Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nada Oršolić | -- | 8304 | 2022-10-13 11:14:04 | | | |
| 2 | Sirius Huang | Meta information modification | 8304 | 2022-10-14 06:07:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Oršolić, N. Development of Allergic Inflammation. Encyclopedia. Available online: https://encyclopedia.pub/entry/29116 (accessed on 07 February 2026).
Oršolić N. Development of Allergic Inflammation. Encyclopedia. Available at: https://encyclopedia.pub/entry/29116. Accessed February 07, 2026.
Oršolić, Nada. "Development of Allergic Inflammation" Encyclopedia, https://encyclopedia.pub/entry/29116 (accessed February 07, 2026).
Oršolić, N. (2022, October 13). Development of Allergic Inflammation. In Encyclopedia. https://encyclopedia.pub/entry/29116
Oršolić, Nada. "Development of Allergic Inflammation." Encyclopedia. Web. 13 October, 2022.
Copy Citation
Inflammation is an adaptive response to stimuli and conditions, such as infection, tissue damage, and cellular stress or malfunction. Regardless of the cause, inflammation likely evolved as an adaptive response to maintaining homeostasis. Many pathological and clinical burdens of allergic disease reflect the long-term consequences of chronic allergic inflammation at sites of persistent or repetitive exposure to allergens.
allergy
inflammatory cells
anti-allergic
anti-inflammatory
1. Introduction
Allergy is an overreaction of the immune system to normally harmless substances and is accompanied by an inflammatory reaction. Allergic inflammation consists of three phases. Early-stage reactions (or type I immediate hypersensitivity reactions) are induced within seconds to minutes after allergen induction, and late-stage reactions occur within a few hours. In contrast, chronic allergic inflammation is a permanent inflammation that occurs at sites of repeated exposure to allergens [1].
IgE immunoglobulins are allergen-specific and are produced by B-cells after the sensitization process. Cytokines, such as interleukin (IL)-4 and IL-13, are crucial in the transition of B-cells from IgG to IgE production. By binding to high-affinity IgE receptors (FcεRI), IgE immunoglobulins trigger the release of mediators from mast cells. Sensitized individuals already have allergen-specific IgE bound to surface IgE receptors on mast cells. The main receptors involved in mast cell activation are: FcεRI, Toll-like receptors, complement receptors (CR1–5) and IgG receptors FcγRI (CD64) and FcγRII (CD32) [2]. Cross-linking of adjacent IgE molecules by bivalent or multivalent allergen, FcεRI aggregation initiates a complex intracellular signaling process that results in the secretion of key biologically active products: (1) mediators stored in primary cytoplasmic granules; (2) mediators formed from lipids and newly synthesized cytokines, chemokines and growth factors, as well as other products. Mediators contribute to an allergic reaction, known as an “immediate hypersensitivity reaction”. Certain genes are involved in the specific immune response (i.e., HLA-D, TCR, CD14, toll-like receptor, STAT6) and Th1/Th2 cell differentiation; others are genes encoding (total) IgE response or IgE receptor functions (i.e., IL-4, IL-4R, FcεRIβ, Fc epsilon RI) and genes involved in the inflammatory process (TNF-γ, IFN-γ, IL-3) [1][2][3].
Two phase reactions trigger the IgE/allergen cross-linking event in acute and chronic inflammation. The first process depends on calcium as the most important trigger, but also the main switch of the cascade allergic reaction and is based on the release of previously formed mediators such as histamine, interleukins, serotonin and Hagemann’s factor from mast cells and basophils (Figure 1). The released chemical mediators are responsible for allergic (inflammatory) processes, such as vasodilation, increased vascular permeability and increased chemotaxis of other inflammatory cells (Figure 1). The second phase of the reaction begins with the synthesis of mediators derived from lipids through the conversion of phospholipids into arachidonic acid by phospholipase A2, followed by the conversion of arachidonic acid into leukotrienes (LT), platelet activation factor (PAF) and prostaglandins [1][2][3]. The inflammatory cascade continues with the synthesis of LTB4 and LTC4 (with their metabolic derivatives LTD4 and LTE4), prostaglandin D2 and other substances that stimulate by acting on vascular smooth muscles, connective tissue, mucous glands and inflammatory cells. Mast cells further increase inflammation through the production of numerous cytokines including TNF-β, IL-4, IL-5, IL-13 [1][4]. Their number is increased in the late phase of inflammation, where mast cells attract leukocytes, especially Th2 cells and eosinophils, by induction and regulation of adhesion molecules on the endothelial cells of blood vessels. Cytokines (IL-4, IL-5 and IL-13) are also secreted by memory Th cells of the Th2 type reactivated by allergens that are presented by local antigen-presenting cells (APC) in the airways, stimulating an inflammatory reaction. Symptoms characteristic of allergic rhinitis are the result of previously formed lipid mediators on the mucous membrane of the upper respiratory tract, and not a property of the allergen itself. Certain patients react to certain allergens, while others do not; reaction to allergens depends on a number of factors including genetics, geography and exposure levels [1][2][3][4].
