
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Haiyoung Jung | + 2593 word(s) | 2593 | 2020-10-20 04:51:33 | | | |
| 2 | Vivi Li | -168 word(s) | 2425 | 2020-11-06 04:41:16 | | | | |
| 3 | Lily Guo | Meta information modification | 2425 | 2021-04-26 09:54:36 | | |
Video Upload Options
Osteoporosis is the most common chronic metabolic bone disease. It has been estimated that more than 10 million people in the United States and 200 million men and women worldwide have osteoporosis. Given that the aging population is rapidly increasing in many countries, osteoporosis could become a global challenge with an impact on the quality of life of the affected individuals. Osteoporosis can be defined as a condition characterized by low bone density and increased risk of fractures due to the deterioration of the bone architecture. Thus, the major goal of treatment is to reduce the risk for fractures. There are several treatment options, mostly medications that can control disease progression in risk groups, such as postmenopausal women and elderly men. Recent studies on the basic molecular mechanisms and clinical implications of osteoporosis have identified novel therapeutic targets. Emerging therapies targeting novel disease mechanisms could provide powerful approaches for osteoporosis management in the future. Here, we present current pharmacological options, and discuss emerging therapies targeting novel mechanisms, investigational treatments, and new promising therapeutic approaches.
1. Introduction
Osteoporosis occurs due to an imbalance between bone resorption and bone formation. As a result, bone breakdown exceeds bone formation. In 1993, the World Health Organization (WHO) defined osteoporosis as a “progressive systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture” [1][2][3]. Osteoporosis is a highly prevalent disorder estimated to affect 200 million women and men worldwide, predominantly those over the age of 60 years. [4]. Osteoporotic fracture is a major health concern that significantly impacts the quality of life of the affected individuals. According to the International Osteoporosis Foundation, worldwide, one in three women and one in five men over the age of 50 years will experience osteoporotic fractures in their lifetime. On the other hand, more than 8.9 million fractures are caused by osteoporosis annually, which means that an osteoporotic fracture occurs every three seconds. Approximately 33% of patients experience a hip fracture and in the year following the fracture, up to 20% die, mainly due to preexisting conditions [5].
Given that life expectancy is increasing globally, osteoporosis will affect the quality of life of individuals and impose an economic burden in most countries. Therefore, osteoporosis should be properly managed using effective approaches, and this can be achieved by understanding the mechanisms underlying the pathogenesis of this disease. To date, bisphosphonates (BPs), which inhibit bone resorption, are one of the most common medications for the treatment of osteoporosis.
2. Therapeutic Approach and Novel Strategies
Numerous medications and therapeutic options have been established for the treatment of osteoporosis [6]. As osteoporosis occurs as a result of an imbalance between bone resorption and bone formation, the pharmacological options for its management are anti-resorptive and anabolic agents.
2.1. Anti-Resorptive Agents
2.1.1. Bisphosphonates (BPs)
BPs come in close contact with osteoclasts and reduce bone resorption by inducing osteoclast apoptosis. BPs are stable analogs of inorganic pyrophosphate and have a core structure of P-C-P bonds, which are responsible for the strong binding affinity toward hydroxyapatite, the major mineral component of bone [7]. This binding to bone minerals enables BPs to be taken up by osteoclasts and inhibit their activity. BPs have been used for the treatment of osteoporosis since the 1990s. They are available in inexpensive generic form, in oral and intravenous formulations, and remain the first-line medications for the treatment of osteoporosis [8]. Alendronate, risedronate, and ibandronate are available as oral tablets, while zoledronic acid and ibandronate are used intravenously. BPs are approved for use in GIO patients who are at increased risk of fracture [9][10][11]. The major adverse event is the risk of atypical femoral fractures and osteonecrosis of the jaw. Gastrointestinal and renal complications have also been reported [12][13], and long-term use of BPs is also associated with a risk for osteomalacia [14]. BPs can be prescribed for less than five years and are supplemented with calcium.
