Bio-membranes exhibit complex but unique mechanical properties as communicative regulators in various physiological and pathological processes. Exposed to a dynamic micro-environment, bio-membranes can be seen as an intricate and delicate system. The systematical modeling and detection of their local physical properties are often difficult to achieve, both quantitatively and precisely. The emerging diamonds hosting quantum defects (i.e., nitrogen-vacancy (NV) center) demonstrate intriguing optical and spin properties, together with their outstanding photostability and biocompatibility, rendering them ideal candidates for biological applications. Notably, the extraordinary spin-based sensing enables the measurements of localized nanoscale physical quantities such as magnetic fields, electrical fields, temperature, and strain. These nanoscale signals can be optically read out precisely by simple optical microscopy systems. Given these exclusive properties, NV-center-based quantum sensors can be widely applied in exploring bio-membrane-related features and the communicative chemical reaction processes. Herein, it is focused on NV-based quantum sensing in bio-membrane fields. The attempts of applying NV-based quantum sensors in bio-membranes The challenges and future directions of this novel technology to be utilized in bio-membranes will also be discussed.
1. Emerging Applications of Diamond Quantum Sensing in Membrane Systems
NV-based quantum sensors can be applied widely and differently in bio-membrane fields regarding the related magnetic, electrical, thermodynamic, and mechanical quantities. Understanding the bio-function of the cell membrane requires the quantitative detection and analysis of the physical properties of the cell membrane. For instance, nanoscale magnetic field fluctuations derived from the fundamental spins are ubiquitous in biological systems and act as a rich source of information about the processes that generate them at the atomic and molecular levels, still waiting to be explored
[1]. Nanoscale thermal gradient variations in the proximity of bio-membranes are also vital for understanding the cross-membrane motions due to biological or chemical interactions
[2]. The following section will mainly demonstrate recent major bio-membrane-related applications from the physical and chemical perspectives to show the superior advantages of NV-based diamond quantum sensing applied in bio-membrane systems at atomic levels.
2. Measurements of Physical Events in Membrane Systems
2.1. Membrane Structure-Related Measurements
Lipid bilayer membranes can serve as a useful and appropriate platform upon suitable modification for various bio-membrane applications such as single ion channel analysis or drug screening
[3]. They can be utilized to develop probes for studying bio-membrane systems, with an emphasis on detecting the nanoscale molecules and atoms to gain information that may be hidden in the ensemble averaging. A certain type of nanoprobe is to be developed for sensing the weak magnetic fields that derive from essential spins in nanoscale biology, from naturally occurring (free radicals) or specifically introduced (spin labels). They are in great demand to study the dynamic processes in bio-membrane systems in situ, such as has been done by magnetic resonance techniques such as electron spin resonance (ESR)