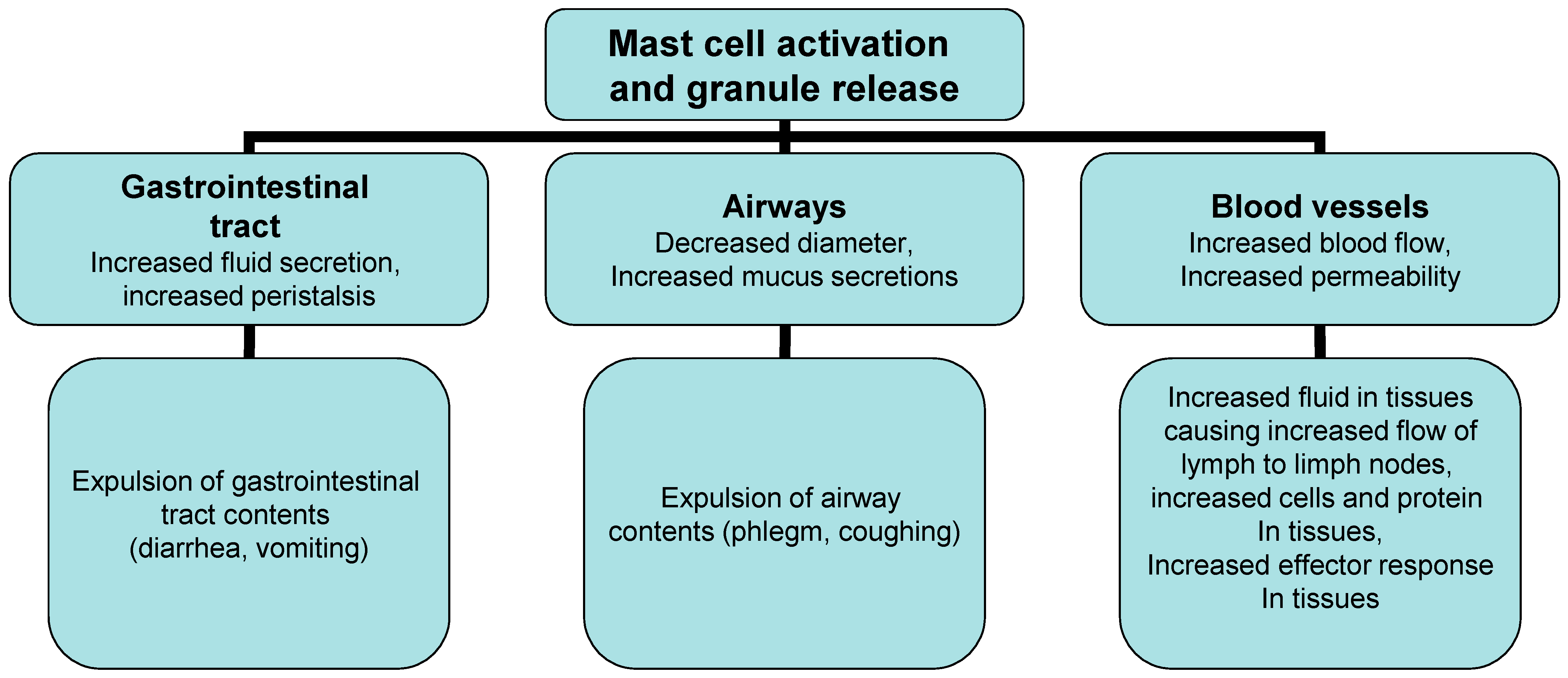
Figure 1. Effector mechanism in allergic reaction.
The late phase of the reaction showing clinical features of inflammation after 6–12 h is based on an increase in the arrival of inflammatory cells in the tissue, especially eosinophils, neutrophils, basophils, macrophages and lymphocytes. The key task of cytokines secreted from mast cells is to: (1) recruit other immune cells either directly or indirectly (TNF-α,LTB4, IL-8 CCL2 and other chemokines); (2) activate innate immune cells (TNF-α and IL-5); (3) affect many aspects of DC biology, T cells and B cells (IL-10, TNF-α, TGF-β, histamine); (4) stimulate the reaction of Th2 cells (IL-4 and IL-13), (5) promote the inflammatory reaction (TNF-α, and IL-3), and (6) stimulate the production and activation of eosinophils (IL-5 and GM-CSF) [1][2][3][4]. Certain mast-cell-derived products can also influence the biology of structural cells, including vascular endothelial cells, epithelial cells, fibroblasts, smooth muscle cells and nerve cells while other molecules, such as IL-10, TGF-β, can have anti-inflammatory or immuno-suppressive functions. Leukocytes recruited in late-phase reactions consist of Th2 cells in the early phases of the response and Th1 cells in the late phases; they release cytokines that attract and activate granulocytes, monocytes and eosinophils, contributing to the inflammatory reaction and damage to the surrounding tissue, especially after the reaction of eosinophils with IgE and their degranulation.
Chronic allergic inflammation occurs when exposure to allergens is constant or repeated and this persistent inflammation is associated with changes in the structural cells at the affected sites, and in many cases with markedly altered function of the affected organs. Thus, it is known that chronic allergic inflammation in atopic dermatitis (AD), allergic rhinitis, and asthma is associated with tissue remodeling, which includes long-term changes to the structural elements of the affected sites (such as increased vascularity) and substantial alterations in the barrier function of the affected epithelia including increased risk of both cutaneous infections with the bacterium such as Staphylococcus aureus and the colonization or the development of nasal polyps in patients with allergic rhinitis. Chronic inflammation is based on a complex interaction of many cells including mast cells, T cells, eosinophils, basophils, neutrophils, monocytes/macrophages, platelets, NK cells (natural killer T-cells), as well as a number of cytokines, including IL-4, IL-5, IL-12, IL-13, IL-15, IL-25 and IL-33 [1][5][6].
2. Cells and Their Mediators Involved in the Inflammatory Process
The inflammatory process begins with the release of various mediators from the resident cells, including granulocytes (neutrophils, mast cells, eosinophils), lymphocytes, macrophages, DCs, platelets, interstitial fibroblasts, and vascular endothelial cells. This signaling changes the local adhesion molecules’ profile and creates a chemotactic gradient that recruits cells from the blood stream to the inflammatory site.
2.1. Neutrophils
Neutrophils, the main cell type in most acute and some chronic inflammatory diseases, are the body’s first line of defense in the innate arm of the immune system. Neutrophils dominate the early stages of inflammation as cells with antimicrobial functions and cells which can interact, directly, or via cytokines and chemokines, with other immune cells to modulate both innate and adaptive immune responses. Their granules contain numerous microbicidal agents; they can destroy invading microorganisms, through phagocytosis and intracellular degradation, release of granules, and formation of neutrophil extracellular traps after detecting pathogens. Several studies have shown that neutrophils can regulate the induction of allergic inflammation through their innate responses. Neutrophils are a major source of oxidative stress through their respiratory chain; they are the main source of superoxide anions (O2−), H2O2 and HOCL which, together with neutrophil proteases, increase the degree of tissue damage. In addition, neutrophils may contribute to allergic inflammation by increasing microvascular permeability, inducing proinflammatory cytokines, elastase, cathepsin G, and proteinase-3, IL-8, lactoferrin, myeloperoxidase (MPO), matrix metalloproteinase 9 (MMP-9), and secreted polymeric mucins MUC5AC [1][2][3][4][5][6]. It has been shown that neutrophil elastase causes MUC5AC mucin synthesis via the EGF receptor, ERK and NF-kB pathways in A549 cells [6]. Activated neutrophils have a role in the allergic sensitization process and thus, may contribute to allergic inflammation in allergic rhinitis and asthma by priming T cells and attracting eosinophils.
