceous materials in aqueous solution. Carbon 2006, 44, 2681–2688.
- Hamza, I.A.A.; Martincigh, B.S.; Ngila, J.C.; Nyamoria, V.O. Adsorption studies of aqueous Pb(II) onto a sugarcane bagasse/ multi-walled carbon nanotube composite. Phys. Chem. Earth 2013, 66, 157–166.
- Varadwaj, G.B.B.; Oyetade, O.A.; Rana, S.; Martincigh, B.S.; Jonnalagadda, S.B.; Nyamori, V.O. Facile synthesis of three-dimensional Mg−Al layered double hydroxide/partially reduced graphene oxide nanocomposites for the effective removal of Pb2+ from aqueous solution. ACS Appl. Mater. Interfaces 2017, 9, 17290–17305.




