| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Janusz Blasiak | + 2480 word(s) | 2480 | 2020-11-06 05:15:06 | | | |
| 2 | Bruce Ren | Meta information modification | 2480 | 2020-11-09 03:21:10 | | |
Video Upload Options
The continuous increase in life expectancy results in a steady increase of cancer risk, which consequently increases the population of older adults with cancer. Older adults have their age-related nutritional needs and often suffer from comorbidities that may affect cancer therapy. They frequently are malnourished and present advanced-stage cancer. Therefore, this group of patients requires a special multidisciplinary approach to optimize their therapy and increase quality of life impaired by aging, cancer, and the side effects of therapy. Evaluation strategies, taking advantage of comprehensive geriatric assessment tools, including the comprehensive geriatric assessment (CGA), can help individualize treatment. As epigenetics, an emerging element of the regulation of gene expression, is involved in both aging and cancer and the epigenetic profile can be modulated by the diet, it seems to be a candidate to assist with planning a nutritional intervention in elderly populations with cancer.
1. Introduction
Aging of societies implies an increasing number of cancer diagnoses in the elderly [1]. As older adults have substantially different nutritional needs than their younger counterparts, the question is whether such differences will result in a different response to cancer therapy in the categories of both the efficacy in the target tissue and unwanted side effects. Any kind of cancer therapy is a serious challenge and burden for the patient, so it should be adjusted to the nutritional status of the patient and vice versa. Nutritional studies among older adults with cancer are considered a major area of interest in geriatric oncology, as most studies on diet and nutrition in cancer have been conducted in younger adults [2].
In general, the care of older adults with cancer is complex due to competing comorbidities, multiple drugs usage, deficit in cognitive functions, and other features complicating the care. On the other hand, cancer chemotherapy may be associated with adverse events, including vomiting and mouth sores, that may influence the nutritional status of cancer patients. Furthermore, cancer is frequently associated with weight loss and a dietary intervention may be recommended in such cases. A European study showed that over 70% of elderly cancer patients presented undernutrition, defined as weight loss of 10% or greater [3].
Epigenetic regulation of gene expression is an emerging field in human molecular genetics, physiology, and pathology. The epigenetic profile of the genome (the epigenome) is established by DNA methylation, chemical modifications of chromatin, and the action of non-coding RNAs. In contrary to its genetic counterpart, the epigenetic profile is erased in the germ cells and can be modulated at any stage of development by environmental and lifestyle influences. This fact is exploited in epigenetic therapies with the use of drugs modulating the epigenetic profile (epidrugs) [4]. Many studies show that diet and nutrition influence epigenetic mechanisms playing a role in the pathogenesis of many diseases, including cancer (reviewed in [5]). On the other hand, the epigenetic profile is modulated by aging. Therefore, epigenetics seems to be a natural candidate to link nutrition with cancer therapy in older adults. In this review, we discuss the main problems associated with nutrition in older adult cancer patients undergoing active therapy, as well as the role of the epigenetic profile in aging and cancer transformation, and present a perspective of epigenetic nutritional intervention in elderly cancer patients.
2. Management of Older Adult Cancer Patients
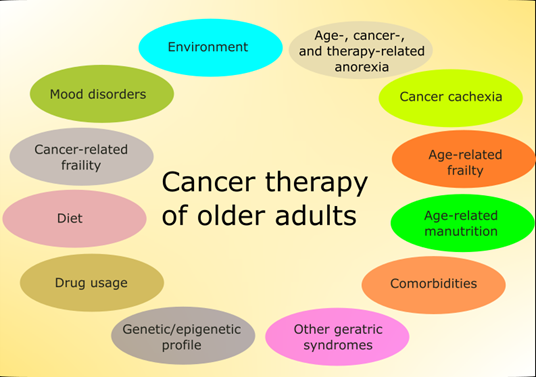
Although chronologic age is one of the main determinants of therapeutic strategy in cancer, older adults have other conditions that may influence morbidity and mortality independently of metrical age (Figure 1) [6].