2.1.2. Denosumab
Denosumab (Prolia) is a human IgG2 monoclonal antibody against RANKL that inhibits osteoclast formation, function, and survival [15][16][17]. The half-life of denosumab is approximately 26 days and it does not appear to form neutralizing antibodies [18]. Denosumab is FDA-approved for the treatment of postmenopausal osteoporosis with a high risk for fracture as well as for bone loss in men with prostate cancer receiving androgen deprivation therapy. It has also been approved for women with breast cancer, who are at risk for osteoporotic fracture. A 60 mg dose is applied subcutaneously every six months and can be supplemented with oral calcium and vitamin D. The adverse effects of denosumab are related to the fact that RANKL is also abundantly expressed by dendritic cells and activated T lymphocytes, and its antagonistic effect could affect the immune system [19]. In the previous study, it was reported that the denosumab treatment group showed skin eczema (3%) and cellulitis (0.3%) compared to the control group [20].
2.1.3. Selective Estrogen Receptor Modulators (SERMs)
Estrogen has been shown to directly regulate the survival of mature osteoclasts via the Fas/FasL system. Consistently, selective ablation of estrogen receptor alpha in the osteoclasts of women could lead to an osteoporotic bone-like phenotype. SERM or estrogen interacts with the RANKL/RANK/OPG system and decreases bone resorption. Raloxifene, representing dual agonistic and antagonistic properties in estrogenic pathways, is a first-line therapy for patients with a high risk for spine fracture.
2.2. Emerging Therapies and Investigational Agents Targeting Bone Resorption
2.2.1. Cathepsin K Inhibitors
Cathepsin K, the primary enzyme released from osteoclasts, digests collagen in bones. It is a more desirable anti-bone resorption target as it prevents osteoclast activity by inhibiting the late differentiation of osteoclasts without affecting normal bone remodeling [21]. The advantage of targeting cathepsin K rather than osteoclastogenesis is to allow continued signals to osteoblasts and consequent bone formation. Odanacatib is a selective cathepsin K inhibitor; unfortunately, Merck discontinued the development of odanacatib due to an increased risk of stroke [22].
2.2.2. Lasofoxifene
Lasofoxifene is a third-generation SERM. It is approved for osteoporosis treatment in Europe, but its approval is pending in the United States [23]. In a clinical study, the group treated with lasofoxifene at a dose of 0.5 mg per day demonstrated a 42% risk reduction for vertebral fractures and a 24% risk reduction for nonvertebral fractures. It has also been found that lasofoxifene treatment was associated with a decrease in breast cancer, coronary heart disease, and stroke occurrence [24]. Recently, the FDA granted a fast track designation to lasofoxifene for the treatment of women with estrogen receptor-positive, HER2-negative metastatic breast cancer.
2.3. Anabolic Agents
PTH and Parathyroid Hormone-Related Protein (PTHrP) Analogues
PTH and PTHrP can increase the number and activity of osteoblasts by stimulating osteoblast differentiation, as a consequence of increased bone formation. PTH is secreted by the parathyroid gland to adjust homeostasis of serum calcium and phosphate mainly in response to low blood calcium levels. Binding of PTH to osteoblasts induces RANKL expression, which increases osteoclast differentiation and function, and results in calcium release by bone resorption. PTH can also reverse the glucocorticoids-induced IGF-1 suppression in GIO.
Teriparatide, the first anabolic treatment approved for osteoporosis, is a recombinant human PTH (1–34) analogue. It is well known that continuous PTH dosing results in a catabolic effect, and conversely, intermittent intake promotes an anabolic effect on bone [25]. Stimulation of osteoblastic activity was shown by intermittent administration of teriparatide at small doses [26], mainly mediated through expression of interleukin-11, suppression of DKK-1, and activation of Wnt signaling [27]. Although therapies with teriparatide showed great improvement on BMD, it is not clear whether teriparatide could prevent fracture efficiently [28][29]. In GIO, however, teriparatide treatment was related to significantly fewer new vertebral fractures compared to alendronate treatment at 18 and 36 months [30]. However, due to its risk for osteosarcoma, the usage of teriparatide is restricted to those at a very high risk for fracture.
Abaloparatide (PTHrP1-34), the second recombinant human PTH analog, received FDA approval in 2017. It is expected to induce a stronger anabolic effect than teriparatide [31]. In a phase three clinical trial, abaloparatide reduced the incidence of new vertebral fracture by 86% and nonvertebral fracture by 43% over an 18-month period [29]. Treatment with this drug is limited to two years. Furthermore, the use of abaloparatide is more cost effective than that of teriparatide.