[4], where the sensitivity and resolution need to be improved. As shown in
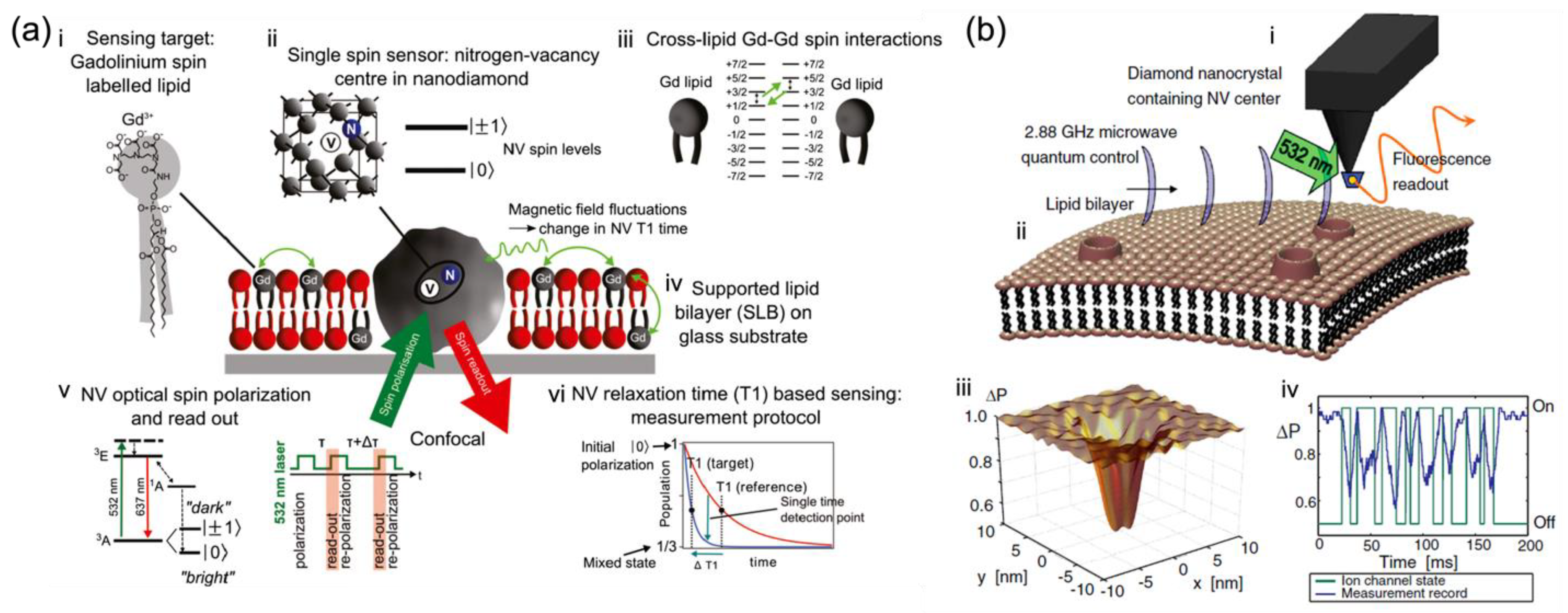
Figure 1a, Kaufmann et al. demonstrated the ND with a single NV as a nanoparticle probe, situated in an artificial lipid bilayer with gadolinium spin labels and acting as a nanoscopic detector for direct weak magnetic field sensing under ambient conditions with noncontact optical readout
[1]. Specifically, the NDs with NV centers were surrounded by a supported lipid bilayer labeled with Gd
3+ for producing characteristic magnetic fluctuations in the lipid environment (
Figure 1(aiii)) as the detected target. Changes in the spin relaxation time (T
1) of this single NV spin probe (
Figure 1(av,vi)) located in the lipid bilayer were optically detected at a projected sensitivity of ~5 Gd spins per Hz
1/2 [1]. This demonstrates that the NV quantum sensor is sensitive to cross-lipid magnetic fluctuations generated from a small number of spins such as Gd labels in the nanoscale vicinity of the ND. The detection of such a small number of spins in a biological lipid model brings new possibilities for bio-membrane information measurement. It also highlights the potential of NV diamond sensors as a magnetic probe in nanoscopic bio-membrane systems, circumventing the fundamental issues related to ensemble averaging.
Ion channels enable selective and passive diffusion of ions across the cellular membrane, while the ion pumps create and uphold the potential gradients actively across membranes in living cells
[5][6]. Recent measurement techniques such as patch-clamp methods are invasive and hard to scale up
[7]. The approach suitable for ion-channel monitoring is to consider a non-invasive and reliable detection method in situ. Based on the quantum properties of a single-atom probe, the NV-based quantum sensors are suitable and applicable.
As shown in
Figure 1b, Hall et al. studied the quantum motions of an NV atomic probe tip near the ion channel and lipid bilayer
[8]. The NV detector (
Figure 1(bi)) was comprised of a diamond nanocrystal hosting an NV center fabricated at the end of an atomic force microscope (AFM) tip. It was locked and protected in the super stable diamond to be kept within nanometers of targeted samples for biological applications. To detect the weak fluctuations of magnetic moments arising from ion-channel operation, the quantum decoherence of the NV center induced by ion flux was measured, which shows an ultra-sensitive monitoring capability for ion-channel issues, far beyond the limits of magnetometer sensitivity
[9]. In addition to the embedded ion channels, Hall et al. also set the lipid membrane and the instant surroundings as fluctuating electromagnetic sources and evaluated the effect of each source on the quantum coherence of NV centers quantitatively to identify the sensitivity of NV detectors to ion channel signals. Their theoretical findings show the possibility that ion channel operation can be detected in real-time with millisecond resolution by direct monitoring of the quantum decoherence of NV sensors. This may have great impacts on the fields of membrane biology and drug discovery.