Figure 1. Main factors affecting therapy in older adults with cancer. Some of these factors are mutually dependent and some partly overlap. Environment is understood here in a broad sense and also includes family and social relationships. Some factors, such as the diet, are of general significance, but have several features specific for this group of patients.
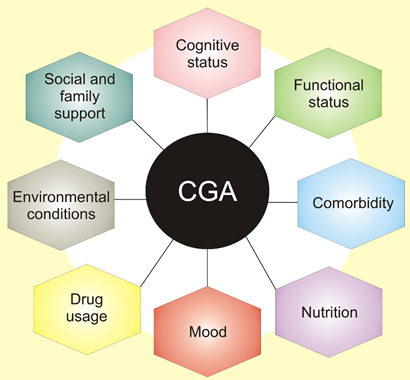
These conditions include cognitive impairment, delirium, incontinence, malnutrition, falls, gait disorders, pressure ulcers, sleep disorders, sensory deficits, fatigue, dizziness, and others. They are widespread in older adults and may have a major influence on quality of life and disability. Therefore, doctors should have a tool to quickly assess various aspects of elderly patients to develop an optimal therapeutic strategy as well as monitor and evaluate its consequences. They are listed in the comprehensive geriatric assessment (CGA), a process used to evaluate and manage fit, frail, or vulnerable older people (Figure 2) [7].
CGA involves not only medical diagnoses but also functional deficiency and the environmental and social matters that disturb patient wellbeing. It creates problem lists and shows aim-driven interventions to face them. Eventually, it delivers and organizes a complex plan for therapy, rehabilitation, support, and long-term care [7].
Figure 2. Comprehensive geriatric assessment (CGA) is an organized evaluation method to provide a multidisciplinary assessment of and care for the elderly. It assesses physical medical conditions, including comorbidity, the disease severity, immunization status, and others. Assessment of functional status refers to an elderly person’s ability to perform daily tasks and determines several core functions, including balance and mobility. Other areas of CGA include issues contained in broad categories of assessment of social health and environment.
CGA factors that may be useful in oncology care of older adults are physical function, comorbid medical conditions, cognitive function, psychological state, social support, polypharmacy, and geriatric syndromes [6]. Financial consideration is also included in these factors, often with social support. These factors should be considered in a decision-making process in the treatment of older adults with cancer. Comorbid medical conditions seem to be critical for life expectancy and treatment tolerance, which is essential to maintain quality of life. Moreover, these comorbid conditions usually, if not always, affect the treatment. Therefore, the basic question a doctor should answer is whether a patient is more likely to die of cancer or other comorbid conditions, which is a complex and challenging task in the case of older adults [6]. From the point of view of this review, comorbidity resulting from nutritional status is of a prime interest. However, it is not easy to determine the involvement of dietary factors in the pathogenesis of many serious diseases influencing cancer treatment in the elderly, including other cancers. That is why we will focus on the existing nutritional status of older cancer patients.
In general, weight loss in late life was associated with an increased mortality [8]. Malnutrition in older adults with cancer may diminish tolerance to therapy and result in a worse response to treatment [9]. The risk associated with nutritional status in the senior population can be quickly evaluated with the Mini-Nutritional Assessment (MNA), a part of CGA, including anthropometric measurements; questions related to lifestyle, mobility, and medications; a brief dietary questionnaire; and self-perception of health and nutrition [10]. It can be an alternative or supplement for self-reported practical markers of frailty, including weight loss and low Body Mass Index (BMI), which was established as less than 18.5 kg/m2 by the World Health Organization[11].
In a multicenter study, Soubeyran et al. enrolled over 300 patients older than 70 years with various types of advanced cancer [12]. They evaluated their state with various aspects of baseline abbreviated CGA and concluded that a low MNA score and poor mobility predicted an early death—within 6 months from the start of chemotherapy. These studies confirm that a poor nutritional status in older adults with cancer is correlated with a bad prognosis. The authors underlined that the MNA test in these patients likely reflected the consequences of advanced disease and that the MNA questionnaire contained 18 questions not related directly with nutrition. Yet another method for comprehensive nutritional assessment of adult oncology patients to determine the strategy of nutritional intervention is SGA (Subjective Global Assessment) and its variant, PG-SGA (Patient Generated-Subjective Global Assessment) [13].