2.4. Emerging Therapies and Investigational Agents for Targeting Bone Formation
Anti-Sclerostin Antibodies
Sclerostin is an osteocyte/osteoclast-secreted protein that interferes with osteoblast differentiation, proliferation, and activity. It competitively binds to LRP-5/6 on osteoblasts and inhibits the Wnt/β-catenin pathway, thereby preventing osteoblast differentiation [32][33][34][35]. An anti-sclerostin antibody, romosozumab, showed a greater increase in BMD than alendronate and teriparatide in phase three clinical trials. Furthermore, it showed a 73% lower risk for new vertebral fracture at 12 months compared with placebo. In July 2017, the FDA rejected the approval of rosomozumab for osteoporosis treatment due to a higher rate of serious cardiovascular events compared with alendronate. In April 2019, romosozumab (Evenity, Amgen/UCB), a humanized monoclonal antibody, finally received FDA approval. It comes with the drug’s label noting an increased risk of myocardial infarction, stroke and cardiovascular death in clinical trials [36]. Other anti-sclerostin monoclonal antibodies, such as blosozumab and BPS804, are in the process of drug development [37].
2.5. Non-Pharmacological Fracture Prevention
2.5.1. Calcium
Calcium intake is the best option only in patients whose osteoporosis pathology is directly related to calcium shortage or patients with secondary hyperparathyroidism. Administration of calcium (800–1200 mg daily) will suppress PTH release and eventually decrease bone resorption and bone turnover. However, excessive calcium intake (more than 1500 mg total daily) is not beneficial, will be excreted, and it might be associated with an increased risk of renal stones [38][39].
2.5.2. Vitamin D
Vitamin D modulates calcium metabolism, including intestinal absorption, renal excretion, and bone resorption. All patients receiving glucocorticoid therapy should improve nutrition to decrease fracture risk, and it can be partially achieved by adequate calcium and vitamin D status (serum level of 25-hydroxyvitamin D, > 20 ng/mL; 50 nmol/L). In order to correct the deficiencies, supplements can be used at a dose of 600–800 IU vitamin D daily. Several reports have shown that active vitamin D has positive effects in increasing BMD and preventing vertebral fractures [40][41]. On the contrary, intermittent high doses of vitamin D (60,000 IU monthly or 500,000 IU annually) have been associated with an increased risk of falls and fractures. Thus, the recommended daily dose should not exceed 4000 IU of vitamin D in normal status [42][43].
2.6. Novel Targets, Novel Approach, and Experimental Materials for the Prevention of Osteoporosis
Stem Cells
Stem cell-based therapies are becoming increasingly important in the treatment of chronic and long-lasting diseases, including osteoporosis, as they could enable curative and personalized regenerative medicine approaches. Several different types of stem cells have been evaluated to modulate osteoporosis, including embryonic, induced pluripotent, and MSCs [44][45][46][47][48]. Among them, MSCs are critical candidates for bone regenerative medicine, as they have advantages over other types of stem cells clinically, including ease of harvesting, immunosuppressive outcomes, and fewer ethical concerns [49][50][51]. It has also been found that bioactive molecules, such as IGF-1, TGF-β, vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), angiogenin, and IL-6 that are derived from MSCs, can support bone regeneration to a great extent [52][53][54][55]. Furthermore, exosomes released by MSCs have been demonstrated to have a promising effect on bone remodeling and the prevention of bone loss in vivo [56][57].
MSC transplantation is feasible and its effects on bone formation have been previously described [58]. MSCs can directly repair the pathological area and also differentiate into osteoblasts, which is their endogenous role in bone formation. Bone marrow-derived MSCs with high osteogenic differentiation potential are considered to be a reliable and effective source for osteoporosis MSC therapies [59][60][61][62]. MSC transplantation has been conducted in osteoporotic animal models and humans, and it could indeed support microenvironment, promote bone regeneration and have paracrine effects. Thus, the secretome and/or exosomes from MSCs transplantation might be key to regulating osteoporosis, in addition to the cell therapy itself [63] In line with this, there are over 1000 clinical trials of MSC transplantation therapies registered with ClinicalTrials.gov (http://www.Clinicaltrials.gov/). Many cases involve bone diseases and conditions, such as osteoarthritis, osteogenesis imperfecta, rheumatoid arthritis, and osteoporosis. Research efforts will be made in the future to clarify the clinical application of MSCs in osteoporosis [64].
The molecular mechanisms of osteoporosis and therapeutic agents are summarized in Figure 1.