Figure 1. In situ nanoscale detection of dynamic processes in separate membrane structures by NV-based quantum sensing as nano-magnetometer probes. (
a) Schematic of the single-spin NV sensor detecting nanoscale spin labels in a supported lipid bilayer (SLB)
[1]. (
i) Gd spin-labeled lipids are inserted into the SLB. (
ii) Nanodiamond (ND) with a single NV optical center acts as a single-spin sensor. (
iii) Magnetic field fluctuations generated by Gd spin labels influence the NV spin state. (
iv) SLB is formed around an ND fixed on a glass substrate. (
v) Electronic energy structure of the NV center showing optical spin readout of the spin sublevels m
S = 0 and m
S = ± 1and the T
1 measurement protocol. (
vi) Schematic of the T
1 measurement.
[1] (
b) NV quantum imaging of the ion-channel operation
[8]: (
i) A single NV defect in a nano diamond is placed on an AFM tip. (
ii) The cell membrane near the host channels permits the flow of ions across the membrane surface, resulting in an effective fluctuating magnetic field affecting the NV quantum spin state. (
iii) The NV decoherence leads to a fluorescence decrease, most distinct in areas close to the ion-channel opening. (
iv) Variations in fluorescence allow the temporal tracking of ion-channel dynamics
[8].
2.2. Membrane Motion Dynamics and Fluidity Measurement
The bulk lipid in the cell membrane makes a fluid matrix for protein rotation and lateral diffusion to achieve physiological functioning
[10]. Such high membrane fluidity enables membrane proteins and molecules to diffuse rapidly in the plane of the bilayer and to interact with one another in cell signaling, which is vital for life
[11]. Though it may seem hard to track the membrane fluidic motion due to the complex membrane model and the dynamic environment, Feng et al. still figure out a way by attaching diamond particles to live cell membranes for 6D tracking in measuring live bio-membrane dynamics
[12]. Feng et al. demonstrated the synchronized 3D translation together with 3D rotation tracking of diamond particles based on NV magnetometry, and the simultaneous precision of ~10–40 nm for translation and ~1−5° for rotation with a 1-s measurement time was achieved. They carried out the 6D tracking of single diamond particles on a giant plasma membrane vesicle to characterize the translation and rotation on a lipid vesicle as a model system for cell membranes
[12]. This provided clues to separate the intrinsic rotation of the diamond particle from the geometric effect due to parallel transport on a curved surface (the plasma membrane vesicle sphere). They further applied the 6D tracking technique to monitor single NDs on live cell membranes. The motion characteristics of the NDs on the cell membranes under various controlled conditions (normal, fixed, necrotized, or ATP-depleted) indicated that the rotation of the NDs is associated with cell metabolic activities. This technique expands the toolbox of single particle tracking and brings a distinct approach to issues where the analysis of translational and rotational correlations is critical.
The cell membrane, attached closely to its cytoskeleton, is crucial in regulating cell mechanics. The overall cell elasticity is an important parameter underlying the dynamics of diverse cellular activities
[13]. The elastic property of the cell membrane can be measured through several contact methods such as optical tweezers and atomic force microscopy (AFM)
[14]. Specifically, AFM indentation is the most widely utilized method to measure the cellular elasticity, but the local indentation data are usually complex and ambiguous to interpret
[15], as they are related not only to the intrinsic cellular mechanical properties but also the contact features between the membrane and the tip, the uncertainty of which would lead to ambiguity in data interpretation.