Aaldriks et al. enrolled 143 patients aged 70 years or older with advanced colorectal cancer receiving adjuvant or palliative chemotherapy [14]. Before chemotherapy, they were assessed by MNA, Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), Groningen Frailty Indicator (GFI), and Mini Mental State Examination (MMSE). The authors observed that malnutrition and frailty were strongly linked with an increased mortality risk in patients undergoing palliative chemotherapy and a poor score on MNA was correlated with a worse tolerance of chemotherapy. Therefore, nutrition was again shown to be an important factor in the cancer care of older adults.
Comorbidity is one of the most important issues addressed in geriatric assessment. As older age is associated with frailty, diabetes, and cancer, Liuu et al. investigated older adults with cancer from the prospective single-center cohort ANCRAGE (Analyses of CanceR in AGEd) in order to determine the influence of type 2 diabetes mellitus (T2DM) and its vascular complications on frailty and adverse outcomes during 8-year follow-up [15]. They recruited nearly 1100 patients ≥ 75 years with cancer, and about 30% of them presented a metastatic disease, and frailty was common in this group (84%). After adjustment for age, gender, and metastatic status, frail T2DM patients with vascular complications displayed the highest risk of all-cause death. In the context of this review, the most important result of this study was that death was more often due to non-cancer causes, which supports the complexity of considerations surrounding the care of older adults with cancer. On the other hand, it is not easy to assess the real role of cancer in deaths whose immediate reason was T2DM, as cancer and T2DM have much in common and affect each other [16].
3. Nutrition, Aging, and Cancer in the Elderly
Older adults show diminished energetic demands, but they still need some essential nutrients, which are especially important as their total intake of food is lower than average. Therefore, their diet should be carefully chosen with limited amounts of products with sugar and fat and a dominating proportion of products with high nutrient density. However, cancer as a systemic disease may enforce alterations to such carefully established diets, and cancer therapy may require further changes. Despite common use of dietary supplements after cancer diagnosis, no consensus has been achieved for their recommendation by medical authorities, including the World Cancer Research Fund and the American Cancer Society [2].
Many dietary supplements administrated to cancer patients contain antioxidants that may neutralize reactive oxygen species (ROS) that play a role in the process of carcinogenesis, as they may induce mutations fueling cancer transformation [17][18]. However, many regimes of chemotherapy and radiotherapy produce ROS that can damage biological molecules, including proteins and DNA, in cancer cells. Supplementary antioxidants add to the cellular antioxidant defense system, containing antioxidant enzymes, DNA repair, and low-weight antioxidants. Many studies suggest that this system declines with aging [19][20]. At present, clinical recommendations say that cancer patients, independently of age, should rather not take antioxidants during therapy [21][22][23]. In a recent study, Ambrosone et al. concluded that the use of antioxidant supplements during chemotherapy, as well as iron and vitamin B12, might increase the risk of breast cancer recurrence and mortality [24]. However, this study did not stratify patients according to age, and the main age of patients enrolled in the study was about 50 years.
Malnutrition arises from an inflammatory state, which advances anorexia and resulting weight loss. Malnutrition is common in cancer patients, as up to 40% of all cancer patients display weight loss at the time of diagnosis [25][26]. However, older adults may show weight loss as a result of various comorbidities and other geriatric syndromes, so it is not easy to precisely determine cachexia among them. On the other hand, obesity, the other face of malnutrition, is increasingly becoming an issue affecting cancer survival [27][28]. This problem may be especially important in older cancer patients, as obesity occurs with aging, despite a reduction in food consumption (reviewed in [29]). Weight gain and obesity among older adults may occur with concomitant reduction in muscle mass and sarcopenia [30]. However, steroids and hormonal therapy in a long-term cancer treatment may stimulate the development of diabetes and cardiovascular disease at which older adults, especially with obesity, are at risk [31][32]. Therefore, nutritional research is needed among obese older cancer patients to establish prognosis of the disease course [33].