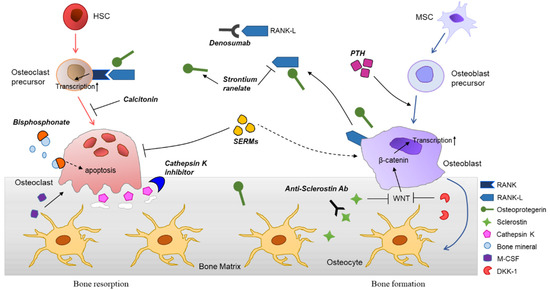
Figure 1. Differentiation of bone cells, bone remodeling process, and various therapeutic agents for osteoporosis. Hematopoietic stem cells (HSCs) are differentiated into osteoclasts, mediated through stimulation of receptor activator of nuclear factor kappaB ligand (RANKL), generated from osteoblasts. Osteoclasts can be further maturated by monocyte-colony stimulating factor (M-CSF). The bone resorption occurs by matrix metalloproteinases and cathepsin K, secreted by mature osteoclasts. Osteoblasts are derived from the mesenchymal stem cells (MSCs) and involved in bone formation. The major roles of osteoblasts in bone remodeling are activation of osteoclasts differentiation and generation of bone cells including osteocytes. Various signaling molecules, such as insulin-like growth factor 1 (IGF-1), transforming growth factor β (TGF-β), and Wnt induce osteoblast differentiation. Osteocytes are embedded in the bone matrix and orchestrate the bone remodeling. They promote bone formation by releasing osteoprotegerin together with osteoblasts, whereas suppress osteoblastogenesis by secretion of sclerostin and Dickkopf-related protein 1 (DKK-1), inhibitors of Wnt signaling. Many therapeutic agents are being developed based on the molecular biology of bone and used clinically. Anti-resorptive agents are bisphosphonates (BPs), anti-RANKL antibodies (e.g., denosumab), selective estrogen receptor modulators (SERMs), and calcitonin. Parathyroid hormone (PTH) analogues, strontium ranelate, and anti-sclerostin antibodies can be categorized as anabolic agents for osteoporosis.
3. Conclusions
Osteoporosis is an increasingly prevalent condition as the aging population grows fast globally. It causes more than 8.9 million fractures per year worldwide [65]. Not only in western countries, but also in East Asian countries, such as China, Korea, and Japan, many elderly women and men already have increased osteoporotic fracture risks. Osteoporotic fractures may lead to significant functional limitations and increased mortality. Thus, a timely diagnosis, prescription of medication, as well as the management of this disease are important. Although the optimal period of pharmacological treatment and the starting age is controversial, novel strategies and emerging therapies could provide more options for patients and clinicians. To date, BPs remain the first-line and most cost-effective medicine for osteoporosis, but there is also concern about their long-term use due to safety issues. As mentioned above, the first goal of osteoporosis treatment using pharmaceuticals is to reduce the risk for fracture. To achieve this, adequate investigations should be conducted and a proper diagnosis should be given. Since BMD alone cannot predict the risk for fracture, new cutting-edge technologies, including next-generation sequencing, genome-wide screening and assessment, and stem cell therapeutics should be considered. In conclusion, by gaining a better understanding of the molecular mechanisms underlying osteoporosis, enabling and using new emerging technologies, it is possible to achieve better outcomes in patients with osteoporosis.
References
- Genant, H.K.; Cooper, C.; Poor, G.; Reid, I.; Ehrlich, G.; Kanis, J.; Nordin, B.C.; Barrett-Connor, E.; Black, D.; Bonjour, J. Interim report and recommendations of the World Health Organization task-force for osteoporosis. Osteoporos. Int. 1999, 10, 259–264.
- Todd, J.; Robinson, R. Osteoporosis and exercise. Postgrad. Med. J. 2003, 79, 320–323.
- Bawa, S. The significance of soy protein and soy bioactive compounds in the prophylaxis and treatment of osteoporosis. J. Osteoporos. 2010, 2010.
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson--Hughes, B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone. Min. Res. 2014, 29, 2520–2526.
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56.
- Ukon, Y.; Makino, T.; Kodama, J.; Tsukazaki, H.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. Molecular-based treatment strategies for osteoporosis: A literature review. Int. J. Mol. Sci. 2019, 20, 2557.
- Russell, R.G.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19.
- Crandall, C.J.; Newberry, S.J.; Diamant, A.; Lim, Y.-W.; Gellad, W.F.; Booth, M.J.; Motala, A.; Shekelle, P.G. Comparative effectiveness of pharmacologic treatments to prevent fractures: An updated systematic review. Ann. Int. Med. 2014, 161, 711–723.