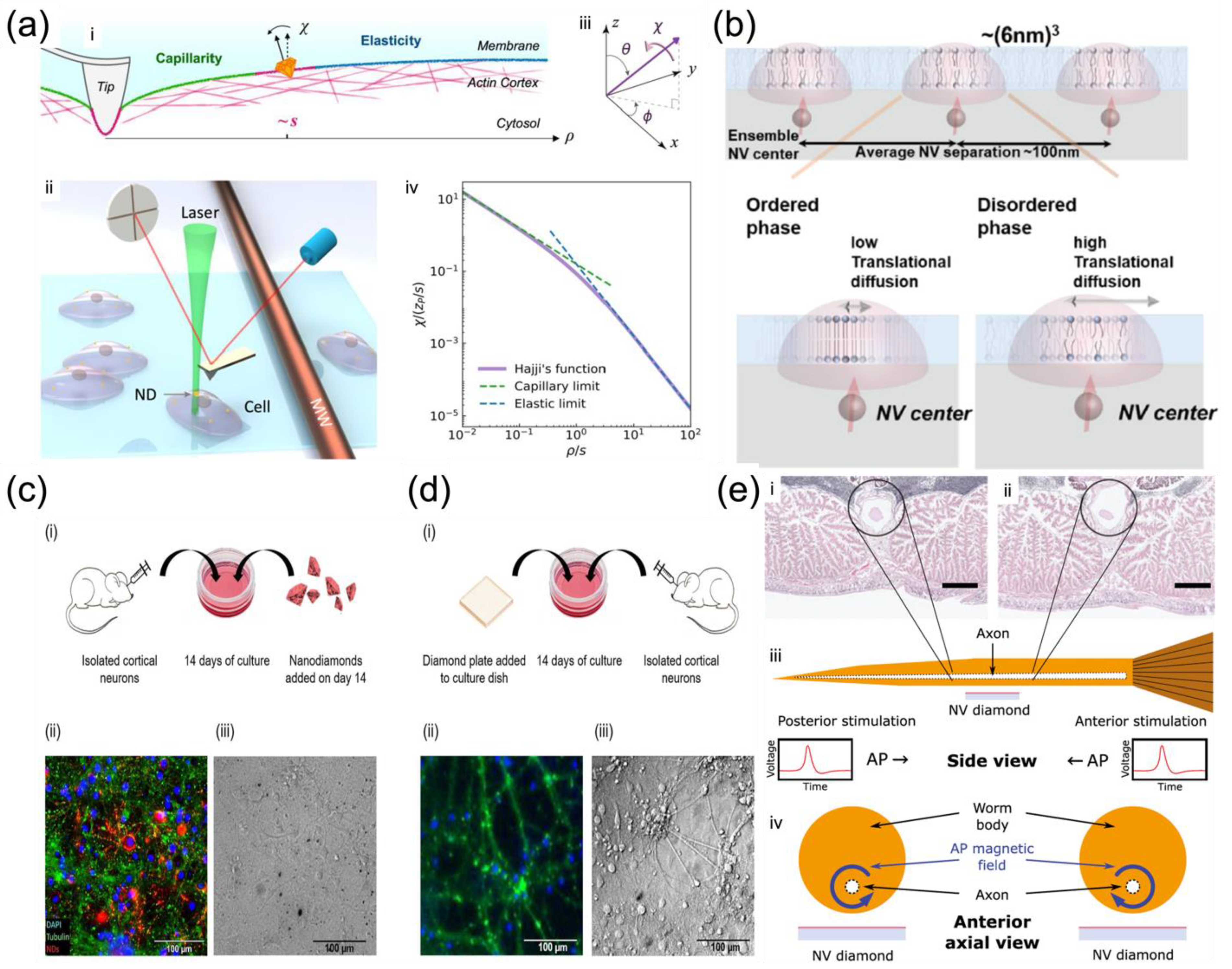
Cui et al. reported a strategy of measuring the non-local deformation of fixed HeLa cells induced by AFM indentation (
Figure 2a) with the assistance of the orientation sensing by NV centers in NDs
[16]. The non-local deformation away from the indentation point is independent of the contact details but relies purely on the intrinsic mechanical properties of biological membranes, irrespective of local indentation uncertainties. This solves the above-mentioned issues in that it does not require the detailed local contact information between the membrane and the indentation tip, making it simpler for measurement. More spectacularly, the spin resonances of the NV center dependent on the magnetic field along the NV axis were applied to measure the orientation of the NDs on the membrane surface, thus achieving surface deformation mapping
[16]. This represents a unique and simple way to provide sufficient resolution and precision for studying bio-membranes. Under controlled structural manipulations, this strategy was able to detect the competition between the elasticity and capillarity on cell membranes soaked in liquid and to evaluate the elastic modulus and surface tension simultaneously measured by NV-center-dependent orientation sensing
[16]. It was concluded that if the capillarity was not considered, the membrane elastic moduli would have been overestimated as in most previous studies using local depth-loading data. In addition, after the depolymerization of the actin cytoskeleton structure, there was a reduction of both elastic moduli and surface tensions. These findings bring the first clear experimental observation of the elastocapillary effect by AFM indentation on cell membranes.