2.2. Monocytes/Macrophages
Monocytes/macrophages are a heterogeneous cell population acting as a bridge between the innate and adaptive immune systems. Macrophages and neutrophils migrate into extravascular tissue by adhesion and diapedesi in the early acute phase of inflammation. Macrophages exert a variety of pro- and anti-inflammatory functions that are correlated with various states of immune activation and are broadly classified into M1- or M2-related classes. Macrophage plasticity is influenced by environmental factors; M1 polarization is characterized by the expression of Th1 and Th17 cells, while in M2 polarization the Th2 response is dominant. M1 macrophages highly express CD80, CD86, MHCII, TLR4, and iNOS, and produce high levels of pro-inflammatory Th1 cytokines (e.g., IL-6, IL-12, IL-1β, and TNF-α) and chemokines (e.g., CCL2, CCL5, MCP-1, MCP-2, IL-8) while M2 macrophages are characterized by high expression of MRC1, CD163, Arg-1 and low expression of iNOS, MHCII and CD86. M2 is generally assumed to play significant roles in wound healing, tissue remodeling, parasite clearance, and resolution of excessive inflammation.
These important immune effector and homeostatic macrophage functions are altered during allergic reactions, and activated macrophages can contribute to the propagation of the inflammatory process by producing a number of inflammatory cytokines, chemokines, complement cascade proteins (C1, C2,C3, and C4), and eicosanoids [e.g., prostaglandin (PG)E2, thromboxane (TX)A2, prostacyclin (PGI2), LTB4, and PAF], procoagulant factors such as tissue factor (TF) and plasmin inhibitor (or alpha 2-antiplasmin). Macrophages are characterized by the presence of numerous receptors on their surface, such as scavenger receptors, TLR, opsonin receptors, including the mannose receptor for recognizing glycoproteins on the surface of microbes, CR1, CR3, CR4, Fcγ RI, Fcγ RII, and Fcγ RIII [1][4][7].
2.3. Eosinophils
Eosinophils play a key role in the symptoms of asthma, allergies, and atopic and adverse drug reactions. Eosinophils can regulate local immune and inflammatory responses, and their accumulation in the blood and tissue is associated with several inflammatory and infectious diseases [8][9]. When activated, eosinophils rapidly produce and secrete four distinct granule cationic proteins: major basic protein, eosinophil peroxidase, eosinophil cationic protein and eosinophil-derived neurotoxin, and inflammatory mediators including GM-CSF, and RANTES, which exert an autocrine effect on eosinophil survival, differentiation, and accumulation. In addition, eosinophils secrete a range of highly toxic granule proteins and other mediators of inflammation presented in Table 1. Eosinophils play an essential role in the development and maintenance of allergic diseases through the secretion of lipid mediators and proteins and may be an important target for treatment and management of allergic diseases. Eosinophils have an important role in late-phase allergic reactions, where they are recruited in high numbers. Recruitment of eosinophils is a complex and dynamic process involving cell adhesion and attraction, diapedesis, and chemotaxis by cytokines such as IL-5 and eotaxin which can regulate eosinophil homing and tissue accumulation in allergic asthma [8][9]. Eosinophils express seven integrin heterodimers [10]: α4β1 (CD49d/29), α6β1 (CD49f/29), αMβ2 (CD11b/18), Lβ2 (CD11a/18), αXβ2 (CD11c/18), αDβ2 (CD11d/18), and α4β7 (CD49d/β7). Rapid apoptosis of eosinophils in the absence of their growth factor IL-5 was observed in the tissues. The IL-5 production by Th2 cell located in the airway mucosa stimulates the migration of eosinophils from the blood and increases their survival by inhibiting apoptosis. The main biological effects of mature eosinophils are considered to occur in tissues, where they may release active mediators upon activation, including proinflammatory cytokines, arachidonic acid-derived mediators, enzymes and reactive oxygen species (ROS) (Table 4). The release of toxic substances by activated eosinophils may contribute to the pathophysiology of eosinophilic disorders, including allergic asthma and rhinitis. Eosinophils also express a number of Ig receptors, including receptors for IgE that are involved in activation and release of prestored toxic granule proteins involved in the destruction of the epithelial cell layer that is characteristic for allergic asthma. In autoimmune diseases, eosinophils have a number of functions, including: (1) tissue damage by cytotoxic granule proteins; (2) antibody-dependent cellular cytotoxicity; (3) activation of tissue remodeling and fibrosis; (4) antigen presentation; (5) modulation of the adaptive immune response; (6) promotion of B cell responses; and (7) induction of tissue repair processes. Eosinophils can induce a protective immune response against helminths, viral and bacterial pathogens. Eosinophils are a source of anti-tumorigenic (e.g., TNF-α, granzyme, cationic proteins, and IL-18) and protumorgenic molecules (e.g., pro-angiogenic factors) depending on the milieu.
Table 1. Eosinophils secrete a range of highly toxic granule proteins and other mediators of inflammation that are released by eosinophils.