4. Nutrition and Cancer Therapy in the Elderly
Nutrition care during active cancer therapy should be directed to increase the efficacy of the therapy, reduce unwanted side effects, prevent nutritional deficiencies, and maintain weight and quality of life [34]. Nutritional status is an independent predictor of survival, and poor nutritional status is associated with worse outcomes for older patients undergoing cancer therapy [35][36]. On the other hand, malnutrition may be a risk factor for unwanted side effects of chemotherapy [37][38].
Chemotherapy influences patients’ nutritional status, as more than half of patients undergoing chemotherapy experience vomiting, mucositis, nausea, and parageusia [39]. Similar effects can be expected in a substantial proportion of cancer patients undergoing radiotherapy [40]. Consequently, malnutrition is an important element that should be considered in the planning of and during cancer therapy. Optimally, malnutrition should be recognized prior to surgery, chemotherapy, and radiotherapy, or any other therapy, and treated with a nutritional intervention [41]. Therefore, nutritional interventions should be fundamental and adjuvant for any kind of cancer therapy as a kind of multidisciplinary follow-up. When patients are of an advanced age, this issue becomes more complex and requires some additional and specific approaches.
Muscle mass loss and fatty muscle infiltration are frequently used to assess malnutrition, sarcopenia, and cachexia and to monitor the side effects of cancer therapy [42]. Cancer-independent, significant muscle mass loss in older adults is an important factor that should be considered in such assessments.
Apart from problems associated with cancer therapy and directly related to the diet and nutrition status, some other factors should be considered in the cancer care of older patients. Hoppe et al. presented data from 12 centers in France with older (age ≥70 years) cancer patients receiving first-line chemotherapy [43]. They observed that a substantial portion of patients, 50 of 364, experienced an early functional decline between the beginning of chemotherapy and its second cycle. This decline was determined as a decrease of ≥0.5 points on the Activities of Daily Living scale [44]. Factors associated with early functional decline were evaluated with the use of various geriatric assessments, including abbreviated CGA, MNA. They observed that early functional decline resulting from first-line chemotherapy was associated with baseline depression and instrumental dependencies. Both these features may cause nutritional problems and impede nutritional interventions.
The diet seems to be the only element during cancer therapy that can be perceived by a patient as a fully controllable means to maintain energy and activity and successfully overcome the therapy [9]. This seems especially important in the case of head and neck cancer as well as cancer of the gastrointestinal tract, as patients with these cancers are particularly prone to problems with nutrition due to the location of tumor and area of treatment [45][46].
From the clinical point of view, future research should concentrate on energy balance among older adults and their body composition during cancer treatment, biomarkers for cachexia, and personalized multi-disciplinary interventions [47]. From a scientific standpoint, it is important to determine the process of cellular aging in cancer cells and relate it to organismal aging.
References
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, S.J. Age and Cancer Risk. Am. J. Prev. Med. 2014, 46, S7–S15, doi:10.1016/j.amepre.2013.10.029.
- Presley, C.J.; Dotan, E.; Soto-Perez-De-Celis, E.; Jatoi, A.; Mohile, S.G.; Won, E.; Alibhai, S.; Kilari, D.; Harrison, R.; Klepin, H.D.; et al. Gaps in nutritional research among older adults with cancer. J. Geriatr. Oncol. 2016, 7, 281–292, doi:10.1016/j.jgo.2016.04.006.
- Paillaud, E.; Caillet, P.; Campillo, B.; Bories, P.N. Increased risk of alteration of nutritional status in hospitalized elderly patients with advanced cancer. J. Nutr. Health Aging 2006, 10, 91–95.
- Kagohara, L.T.; Stein-O’Brien, G.L.; Kelley, D.; Flam, E.; Wick, H.C.; Danilova, L.V.; Easwaran, H.; Favorov, A.V.; Qian, J.; Gaykalova, D.A.; et al. Epigenetic regulation of gene expression in cancer: Techniques, resources and analysis. Briefings Funct. Genom. 2017, 17, 49–63, doi:10.1093/bfgp/elx018.
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425, doi:10.3390/ijms19113425.