The cell membrane has different domains that are vital for cellular functions, such as molecule transport, communication, and metabolic interactions with the surrounding medium
[17][18][19]. Those functional domains are compartmentalized by distinct phases of lipid membranes, resulting in extensive research on understanding their structural and dynamic characteristics. The phase behavior of a lipid bilayer shows the relative fluidity of the individual lipid molecules and how this fluidity varies with temperature
[20]. Broadly, a lipid bilayer may exist as a liquid or gel phase across a wide range of temperatures. All lipids can undergo a phase change from a solid to a liquid at the characteristic temperature
[21]. The lipid bilayer fluidity, characterized by the two-dimensional translational diffusion of lipid molecules
[22], determines the fundamental property of lipids with different phases and hence the different domains. Recently, fluorescent probes were utilized for the detection of nanoscale diffusion and the identification of nanoscale domains
[23]. However, they generally alter the mass and structure of the target molecule and deteriorate the dynamics to be observed
[24]. To address these issues, NV center-based quantum measurement can be used as a label-free technique with nanoscale detection volume for direct diffusion measurement without causing perturbations in biological surroundings.
Ishiwata et al. investigated nanoscale phase change detection of lipid bilayers utilizing nuclear spin detection within a small interval (~10 nm) (
Figure 2b), determined by the depth of the NV center
[25]. It was observed that the NV center showed a variation of the relaxation time as a function of the change in temperature. By combining the Monte Carlo translational diffusion and molecular dynamics simulation, the translational diffusion constant varied from 1.5 ± 0.25 nm
2/μs to 3.0 ± 0.5 nm
2/μs when the temperature changed from 26.5 °C to 36.0 °C, depicting a phase change from the ordered to disordered
[25]. The phase change in the lipid bilayer was observed directly for the first time by NV magnetometry, paving the way for the label-free identification of domains in the cell membrane to reveal the relationship between cell membrane dynamics and cell function
[26].
2.3. Membrane Potential and Polarity Measurement
Understanding of the biological neural network dynamics needs quantitative detection and analysis, which are vital for gaining insight into information processing in the brain. The information transferred in neurons is in the form of action potentials (APs), where the propagation of signals is crucial for intercellular communication. Magnetic fields arising from neuronal APs can pass through biological tissue largely without perturbations, making magnetic measurements of AP dynamics possible to be carried out outside the cell or even outside an intact organism
[27]. However, it is challenging to achieve single-neuron spatial resolution and scalable measurements for functional networks or intact organisms.
Barry et al. demonstrated the non-invasive magnetic sensing of neuronal AP dynamics with single-neuron sensitivity by optically probed NV centers in diamond, which is applicable in intact organisms (
Figure 2e)
[27]. This approach enables precise measurement of AP waveforms from individual neurons and correlates magnetic fields with the AP conductive velocity, which determines the AP propagation direction directly by the fact that NV centers are sensitive to the AP magnetic field vector. More recently, Price et al. performed the NV sensing protocol both intracellularly by NDs (
Figure 2c) and extracellularly by diamond plates (
Figure 2d) with cultures of biological neuronal networks, which are highly interconnected for generating synchronized waves of endogenous neuronal activity
[28]. The NV sensing protocols based on the ODMR technique and MW modulation of photoluminescence were performed under a widefield fluorescent microscope, enabling high-resolution spatial and temporal mapping of the endogenous neuronal activity under biological conditions.
2.4. Nanoscale Thermometry in Membrane
Generally, the recent understanding of thermal effects in nanoscale biological systems is on the basis of macroscopic measurements
[29]. Less is known about the nanoscale local thermostability or heat tolerance of subcellular organelles. Tsai et al. presented the hybrids of gold nanorod–fluorescent ND (GNR–FND) (
Figure 3a–c) as a combined nano-heater and nanothermometer inside living cells
[30]. With both heating and probing by the 594 nm laser, the temperature changes were measured by recording the spectral shifts of the zero-phonon lines of NV centers in fluorescent NDs
[30]. This method is capable of identifying the rupture temperatures of membrane nanotubes in human embryonic kidney cells and generating high-temperature gradients on the cellular membrane for optically controlled hyperthermia. The results demonstrated a new paradigm for hyperthermia research and application.