| Class of Product | Mediators | Biological Effect |
|---|---|---|
| Enzyme | Eosinophil peroxidase | Toxic to target by catalysing halogenation. Triggers histamine release from mast cells |
| Eosinophil collagenase | Remodeling of conective tissue matrix | |
| Lizosim | Higly toxic granule proteins which can kill microorganisms and parasites | |
| Acid phosfatase | ||
| Arilsulfatase B | ||
| Catalase | ||
| Enoil-CoA hidratase | ||
| 3-Ketoacyl-CoA tiolase | ||
| β-glucoronidase | ||
| Elastase | ||
| Katepsin D | ||
| Toxic proteins | Major basic protein | Toxic to parasites and mammalian cells. Triggers histamine release from mast cell, Stimulation of neutrophils, Mast cells, and basophils, Respiratory epithelial desquamation, M2 receptor dysfunction |
| Eosinophil cationic protein | Toxic to parasites. Neurotoxin, bronchial hyperresponsiveness, leads to bronchoconstriction, respiratory epithelial desquamation, cell and parasite toxicity, Generation of radical species, stimulation of mast cells | |
| Eosinophil-derived neurotoxin | Neurotoxin | |
| Cytokine | IL-3, IL-5, IL-9, GM-CSF, IFN-γ, TNF-α, IL-2, IL-13, IL-16, IL-17, IL-4, IL-6, IL-8 |
Sustained inflammation, amplify eosinophil production by bone marrow. Cause eosinophil activation eosinophil migration, development, and survival |
| Chemokine | CCL2, CCL3, CCL5 (RANTES), CCL-7, CCL8, CCL11, CCL13, CXCL1, CXCL10, CXCL12, and IL-8 | Promote influx of leukocytes Migration of monocytes, macrophages, neutrophils, T cells, and eosinophils Increase adhesion molecule expression, airway wall remodeling Increase eosinophil survival, increased adhesion molecules expression, airway wall remodeling |
| eotaxin | Cause ckemotaxis for eosinophils | |
| Lipid mediators | Leukotrienes (LTC4, LTD4, LTB4), tromboxan A2 (TXA2), prostagladins (PGE2, PGG2, PGF2, PGI2), Cysteinyl leukotrienes, | Smooth muscle contraction, increased vascular permeability, mucus secretion Increased mucus secretion, increased vascular permeability, activation of eosinophils, mast cells, basophils, neutrophils, and platelets, smooth muscle cell contraction Increased adhesion molecules expression Chemotaxis of eosinophil and neutrophil |
| 5-HETE; 12-HETE; 15-HETE (monohydroxyeicosatetraenoic acids); 5,15-diHETE; 8,15-diHETE; 14,12-diHETE | Play a vital role in inflammation by controlling the intesity and duration of pain, fever, swelling, and heat of an affected area | |
| Lipoxin A4 and C4 (LXA4, LXC4) | Inhibits PMN adherence, migration, degranulation, and superoxide anion generation, as well as eosinophil migration and lymphocyte activation, and has potent actions on cytokine formation and release. Blocks P-selectin mobilization induced by peptidoleukotrienes and attenuated P-selectin-mediated PMN-endothelial cell adhesion. | |
| 13-hydroxilinoleic acid (13HODE) | ||
| Platelet-activating factor (PAF) | Chemotactic to leukocytes. Amplifies production of lipid mediators, neutrophil, eosinophil, and platelet activation. | |
| Growth factor of eosinophils | Tumor necrosis factor α and β (TNF-α, TNF-β); Platelet-derived growth factor (PDGF); Vascular endothelial growth factor (VEGF); Heparin-binding epidermal growth factor (HB-EGF); Nerve growth factor (NGF); Endothelin (ET); Corticotropin releasing factor (SCF); A proliferation-inducing ligand (APRIL) | Control of maturation and differentiation of eosinophils |
2.4. Basophils
Basophils, the least abundant granulocyte population (less than 1%) of all circulating leukocytes, are initiators, regulators and effectors of type 2 inflammation. Type 2 inflammation, a specific type of immune response, which includes Th2 cells that secrete IL-4, IL-5, and IL-13 and stimulate type 2 immunity, which is characterized by high levels of immunoglobulin E (IgE) and eosinophils. In addition to the accumulation of eosinophils, there is an accumulation of other cells such as basophils, mast cells, Th2 cells, type 2 innate lymphoid cells (ILC2s) and IgE-producing B cells and their cytokines. An excessive response of eosinophils within the airways leads to airway remodeling and causes smooth muscle cell changes (hyperplasia and hypertrophy), mucus cell changes (hyperplasia, metaplasia) and vascular remodeling (more blood vessels and more leaky vessels). Such changes create a predisposition of the mucous membrane of the respiratory tract for an exaggerated response to inhaled allergens or environmental stimuli, such as viruses, cigarette smoke or other air pollutants. Murine basophils are characterized by the presence of surface markers (FcεRI+, CD49b+, CD69+, Thy-1.2+, CD123+, CD200R+, CD117−, CD19−, CD14−, CD122−, CD11c−, Gr-1−, NK1.1−, B220−, CD3−, γδTCR−, αβTCR−) 2, 8, 9, 11, 12 that can also be found in humans. Basophils can be activated by a variety of signals, including cytokines such as IL-3, IL-5, IL-33, IL-18, and GM-CSFs, antibodies (IgE, IgD, and IgG), allergens, proteases, TLRs ligands, and complement factors (C5a) (Table 2) [1][11]. Numerous TLRs receptors are present on human basophils such as TLR1, TLR2, TLR4, TLR6 and TLR965–67 while murine basophils express TLR1, TLR2, TLR4 and TLR668. In addition to TLRs, human basophils have also been shown to express the complement receptors CR1, CR3, CR4 and CD88. Substances that lead to degranulation of mast cells/basophils are: (1) At/Ag interactions (IgE, IgG); (2) complements (C3a, C4a, C5a); (3) HRF (histamine release factor). When activated, basophils release mediators (Table 2) such as histamine, leukotriene (LT)C4, platelet-activating factor (PAF), all major mediators of acute bronchoconstriction, chemokines, and cytokines (IL-4, IL-5, IL-13, and thymic stromal lymphopoietin). In addition, basophils play an important role in allergic reactions, through B-cell activation, by increasing the production of IL-4 and B-cell-activating factor and inducing production of immunoglobulins IgM, IgG, and IgA, mediated by IgD. Basophils are involved in both IgE-dependent and IgE-independent allergic inflammation. In IgE-dependent allergic inflammation, basophils are activated by antigen and IgE stimulation, causing degranulation and secretion of cytokines. Basophil-derived mediators induce the recruitment of other inflammatory cells, leading to the chronic allergic inflammation in antigen-challenged sites. IgE-independent allergic inflammation mediated by basophils is mostly associated with thymic stromal lymphopoietin (TSLP). TSLP-elicited basophils are activated by cytokine stimulation, such as IL-3, IL-18, and IL-33, leading to the secretion of IL-4 [5][11]. Basophil-derived IL-4 induces the recruitment of inflammatory cells, including eosinophils, to the site of inflammation. In addition, basophils play a major role in the regulation of monocytes and macrophages in M1 or M2 macrophages under the influence of environmental factors [11]. Thus, in the early phase of inflammation, basophil-derived IL-4 acts on skin-resident cells, including fibroblasts, endothelial cells, and ILCs, leading to the accumulation of eosinophils to the site of inflammation while in the later phase of inflammation, basophil-derived IL-4 acts on inflammatory monocytes, leading to the differentiation into M2 macrophages. M2 macrophages promote either the resolution of allergic inflammation or protective immunity against helminthic parasites [11]. Finally, basophils have been shown to act as antigen-presenting cells for TH2 differentiation in response to protease allergens and may act independently of or cooperate with DCs and other professional APC populations [11].