- Klepin, H.; Mohile, S.; Hurria, A. Geriatric assessment in older patients with breast cancer. J. Natl. Compr. Cancer Netw. 2009, 7, 226–236, doi:10.6004/jnccn.2009.0016.
- Welsh, T.J.; Gordon, A.L.; Gladman, J.R. Comprehensive geriatric assessment a guide for the non‐specialist. Int. J. Clin. Pr. 2013, 68, 290–293, doi:10.1111/ijcp.12313.
- Li, X.; Ploner, A.; Wang, Y.; Magnusson, P.K.; Reynolds, C.; Finkel, D.; Pedersen, N.L.; Jylhävä, J.; Hägg, S. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife 2020, 9, doi:10.7554/elife.51507.
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211, doi:10.3390/jcm8081211.
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.-L. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122, doi:10.1016/s0899-9007(98)00171-3.
- Howlader, N.N.A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; Chen, H.S.; et al. (Eds.). SEER Cancer Statistics Review; National Cancer Institute: Bethesda, MD, USA, 2019.
- Soubeyran, P.; Fonck, M.; Blanc-Bisson, C.; Blanc, J.-F.; Ceccaldi, J.; Mertens, C.; Imbert, Y.; Cany, L.; Vogt, L.; Dauba, J.; et al. Predictors of Early Death Risk in Older Patients Treated with First-Line Chemotherapy for Cancer. J. Clin. Oncol. 2012, 30, 1829–1834, doi:10.1200/jco.2011.35.7442.
- Thompson, K.L.; Elliott, L.; Fuchs-Tarlovsky, V.; Levin, R.M.; Voss, A.C.; Piemonte, T. Oncology Evidence-Based Nutrition Practice Guideline for Adults. J. Acad. Nutr. Diet. 2017, 117, 297–310.e47, doi:10.1016/j.jand.2016.05.010.
- Aaldriks, A.A.; Van Der Geest, L.G.M.; Giltay, E.J.; Le Cessie, S.; Portielje, J.E.; Tanis, B.C.; Nortier, J.W.; Maartense, E. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J. Geriatr. Oncol. 2013, 4, 218–226, doi:10.1016/j.jgo.2013.04.001.
- Liuu, E.; Saulnier, P.-J.; Gand, E.; Ragot, S.; Valero, S.; Jamet, A.; Hadjadj, S.; Paccalin, M. Frailty and diabetes status in older patients with cancer: Impact on mortality in the ANCRAGE cohort. Aging Clin. Exp. Res. 2020, 32, 1809–1819, doi:10.1007/s40520-019-01362-9.
- Kaleru, T.; Vankeshwaram, V.K.; Maheshwary, A.; Mohite, D.; Khan, S. Diabetes Mellitus in the Middle-Aged and Elderly Population (>45 Years) and Its Association with Pancreatic Cancer: An Updated Review. Cureus 2020, 12, e8884, doi:10.7759/cureus.8884.
- Forcados, G.E.; James, D.B.; Sallau, A.B.; Muhammad, A.; Mabeta, P.L. Oxidative Stress and Carcinogenesis: Potential of Phytochemicals in Breast Cancer Therapy. Nutr. Cancer 2017, 69, 365–374, doi:10.1080/01635581.2017.1267777.
- Saha, S.K.; Bin Lee, S.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.-M.; Dayem, A.A.; Cho, S.G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544, doi:10.3390/ijms18071544.
- Bryll, A.; Krzyściak, W.; Jurczak, A.; Chrzan, R.; Lizoń, A.; Urbanik, A. Changes in the Selected Antioxidant Defense Parameters in the Blood of Patients after High Resolution Computed Tomography. Int. J. Environ. Res. Public Health 2019, 16, 1476, doi:10.3390/ijerph16091476.
- Kozakiewicz, M.; Kornatowski, M.; Krzywińska, O.; Kędziora-Kornatowska, K. Changes in the blood antioxidant defense of advanced age people. Clin. Interv. Aging 2019, 14, 763–771, doi:10.2147/cia.s201250.
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; Gapstur, S.; Patel, A.V.; Andrews, K.; Gansler, T.; et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention. CA Cancer J. Clin. 2012, 62, 30–67, doi:10.3322/caac.20140.