- Saag, K.G.; Emkey, R.; Schnitzer, T.J.; Brown, J.P.; Hawkins, F.; Goemaere, S.; Thamsborg, G.; Liberman, U.A.; Delmas, P.D.; Malice, M.-P. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N. Eng. J. Med. 1998, 339, 292–299.
- Reid, D.M.; Hughes, R.A.; Laan, R.F.; Sacco--Gibson, N.A.; Wenderoth, D.H.; Adami, S.; Eusebio, R.A.; Devogelaer, J.P. Efficacy and safety of daily risedronate in the treatment of corticosteroid--induced osteoporosis in men and women: A randomized trial. J. Bone Min. Res. 2000, 15, 1006–1013.
- Reid, D.M.; Devogelaer, J.-P.; Saag, K.; Roux, C.; Lau, C.-S.; Reginster, J.-Y.; Papanastasiou, P.; Ferreira, A.; Hartl, F.; Fashola, T. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): A multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2009, 373, 1253–1263.
- Zhou, J.; Ma, X.; Wang, T.; Zhai, S. Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: A systematic review with network meta-analyses. Osteoporos. Int. 2016, 27, 3289–3300.
- Zhou, J.; Wang, T.; Zhao, X.; Miller, D.R.; Zhai, S. Comparative efficacy of bisphosphonates to prevent fracture in men with osteoporosis: A systematic review with network meta-analyses. Rheumatol. Ther. 2016, 3, 117–128.
- Hoppé, E.; Masson, C.; Laffitte, A.; Chappard, D.; Audran, M. Osteomalacia in a patient with Paget’s bone disease treated with long-term etidronate. Morphologie 2012, 96, 40–43.
- Lin, T.; Wang, C.; Cai, X.Z.; Zhao, X.; Shi, M.M.; Ying, Z.M.; Yuan, F.Z.; Guo, C.; Yan, S.G. Comparison of clinical efficacy and safety between denosumab and alendronate in postmenopausal women with osteoporosis: A meta--analysis. Int. J. Clin. Pr. 2012, 66, 399–408.
- Von Keyserlingk, C.; Hopkins, R.; Anastasilakis, A.; Toulis, K.; Goeree, R.; Tarride, J.-E.; Xie, F. Clinical efficacy and safety of denosumab in postmenopausal women with low bone mineral density and osteoporosis: A meta-analysis. Semin. Arthritis Rheum 2011, 41, 178–186.
- Cummings, S.R.; Martin, J.S.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Eng. J. Med. 2009, 361, 756–765.
- Hanley, D.; Adachi, J.; Bell, A.; Brown, V. Denosumab: Mechanism of action and clinical outcomes. Int. J. Clin. Pr. 2012, 66, 1139–1146.
- Walsh, M.C.; Choi, Y. Biology of the RANKL–RANK–OPG system in immunity, bone, and beyond. Front. Immunol. 2014, 5, 511.
- Anastasilakis, A.D.; Toulis, K.A.; Polyzos, S.A.; Anastasilakis, C.D.; Makras, P. Long-term treatment of osteoporosis: Safety and efficacy appraisal of denosumab. Clin. Risk Manag. 2012, 8, 295–306.
- Martin, T.J.; Sims, N.A. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends. Mol. Med. 2005, 11, 76–81.
- Mullard, A. Merck & Co. drops osteoporosis drug odanacatib. Nat. Rev. Drug Discov. 2016, 15, 669–670.
- Schmidt, C. Third-generation SERMs may face uphill battle. J. Natl. Cancer Inst. 2010, 102, 1690–1692.
- Cummings, S.R.; Ensrud, K.; Delmas, P.D.; LaCroix, A.Z.; Vukicevic, S.; Reid, D.M.; Goldstein, S.; Sriram, U.; Lee, A.; Thompson, J. Lasofoxifene in postmenopausal women with osteoporosis. N. Eng. J. Med. 2010, 362, 686–696.
- Hock, J.; Gera, I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J. Bone Min. Res. 1992, 7, 65–72.
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.-Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Eng. J. Med. 2001, 344, 1434–1441.
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast differentiation at a glance. Med. Sci. Monit. 2016, 22, 95–106.