Table 2. Mediators of basophils.
| Class of Product | Mediators | Examples of Function |
|---|---|---|
| Granule-associated | Histamine and serotonin | Alter vascular permeability, smooth-muscle contraction |
| Heparin and/or chondroitin sulphate peptidoglycans | Enhance chemokine and/or cytokine function and angiogenesis | |
| Neutral proteases Tryptase, chymase, carboxypeptidase A, cathepsin G, elastase, and other proteases |
Remodel tissue and recruit effector cells | |
| Growth factor | VEGF and FGF2, SCF | Recruit effector cells and enhance angiogenesis |
| Enzymes | Matrixmetalloproteinases 9 (MMP9), β-hexosaminidases and heparanases, plasminogen activator, renin, β-glucuronidase Arylsulfatase |
Tissue damage/remodelling |
| Lipid-derived | Prostaglandin D2 (PGE2) Leukotriene C4, D4, E4 (LTC4, LTD4,LTE4) Platelet activating factor |
Vasodilation, bronchoconstriction, neutrophil chemotaxis Prolong bronchoconstriction, mucus secretion, increase vascular permeability, Bronchoconstriction, increase vascular permeability, chemotaxis of leukocytes |
| Cytokine | IL3, TNF-α, MIP-α IL-4, IL-6, IL-8, IL-13, IL-18, IL-33, Thymic stromal lymphopoietin (TSLP) IL-5 |
Promotes mast cell proliferation Inflammation Th2 differentiation Basophils regulation, IgE-independent disorders Promotes eosinophil production and activation |
| IL-3, IL-4, IL-5, IL-9, IL-13, IL-15 and IL-16 | Functions of T helper 2-type cytokines | |
| IL-12 and IFN-γ | Functions of T helper 1-type cytokines | |
| IL-10, TGF-ß and VEGF | Regulate inflammation and angiogenesis | |
| Chemokine | MIP-1α (CCL3), MIP-1β (CCL4), CCL12, and Cxcl2, CXCL11, innate lymphoid cells (ILC2s) | Recruit effector cells, including DCs, and regulate immune responses |
| Neuropeptides | ACTH, CRF, urocortin, VIP | Inflamation, sensory nerve modulation, vasodilatation |
| Other | Nitric oxide and superoxide radicals Antimicrobial peptides |
Bactericidal Bactericidal |
2.5. Mast Cells
Mast cells are effector cells in the regulation of numerous processes, including the regulation of immunity, inflammation, crossing the blood–brain barrier and cancer growth [2][3][4]. Mast cells play an important role in the early phase of allergic reactions due to their localization and rapid encounter with environmental or food allergens in the submucosa of the respiratory or digestive tract. Changes in the surrounding tissue such as swelling, itching, sneezing in allergic rhinitis are attributed to the secretion of histamine. Alongside pro-inflammatory cytokines (IL-1, IL-6, IL-8 and TNF-α), upon stimulation, mast cells secrete number different mediators (Table 3), including biogenic amines serglycin proteoglycans, serine proteases, and various other enzymes and growth factors; these molecules can be associated with the granules, such as tumor-necrosis factor-α (TNF-α) and vascular endothelial growth factor A (VEGFA). Mast cells activated by the aggregation of FcεRI also release an abundance of arachidonic acid metabolites, notably leukotriene (LT) C4, prostaglandin (PG) D2, and platelet activating factor (PAF) [12]. The main classes of mediators that are released by mast cells are presented in Table 3. Mast cells contribute to the expression and synthesis of cytokines depending on: (1) the maturation of mast cells; (2) the location of mast cells within different tissues [13]; and (3) cytokine activation of mast cells [13].
Table 3. Main classes of mediators that are released by mast cells.
| Class of Product | Mediators | Biological Effect |
|---|---|---|
| Granule-associated | Histamine and serotonin | Alter vascular permeability, smooth-muscle contraction |
| Heparin and/or chondroitin sulphate peptidoglycans | Enhance chemokine and/or cytokine function and angiogenesis | |
| Tryptase, chymase, carboxypeptidase and other proteases | Remodel tissue and recruit effector cells | |
| TNF, VEGF and FGF2 | Recruit effector cells and enhance angiogenesis | |
| Lipid-derived | LTC4, LTB4, PGD2 and PGE2 | Increased vascular permeability, vasodilatation, recruit effector cells, regulate immune responses, and promote angiogenesis, platelet aggregation, oedema and bronchoconstriction |
| Platelet-activating factor (PAF) | Platelet aggregation and degranulation, contraction of pulmonary smooth muscles, activates effector cells, enhances angiogenesis and induces physiological inflammation | |
| Cytokine | TNF, IL-1α, IL-1ß, IL-6, IL-18 GM-CSF, LIF, IFN-γ. and IFN-ß | Induce inflammation |
| IL-3, IL-4, IL-5, IL-9, IL-13, IL-15 and IL-16 | Functions of T helper 2-type cytokines | |
| IL-12 and IFN-γ | Functions of T helper 1-type cytokines | |
| IL-10, TGF-ß and VEGF | Regulate inflammation and angiogenesis | |
| Chemokine | CCL2, CCL3, CCL4, CCL5, CCL11 and CCL20 | Recruit effector cells, including DCs, and regulate immune responses |
| CXCL1, CXCL2, CXCL8, CXCL9, CXCL10 and CXCL11 | Recruit effector cells and regulate immune responses | |
| Neuropeptides | Adrenocorticotropin hormone (ACTH), CRF, urocortin, vasoactive intestinal peptide (VIP) | Inflamation, sensory nerve modulation, vasodilatation |
| Other | Nitric oxide and superoxide radicals Antimicrobial peptides |
Bactericidal Bactericidal |
Mast cells produce Th2 cytokines, such as IL-4 and IL-13, which are increased in AD. These cytokines facilitate the development of skin infections by inhibiting the production of antimicrobial peptides through keratinocytes [2][3][4]. Furthermore, the cytokine profile of mast cells is crucial in their physiological and pathological role in innate and acquired immunity [2][3][4], inflammation [2][12][13], wound healing and tumor growth [2]. In addition to cytokines, mast cells secrete proinflammatory, vasoactive, and neurosensitizing molecules. Regardless of the activation mechanism, vasoactive, proinflammatory, and neurosensitizing molecules act on keratinocytes, endothelial cells, or nerve endings to stimulate the release of additional molecules leading to chronic inflammation and neuropathic hypersensitivity or pain [14][15]. Chronic stress weakens immune processes, while acute stress stimulates these processes. In addition to the pathological activation of mast cells via FcεRI, under physiological conditions, mast cells play a protective role in host defense against bacteria through the production of tumor necrosis factor (TNF)-α, mainly as a result of Toll-like receptor (TLR)4- or CD48 (a mannose-containing GPI-anchored molecule)-mediated activation [14].