- Norman, H.A.; Butrum, R.R.; Feldman, E.; Heber, D.; Nixon, D.; Picciano, M.F.; Rivlin, R.; Simopoulos, A.; Wargovich, M.J.; Weisburger, E.K.; et al. The Role of Dietary Supplements during Cancer Therapy. J. Nutr. 2003, 133, 3794S–3799S, doi:10.1093/jn/133.11.3794s.
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 242–274, doi:10.3322/caac.21142.
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients with Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814, doi:10.1200/jco.19.01203.
- DeWys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic effect of weight loss prior tochemotherapy in cancer patients. Am. J. Med. 1980, 69, 491–497, doi:10.1016/s0149-2918(05)80001-3.
- Wigmore, S.J.; Plester, C.E.; Ross, J.A.; Fearon, K.C. Contribution of anorexia and hypermetabolism to weight loss in anicteric patients with pancreatic cancer. Br. J. Surg. 1997, 84, 196–197.
- Ligibel, J.A.; Alfano, C.M.; Hershman, D.; Ballard, R.M.; Bruinooge, S.S.; Courneya, K.S.; Daniels, E.C.; Demark-Wahnefried, W.; Frank, E.S.; Goodwin, P.J.; et al. Recommendations for Obesity Clinical Trials in Cancer Survivors: American Society of Clinical Oncology Statement. J. Clin. Oncol. 2015, 33, 3961–3967, doi:10.1200/jco.2015.63.1440.
- Ligibel, J.A.; Alfano, C.M.; Hershman, D.L.; Merrill, J.K.; Basen-Engquist, K.; Bloomgarden, Z.T.; Demark-Wahnefried, W.; Dixon, S.; Hassink, S.G.; Jakicic, J.M.; et al. American Society of Clinical Oncology Summit on Addressing Obesity Through Multidisciplinary Provider Collaboration: Key Findings and Recommendations for Action. Obesity 2017, 25, S34–S39, doi:10.1002/oby.21987.
- Wysokiński, A.; Sobow, T.; Kłoszewska, I.; Kostka, T. Mechanisms of the anorexia of aging—A review. AGE 2015, 37, doi:10.1007/s11357-015-9821-x.
- Morley, J.E. Anorexia of aging: Physiologic and pathologic. Am. J. Clin. Nutr. 1997, 66, 760–773, doi:10.1093/ajcn/66.4.760.
- Brocco, D.; Florio, R.; De Lellis, L.; Veschi, S.; Grassadonia, A.; Tinari, N.; Cama, A. The Role of Dysfunctional Adipose Tissue in Pancreatic Cancer: A Molecular Perspective. Cancers 2020, 12, 1849, doi:10.3390/cancers12071849.
- Prieto-Hontoria, P.L.; Pérez-Matute, P.; Fernández-Galilea, M.; Bustos, M.; Etxeberria, U.; Moreno-Aliaga, M.J. Role of obesity-associated dysfunctional adipose tissue in cancer: A molecular nutrition approach. Biochim. Biophys. Acta (BBA) Bioenerg. 2011, 1807, 664–678, doi:10.1016/j.bbabio.2010.11.004.
- Naimo, G.D.; Gelsomino, L.; Catalano, S.; Mauro, L.; Andò, S. Interfering Role of ERα on Adiponectin Action in Breast Cancer. Front. Endocrinol. 2020, 11, 66, doi:10.3389/fendo.2020.00066.
- Cotogni, P.; Caccialanza, R.; Pedrazzoli, P.; Bozzetti, F.; De Francesco, A. Monitoring Response to Home Parenteral Nutrition in Adult Cancer Patients. Health 2020, 8, 183, doi:10.3390/healthcare8020183.
- Kanesvaran, R.; Li, H.; Koo, K.-N.; Poon, D. Analysis of Prognostic Factors of Comprehensive Geriatric Assessment and Development of a Clinical Scoring System in Elderly Asian Patients with Cancer. J. Clin. Oncol. 2011, 29, 3620–3627, doi:10.1200/jco.2010.32.0796.