- Reid, I.R. Short-term and long-term effects of osteoporosis therapies. Nat. Rev. Endocrinol. 2015, 11, 418–428.
- Miller, P.D.; Hattersley, G.; Riis, B.J.; Williams, G.C.; Lau, E.; Russo, L.A.; Alexandersen, P.; Zerbini, C.A.; Hu, M.-y.; Harris, A.G. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: A randomized clinical trial. JAMA 2016, 316, 722–733.
- Saag, K.G.; Zanchetta, J.R.; Devogelaer, J.P.; Adler, R.A.; Eastell, R.; See, K.; Krege, J.H.; Krohn, K.; Warner, M.R. Effects of teriparatide versus alendronate for treating glucocorticoid--induced osteoporosis: Thirty--six–month results of a randomized, double--blind, controlled trial. Arthritis Rheum. 2009, 60, 3346–3355.
- Hattersley, G.; Dean, T.; Corbin, B.A.; Bahar, H.; Gardella, T.J. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology 2016, 157, 141–149.
- Ott, S.M. Sclerostin and Wnt signaling—the pathway to bone strength. J. Clin. Endocrinol. Metab. 2005, 90, 6741–6743.
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A. Romosozumab treatment in postmenopausal women with osteoporosis. N. Eng. J. Med. 2016, 375, 1532–1543.
- Langdahl, B.L.; Libanati, C.; Crittenden, D.B.; Bolognese, M.A.; Brown, J.P.; Daizadeh, N.S.; Dokoupilova, E.; Engelke, K.; Finkelstein, J.S.; Genant, H.K. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: A randomised, open-label, phase 3 trial. Lancet 2017, 390, 1585–1594.
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N. Eng. J. Med. 2017, 377, 1417–1427.
- Mullard, A. FDA approves first-in-class osteoporosis drug. Nat. Rev. Drug Discov. 2019, 18, 411–412.
- MacNabb, C.; Patton, D.; Hayes, J. Sclerostin antibody therapy for the treatment of osteoporosis: Clinical prospects and challenges. J. Osteoporos. 2016, 2016, 6217286.
- Jackson, R.D.; LaCroix, A.Z.; Gass, M.; Wallace, R.B.; Robbins, J.; Lewis, C.E.; Bassford, T.; Beresford, S.A.; Black, H.R.; Blanchette, P. Calcium plus vitamin D supplementation and the risk of fractures. N. Eng. J. Med. 2006, 354, 669–683.
- Bolland, M.J.; Grey, A.; Avenell, A.; Gamble, G.D.; Reid, I.R. Calcium supplements with or without vitamin D and risk of cardiovascular events: Reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 2011, 342, d2040.
- O’Donnell, S.; Moher, D.; Thomas, K.; Hanley, D.A.; Cranney, A. Systematic review of the benefits and harms of calcitriol and alfacalcidol for fractures and falls. J. Bone Min. Res. 2008, 26, 531–542.
- Papadimitropoulos, E.; Wells, G.; Shea, B.; Gillespie, W.; Weaver, B.; Zytaruk, N.; Cranney, A.; Adachi, J.; Tugwell, P.; Josse, R. Meta-analyses of therapies for postmenopausal osteoporosis. VIII: Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr. Rev. 2002, 23, 560–569.
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010, 303, 1815–1822.
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Orav, E.J.; Staehelin, H.B.; Meyer, O.W.; Theiler, R.; Dick, W.; Willett, W.C.; Egli, A. Monthly high-dose vitamin D treatment for the prevention of functional decline: A randomized clinical trial. JAMA Intern. Med. 2016, 176, 175–183.
- Li, F.; Zhou, C.; Xu, L.; Tao, S.; Zhao, J.; Gu, Q. Effect of stem cell therapy on bone mineral density: A meta-analysis of preclinical studies in animal models of osteoporosis. PLoS ONE 2016, 11, e0149400.
- Ilic, D.; Miere, C.; Lazic, E. Umbilical cord blood stem cells: Clinical trials in non-hematological disorders. Br. Med. Bull. 2012, 102, 43–57.
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018, 2495848.
- Paspaliaris, V.; Kolios, G. Stem cells in osteoporosis: From biology to new therapeutic approaches. Stem Cells Int. 2019, 2019, 1730978.