2.6. Lymphocytes
Lymphocytes comprise three cell types: natural killer (NK) cells as effector lymphocytes of the innate immune system, B cells, and T cells as part of the adaptive immune response, either in humoral immunity or in cell-mediated immunity [1][5][6][16][17][18][19][20]. During inflammation, there are close interactions between lymphocytes and macrophages and their cytokines, in a bidirectional way, which leads to a persistent inflammatory response. Allergen-reactive T helper cells type 2 (Th2) are thought to play an important role in the induction and maintenance of the allergic inflammatory cascade through increased production of cytokines and chemokines (GM-CSF, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, macrophage-derived chemokine). The reaction of other cells activated by Th2 cytokines, and the reaction of damaged tissue associated with Th2 (eotaxin, transforming growth factor-β, IL-11), contributes to pathophysiological allergic disorders. Increased production of cytokines and other factors consequently leads to the increased production of IgE antibodies, recruitment or activation of mast cells, basophils, and eosinophilia, mucus hypersecretion, subepithelial fibrosis, and tissue remodeling. Cytokines produced by Th2 cells induce differentiation, activation, and in situ survival of eosinophils (through IL-5); promote the production of high amounts of antibodies by B lymphocytes, including IgE (through IL-4 or IL-13); as well as the growth of mast cells and basophils (through IL-4, IL-9, and IL-10) [17][18]. Certain cytokines inhibit several macrophage functions or the development of Th1 cells, which are important against the majority of infectious agents, with the exception of some gastro-intestinal nematodes. Thus, the phagocyte-independent Th2 response is usually less protective than the Th1 response. However, Th2 cells likely play an important regulatory role in the immune system because switching from Th1 to Th2 may provide a protective effect when the Th1 response threatens to become a dangerous for the host.
T-regulatory (Treg) cells play a key role in maintaining immune tolerance to allergens. Knowledge about their role in the regulation of infectious, autoimmune diseases, asthma and allergen immunotherapy has increased in the last few years; however, the mechanisms by which Treg cells fail to maintain tolerance in patients with allergic diseases are not fully understood. Many different Treg subsets have been described [19][21], including CD8+ Treg cells, NK cells and several different CD4+ Treg cell subsets. Major populations of natural Treg cells (nTreg) are generated in the thymus but can also develop from conventional CD4+ cells in specific conditions or signals. These cells are known as CD4+ FOXP3+ nTreg cells. Inducible Treg cells are a second population. CD4+ FOXP3+ iTreg cells arise extra-thymically in peripheral lymphoid tissues after exposure to antigens and in the presence of TGF-β. These cells are found in the gastrointestinal tract and the lungs during chronic inflammation. It should be noted that iTreg cells are less stable than nTreg cells, regardless of the fact that they share similar levels of markers, such as FOXP3, CTLA-4, GITR, ICOS, CD103 and CD25. In addition, iTreg can lose FOXP3 expression and secrete cytokines such as IFN-γ and IL-17 under inflammatory conditions. The third population represents CD4+ type 1 T regulatory cells (Tr1) that have been defined by the expression of IL-10 and the surface marker LAG-3 and CD49b when FOXP3 and CD25 expression is absent. Tr1 cells express a number of transcription factors common to other T cell populations including Ahr (Aryl hydrocarbon receptor), and others. Th3 are CD4 T lymphocytes are induced from naïve CD4 T cells by TGF-β, with a similar phenotype to conventional Treg cells that secrete TGF-β and IL-10 and are characterized by the expression of IL-4. The suppressive role of Treg cells is mediated by multiple mechanisms from the release of inhibitory cytokines (TGF-β, IL-10, and IL-35) and cytolytic molecules (granzymes A and B), or the reduction in the function of antigen-presenting cells (cytotoxic T lymphocyte antigen 4 (CTLA-4) and lymphocyte activation gene 3 (LAG-3) to withdrawal of trophic cytokines (IL-2 via CD25) and modulation of metabolic pathways (CD73 and CD39).
In the suppression of effector Th2 cells by nTreg cells, the key cell surface molecules are CTLA-4, Notch-3 and LAG-3 and the intracellular enzyme heme oxygenase (HO)-1. CTLA-4 and CD28 deliver opposite signals to APCs, thereby regulating indoleamine 2,3-dioxygenase (IDO) activity and its anti-proliferative effects on effector T-cells. The suppressive effects of nTreg cells are abrogated by GITRL and interleukin (IL)-6 that can be expressed by APCs. Immature or semi-mature APCs producing IL-10 or transforming growth factor (TGF)-β generate T-regulatory cell type 1 (Tr1)- and Th3-type of adaptive CD4+ T cells that promote immune tolerance (aTreg cells), respectively. The production of these cytokines by APCs can be induced by regulatory-type pathogen-associated molecular patterns (PAMPs). Data suggest that nTreg cells also mediate the development of aTreg (Tr1, Th3) cells.
In addition, other Th lymphocytes have also been used to characterize allergic diseases: Th9 lymphocytes, Th22 lymphocytes, T follicular helper cells (Tfh) lymphocytes and invariant natural killer T (iNKT) lymphocytes [16][17][20]. Th17 cells, characterized by the secretion of IL-17A (also called IL-17), IL-17F, IL-22 and other cytokines, can induce autoimmunity by promoting tissue inflammation and mobilizing innate immunity. Abnormal Th17 immunity may also contribute to the pathogenesis of classically recognized Th2-mediated allergic disorders. Th9 cells have an important role in the immune response regulation. Th9 express predominantly IL-9. IL-9 causes the induction of lung eosinophilia, increased serum total IgE levels, airway hyperreactivity, and generation of cytokines from active mast cells; it also upregulates high-affinity IgE receptors on mast cells. Th22 cells are positive for chemokine receptors CCR4, CCR6 and CCR10 and produce mostly IL-22. High levels of IL-22 have been found in patients with allergic rhinitis and asthma. T follicular helper cells (Tfh) represent a specialized CXCR5-expressing CD4 T cell population, regulated by Bcl-6 in child and adult asthma patients. In addition to the mentioned T cells, a part of unconventional T lymphocytes is also involved in the pathophysiology of asthma, especially iNKT and mucosa-associated invariant T cells (MAIT) [17][20] that produce low to moderate levels of IL-4 and IL -13. MAIT-17 can produce several cytokines, including IL-17 and IFN-γ [17], which are considered to be associated with asthma symptoms in children.