Figure 3. Membrane thermostability and temperature variations measured by NV nanoscale thermometry. (
a) Schematic of the hybrid GNR-FNDs in the endosomes of membrane-tunneling nanotubes (TNTs) for both heating and temperature sensing activated by a 594 nm laser beam
[30]. (
b) Empirical cumulative distribution chart of the membrane temperatures where TNTs are ruptured by local heating and global heating of GFP-transduced human embryonic kidney (HEK) cells
[30]. (
c) (
i) Fluorescence and (
ii) merged bright-field with fluorescence images of HEK cells transduced with actin-GFP fusion proteins (green) and labeled with GNR–FNDs (red). (
iii) Fluorescence and (
iv) merged bright-field with fluorescence images of GNR–FND-labeled, GFP-transduced HEK cells after irradiation by a 594 nm laser with 330 mW for 6 s. Insets of (
ii) and (
iv): Enlarged views of the areas with the particle irradiated by the 594 nm laser enclosed in yellow. White arrows indicate the particles being irradiated
[30]. (
d) Fiber-optic thermometry for single thermo-genetically activated neurons
[31]: (
i) Fluorescence image of a laser-activated neuron. (
ii) Fluorescence of the Ca
2+ sensor in a laser-activated neuron as a function of time (solid line) with the temperature within the laser-irradiated area inside the neuronal culture increased in a stepwise fashion (dashed line) for TRPA1-expressing neurons. (
iii) Photoluminescence intensity from NV centers in a diamond microcrystal on the fiber tip, varies according to the MW frequency at 22 °C (green), 29 °C (blue), and 36 °C (maroon). (
iv) The central frequency of the EPR spectrum of NV centers recorded by ODMR, changes with ambient temperature (filled circles) and its linear fit (solid line). (
v) Laser-induced temperature change ΔT of water (filled circles, triangles, and diamonds) and the NV-diamond microcrystal in air (open circles and triangles) changes with different power of heating radiations with λ ≈ 1040 nm (triangles), 1350 nm (diamonds), and 1450 nm (circles). The linear fits are shown by solid and dashed lines. (
vi) Absorption spectrum of water (dashed line) versus the temperature change ΔT induced by the optical radiation filled with water by 43-mW (circles) and 86-mW (rectangles), measured as a function of the wavelength λ
[31].
Lanin et al. demonstrated fiber-coupled optical NV thermometry for individual thermo-genetically activated neurons based on ODMR technique
[31]. The temperature variations from single neurons were generated by laser and were read out with the NV fiber sensor, strongly related to the fluorescence of Ca
2+ ion sensors, serving as the real-time indicators of the inward Ca
2+ current across the neuron cell membrane expressing transient receptor potential (TRP) cation channels (
Figure 3d)
[31]. This brings the possibility for measuring the neuronal activities with regards to the temperature in real time.
3. Measurement of Chemical Events in Membrane Systems
Chemical reactions attracting strong scientific interest are the cellular oxidation-reduction (redox) processes. Redox reactions are crucial in maintaining metabolic functions in living cells
[32][33], such as energy generation, cellular respiration, and differentiation
[32]. The local monitoring of redox reactions is critical for gaining insight into the cellular processes for maintaining cell viability, where the free radicals play a key role
[34]. Detection of free radicals utilizing fluorescent NDs has recently been demonstrated. Barton et al. showed that the spin relaxation time of NV centers is sensitive to the number of nitroxide radicals, showing a resolution down to ~10 spins per ND (10
−23 mol in a localized volume), with the NV centers in NDs coupled magnetically with nitroxide radicals inside a bioinert polymer coating
[35]. This colloidally stable system can be used dynamically for spatial and temporal readout of the redox chemical process near the ND surface in the liquid environment.
As for cellular metabolic activities, Nie et al. also showed that the T
1 spin relaxometry measurement of NV quantum defects can be applied in detecting free radicals and its generating process both in living cells and isolated mitochondria with subcellular resolution
[36]. Fluorescent NDs were functionalized to target a single mitochondrium inside cells for metabolic activity monitoring.