- Goodarzi, P.; Payab, M.; Alavi-Moghadam, S.; Larijani, B.; Rahim, F.; Bana, N.; Sarvari, M.; Adibi, H.; Heravani, N.F.; Hadavandkhani, M. Development and validation of Alzheimer’s disease animal model for the purpose of regenerative medicine. Cell Tissue Bank. 2019, 20, 141–151.
- Aghebati--Maleki, L.; Dolati, S.; Zandi, R.; Fotouhi, A.; Ahmadi, M.; Aghebati, A.; Nouri, M.; Kazem Shakouri, S.; Yousefi, M. Prospect of mesenchymal stem cells in therapy of osteoporosis: A review. J. Cell. Physiol. 2019, 234, 8570–8578.
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.-Y. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013, 19, 35–42.
- Su, P.; Tian, Y.; Yang, C.; Ma, X.; Wang, X.; Pei, J.; Qian, A. Mesenchymal stem cell migration during bone formation and bone diseases therapy. Int. J. Mol. Sci. 2018, 19, 2343.
- Xiao, L.; Sobue, T.; Esliger, A.; Kronenberg, M.S.; Coffin, J.D.; Doetschman, T.; Hurley, M.M. Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone 2010, 47, 360–370.
- Ugarte, F.; Ryser, M.; Thieme, S.; Fierro, F.A.; Navratiel, K.; Bornhäuser, M.; Brenner, S. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp. Hematol. 2009, 37, 867–875.
- Byun, M.R.; Jeong, H.; Bae, S.J.; Kim, A.R.; Hwang, E.S.; Hong, J.-H. TAZ is required for the osteogenic and anti-adipogenic activities of kaempferol. Bone 2012, 50, 364–372.
- James, A.W. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica 2013, 2013, 684736.
- Chu, C.; Wei, S.; Wang, Y.; Wang, Y.; Man, Y.; Qu, Y. Extracellular vesicle and mesenchymal stem cells in bone regeneration: Recent progress and perspectives. J. Biomed. Mater. Res. Part A 2019, 107, 243–250.
- Behera, J.; Tyagi, N. Exosomes: Mediators of bone diseases, protection, and therapeutics potential. Oncoscience 2018, 5, 181.
- Ye, X.; Zhang, P.; Xue, S.; Xu, Y.; Tan, J.; Liu, G. Adipose-derived stem cells alleviate osteoporosis by enchancing osteogenesis and inhibiting adipogenesis in a rabbit model. Cytotherapy 2014, 16, 1643–1655.
- Chen, H.T.; Lee, M.J.; Chen, C.H.; Chuang, S.C.; Chang, L.F.; Ho, M.L.; Hung, S.H.; Fu, Y.C.; Wang, Y.H.; Wang, H.I. Proliferation and differentiation potential of human adipose--derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J. Cell. Mol. Med. 2012, 16, 582–592.
- Liu, H.-Y.; Chiou, J.-F.; Wu, A.T.; Tsai, C.-Y.; Leu, J.-D.; Ting, L.-L.; Wang, M.-F.; Chen, H.-Y.; Lin, C.-T.; Williams, D.F. The effect of diminished osteogenic signals on reduced osteoporosis recovery in aged mice and the potential therapeutic use of adipose-derived stem cells. Biomaterials 2012, 33, 6105–6112.
- Phetfong, J.; Sanvoranart, T.; Nartprayut, K.; Nimsanor, N.; Seenprachawong, K.; Prachayasittikul, V.; Supokawej, A. Osteoporosis: The current status of mesenchymal stem cell-based therapy. Cell. Mol. Biol. Lett. 2016, 21, 1–20.
- Oryan, A.; Kamali, A.; Moshiri, A.; Eslaminejad, M.B. Role of mesenchymal stem cells in bone regenerative medicine: What is the evidence? Cells Tissues Organs 2017, 204, 59–83.
- Nimiritsky, P.P.; Eremichev, R.Y.; Alexandrushkina, N.A.; Efimenko, A.Y.; Tkachuk, V.A.; Makarevich, P.I. Unveiling mesenchymal stromal cells’ organizing function in regeneration. Int. J. Mol. Sci. 2019, 20, 823.
- Arjmand, B.; Sarvari, M.; Alavi-Moghadam, S.; Payab, M.; Goodarzi, P.; Gilany, K.; Mehrdad, N.; Larijani, B. Prospect of Stem Cell Therapy and Regenerative Medicine in Osteoporosis. Front. Endocrinol. 2020, 11, 430.
- Johnell, O.; Kanis, J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733.