2.7. Plateles
Platelets are small cellular fragments deriving from megakaryocytes that maintain homeostasis, rapidly respond to vascular injury, become activated, and induce platelet aggregation and thrombus formation. Activated platelets and platelet-activating factor (PAF), as a potent inflammatory mediator released by inflammatory cells such as macrophages, neutrophils, eosinophils, platelets and endothelial cells, play an important role in the pathogenesis of inflammatory diseases through the induction of inflammatory mediators (Table 4) that: (1) stimulate vascular endothelial cells to produce cytokines (e.g., IL-1 and IL-18) and platelet expression of CD40/CD40 ligands to promote increased thrombotic activity; (2) stimulate the synthesis of lipid mediators (TXA2, hydroxy-eicosatetraenoate (12-HETE) and PAF); (3) release peptide 2 (activates neutrophils), sphingosine 1-phosphate, growth factors (PDGF and TGF-β), nitric oxide (NO), cytokines (IL-1β and IL-7) and chemokines (CXCL5, MCP-3, RANTES, MIP-1α, platelet factor 4); (4) proinflammatory mediators such as histamine, serotonin; (5) growth-regulating oncogene α (GRO-α); (6) high-mobility group box 1 and P-selectin. The expression of CD40/CD40 ligand on activated platelets is crucial in antigen presentation to effector cells (T lymphocytes), maturation and activation of DCs and production of T-dependent isotype switching as well as interaction between other immune cells such as B cells, T cells, neutrophils, macrophages, endothelial cells, NK cells and DCs [12][22]. PAF plays an important role in inflammatory and thrombotic diseases and in both immune-mediated and non–immune mediated anaphylaxis. It is a neutrophil chemoattractant, which increases eosinophilic and neutrophilic infiltration, platelet aggregation and activation through the release of vasoactive amines in an inflammatory reaction leading to vascular permeability, nasal hyperreactivity to histamine, bradykinin or kinins, circulatory collapse, decreased cardiac output, bronchoconstriction, mucus hypersecretion, and inflammation of bronchi. As a signaling molecule, it plays a role in inflammatory diseases such as asthma, allergic rhinitis, sepsis, atherosclerotic disease, liver cirrhosis, inflammatory reactions in the skin and malignancy. Its production requires phospholipase A2 and acetyltransferase while its degradation is catalyzed by PAF acetyl-hydrolase (PAF-AH). PAF is rapidly hydrolyzed and degraded to an inactive metabolite, lysoPAF; and its elimination time is short (~3–13 min). Platelets have the ability to modulate early inflammation as well as the link between inflammation and coagulation. Platelets are the main source of serotonin that is released during allergic inflammatory responses. It should be emphasized that platelets also contain substances that limit inflammation through the production of lipoxin during the interaction of platelets and leukocytes. They play an important role in the adaptive immune response through the expression of high and low affinity receptors for immunoglobulins (FcγRI, FcγRII, FcγRIII, FcεRI, FcεRII, FcαRI, etc.). Increased levels of blood markers of activated platelets (PF4, P-selectin, β-thromboglobulin (β-TG), and PMP) have been observed in numerous human diseases, including systemic lupus erythematosus (SLE), systemic sclerosis (SSc), and small vessel vasculitis, AD and psoriasis, but also in a mouse model of AD. Some of the platelet factors including histamine, 5-HT, acid proteases, IL-1β, TGF-β, PAF and prostaglandin E2 induce pruritus and bleeding in AD [21][22][23]. The interaction of platelets with the endothelium and leukocytes leads to the activation of platelets and increased deposition of fibrin by platelets, as well as stimulation of neutrophils in the release of chromatin and formation of extracellular traps (NETs), which directly promote activation and aggregation of platelets [24].
Table 4. Inflammatory mediators synthesized by and stored in platelets.
| Class of Product | Mediators | Biological Effects |
|---|---|---|
| Cytokine | IL-1β, IL-1α,IL-7, Tumor necrosis factor α (TNF-α), CD40L, HMGB1, ENA-78 (CXCL5), SDF-1(CXCL12) | IL-1β, CD40L induces adhesion receptor expression on endothelium and release of chemokines Increse leukocyte recruitment |
| Chemokine | IL-8 (CxCL8), platelet factor 4-PF4(CxCL4), β-thromboglobulin (CXCL7/NAP-2), Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES or CCL5), Macrophage Inflammatory Protein-MIP-1α (CCL3), MCP-3 (CCL7), NAP-2 (CXCL7) TARC (CCL17) | RANTES-promote leucocyte recruitment and potent eosinophil chemoattractant; MIP-1α is a chemoattractant for monocytes, macrophages, T-cells and neutrophils Recruits monocytes |
| Enzymes | Matrixmetalloproteinases (MMP), β-hexosaminidases and heparanases | Disruption of the composition and integrity of cell membranes by degrading glycoproteins, glycolipids and glycosaminoglycans |
| Lipid mediators | tromboxan A2, PGE2 and 12-HETE (12-hydroxyeicosatetraenoic acid) | Vasoconstriction; platelet activation and aggregation; smooth muscle contraction Vasodilation (PGE2), naive T cell priming (PGE2),chemotaxis (12-HETE) |
| Growth factor | Platelet-Derived Growth Factor (PDGF),Tumor necrosis factor β(TNF-β), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), growth-related oncogene (GRO-α or CXCL1) | Contribute to cellular proliferation and amplification of the inflammatory response. Proliferative actions on structural cells of the airways; PDGF, a chemoattractant for monocytes and eosinophils |
| Polyphosphates | Phosphates | Pro-inflammatory and procoagulant functions, accumulation of neutrophils and increased vascular permeability |
| ATP | Nucleotide | |
| Serotonin | Monoamine | Vasoactive mediator; stimulation of fibrosis, by increasing collagen synthesis by fibroblasts, as well as neurotransmission |
| Glutamate | Amino acid |
2.8. Epithelial Cells
A healthy epithelium is key to maintaining mucosal homeostasis and may potentiate immune tolerance to frequently encountered allergens. Specialized epithelial subset cells, including secretory and ciliated cells, are important tissue barriers whose dysfunction is a fundamental component of chronic human inflammatory diseases, including allergies. Epithelial cells at mucosal surfaces play a dominant role in allergic diseases and have an active role in the inflammation process. In susceptible individuals, after exposure of the epithelium to environmental allergens, mucosal epithelial cells release cytokines, such as IL-1, IL-25, IL-33, thymic stromal lymphopoietin (TSLP) and GM-CSF, and endogenous danger signals, such as uric acid, ATP and HMGB1. These factors lead to the production of inflammatory mediators and activate a network of DCs and other innate immune cells including basophils and innate lymphoid cells type 2 (ILC2) through the signaling pathways of nuclear factor kappa B (NF-ĸB) and IĸB [25]. Inflammatory mediators of epithelial cells include cytokines (e.g., TNF-α and IL-1β), chemokines (e.g., IL-8, MIP-2, CXC chemokines (MCP-1, IL-7 and IL-15), adhesion molecules [e.g., β-integrins and intercellular adhesion molecule 1 (ICAM-1)], O, and various TLR (2 and 4) receptors, growth factor receptors, TNF-α receptors (TNFR1 and TNFR2), and plasminogen activator receptors [25][26][27]. The relative importance of each mediator appears to differ in various models of Th2 inflammation. For example, IL-33 mediates its Th2-promoting effect via signaling to various immune cells, such as mast cells, T cells, DCs; and ILC2 might be a potent driver of IL-5 and IL-13 in mast cells and ILC2. IL-25 secreted from epithelial cells can activate DCs by upregulation of co-stimulatory molecule expression (CD80, CD86), which promotes the differentiation of naïve T cells into Th2 lineage. IL-25 also enhances collagen deposition in the lungs by promoting IL-13 from ILC2 while IL-13 and ILC2 is necessary for worm clearance in the lamina propria. It has been shown that epithelial cells in the airways can react to IL-25 through an autocrine-paracrine mechanism, stimulating the production and secretion of leukocyte chemoattractants CCL5 (RANTES) and CXCL1 (GROa). In addition, IL-25 plays a role in viral-exacerbations of asthma. Elevated TSLP production from epithelial cells of the human airways, gut and skin is observed in multiple allergic diseases such as AD, asthma, and food allergies. Alongside Th2 differentiation, TSLP can promote Th9 cells and production of IL-9 leading to allergic airway inflammation. TSLP can induce the differentiation of splenic progenitor-like cells resembling granulocyte-monocyte precursors (GMP) into different APC cells of the myeloid lineage.