- Lagro, J.; Timmer-Bonte, J.; Maas, H.A.A.M. Predictors of Early Death Risk in Older Patients Treated with First-Line Chemotherapy for Cancer and the Importance of Geriatric Assessment. J. Clin. Oncol. 2012, 30, 4443, doi:10.1200/jco.2012.45.1310.
- Extermann, M.; Boler, I.; Reich, R.R.; Lyman, G.H.; Brown, R.H.; DeFelice, J.; Levine, R.M.; Lubiner, E.T.; Reyes, P.; Schreiber, F.J.; et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2011, 118, 3377–3386, doi:10.1002/cncr.26646.
- Ferrat, E.; Paillaud, E.; Laurent, M.; Le Thuaut, A.; Caillet, P.; Tournigand, C.; Lagrange, J.-L.; Canouï-Poitrine, F.; Bastuji-Garin, S. Predictors of 1-Year Mortality in a Prospective Cohort of Elderly Patients with Cancer. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 1148–1155, doi:10.1093/gerona/glv025.
- Drareni, K.; Bensafi, M.; Giboreau, A.; Dougkas, A. Chemotherapy-induced taste and smell changes influence food perception in cancer patients. Support. Care Cancer 2020, 1–8, doi:10.1007/s00520-020-05717-1.
- Donaldson, S.S. Nutritional consequences of radiotherapy. Cancer Res. 1977, 37, 2407–2413.
- Arends, J.J.; Baracos, V.V.; Bertz, H.H.; Bozzetti, F.; Calder, P.P.; Deutz, N.; Erickson, N.N.; Laviano, A.A.; Lisanti, M.M.; Lobo, D.N.D.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196, doi:10.1016/j.clnu.2017.06.017.
- Peñas, R.D.L.; Majem, M.; Perez-Altozano, J.; Virizuela, J.A.; Cancer, E.; Diz, P.; Donnay, O.; Hurtado, A.; Jimenez-Fonseca, P.; Ocon, M.J. SEOM clinical guidelines on nutrition in cancer patients (2018). Clin. Transl. Oncol. 2019, 21, 87–93, doi:10.1007/s12094-018-02009-3.
- Hoppe, S.; Rainfray, M.; Fonck, M.; Hoppenreys, L.; Blanc, J.-F.; Ceccaldi, J.; Mertens, C.; Blanc-Bisson, C.; Imbert, Y.; Cany, L.; et al. Functional Decline in Older Patients with Cancer Receiving First-Line Chemotherapy. J. Clin. Oncol. 2013, 31, 3877–3882, doi:10.1200/jco.2012.47.7430.
- Georlee, G.M.; Abiram, U.; Dat, P.N.; Tuan, N.K.; Mehrotra, S. Home-modification interventions addressing falls and participation in activities of daily living among older adults: A scoping review protocol. BMJ Open 2020, 10, e039742, doi:10.1136/bmjopen-2020-039742.
- Novelli, I.R.; Araújo, B.A.D.; Grandisoli, L.F.; Furtado, E.C.G.; Aguchiku, E.K.N.; Bertocco, M.C.G.; Sudbrak, T.P.; De Araújo, I.C.; Bosko, A.C.F.; Damasceno, N.R.T. Nutritional Counseling Protocol for Colorectal Cancer Patients after Surgery Improves Outcome. Nutr. Cancer 2020, 1–9, doi:10.1080/01635581.2020.1819345.
- Paleri, V.; Urbano, T.G.; Mehanna, H.; Repanos, C.; Lancaster, J.; Roques, T.; Patel, M.; Sen, M. Management of neck metastases in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S161–S169, doi:10.1017/s002221511600058x.
- Presley, C.J.; Krok-Schoen, J.L.; Wall, S.A.; Noonan, A.M.; Jones, D.C.; Folefac, E.; Williams, N.; Overcash, J.; Rosko, A.E. Implementing a multidisciplinary approach for older adults with Cancer: Geriatric oncology in practice. BMC Geriatr. 2020, 20, 1–9, doi:10.1186/s12877-020-01625-5.