2.9. Endothelial Cells
Pathogenesis of chronic allergic diseases, such as bronchial asthma, allergic rhinitis, eosinophilic gastrointestinal disorders and AD, involves chronic inflammation and tissue remodeling caused by immune reactions to various antigens on the tissue surface. Due to their anatomical location, vascular endothelial cells are the final responders that interact with various exogenous antigens and pathogen-associated molecular patterns (PAMPs) that come into contact with the epithelial surface. Endothelial cells line the inner walls of blood vessels, forming a selective permeable barrier between the blood inside the vessels and the surrounding tissues. Vascular endothelial cells are the final responders to interact with various alarmins on the epithelial surface which spontaneously express MHC-I molecules and a wide variety of functional PRRs, including TLRs, and NLRs. Endothelial cells form a single-cell-thick layer called the endothelium, which lines the inner wall of blood vessels. The endothelium, as a selective permeable barrier between the bloodstream and vessel walls in physiological conditions, is composed of 1 − 6 × 1013 cells and covers more than 1000 m2 of the entire body surface. The main functions of the endothelium are: (1) maintenance of metabolic homeostasis, (2) regulation of blood vessel hemodynamics, (3) regulation of cell permeability, coagulation and extravasation. Endothelial cells are crucial in the regulation and propagation of the inflammatory response and the development and exacerbation of allergic disorders.
Inflammatory conditions and increased oxidative stress by inflammatory cells lead to the disruption and opening of interendothelial junctions, increasing the migration of inflammatory cells across the endothelial barrier. This migration of inflammatory cells leads to tissue injury in addition to removing pathogens and foreign particles [28]. Activated endothelial cells contribute to inflammation by secreting numerous inflammatory molecules, including inflammatory cytokines (TNF-α, IL-1α, IL-1β, IL-6 and IL-8), chemokines (IL-8, RANTES and MIP-1), ROS and RNS, adhesion molecules [ICAM-1, ICAM-2, PECAM, vascular cell adhesion molecule 1 (VCAM-1), E-selectin and P-selectin], growth factors (VEGF and TGF-β), proteases, leukotrienes (LTs), and prostaglandins (PG). IL-33 can stimulate pulmonary microvascular endothelial and epithelial cells via the IL1RL1/ST2 receptor and stimulate type 2 cytokines, the production of CXCR2 chemokine (IL-8) as the main neutrophil chemoattractant and proangiogenic factor. Endothelial cells play a key role in innate and adaptive immune responses, expressing cytokine and chemokine receptors (TFNR1 and IL-1R), TLRs, and procoagulants and protease-activated receptors. During inflammation, changes in the cytoskeleton and intercellular proteins in these cells are crucial for vascular permeability and leukocyte migration [28][29].
2.10. Fibroblasts
Fibroblasts, a heterogeneous population of stromal cells, are recognized for their structural role in synthesizing (e.g., collagen and fibronectin, proteoglycans, etc.) and remodeling the ECM in tissues. Fibroblasts are mainly responsible for ECM production, but also for the reparative role of damaged tissue through collagen synthesis. ECM remodeling via fibroblasts takes place through the production of metalloproteinase (MMP) and tissue inhibitors of MMP (TIMP) and communication with nearby cells. High levels of MMP2 and IL10 in patients with allergic contact dermatitis and AD are associated with an impaired Th1/Th2 cell ratio. It was demonstrated that there is a bidirectional interaction between eosinophils, mast cells and fibroblasts via numerous soluble factors (growth factors, cytokines and MMPs) which, by activating fibroblasts, leads to the development of tissue remodeling/fibrosis. Prolonged or repeated activation of both mast cells and eosinophils can lead to their extended survival, recruitment of other inflammatory cells and further secretion of inflammatory mediators resulting in tissue damage [30]. Eosinophils also affect collagen production depending on the source of fibroblasts and collagen deposition by MMP-9, TIMP-1 and TIMP-2. Collagen production increased in skin fibroblasts but decreased in lung fibroblasts. Numerous mediators of mast cells and eosinophils could be responsible for the antagonistic or synergistic effects on fibroblast functions. Thus, under certain conditions, there is an excessive and unbalanced repair process, which eventually leads to fibrosis and tissue remodeling. High concentrations of cytokines (TGF-β, IL-4 and TNF-α) from mast cells can stimulate the synthesis of collagen and fibronectin in fibroblasts, contributing to fibrosis that disrupts the structure and function of organs. TNF-α is a growth factor that stimulates fibroblast proliferation and chemotaxis and secretion of MMPs, collagenase and cytokines, including TNF-α, IL-6, and IL-1β [31]. Crosstalk between fibroblasts and innate and adaptive inflammatory cells directly promotes fibroblast activation by cytokines and growth factors and indirectly leads to myofibroblast activation via further induction of pro-inflammatory, pro-fibrotic factors in other immune cells [30].
2.11. Dendritic Cells
Based on ontogeny, DCs consist of heterogeneous groups and can be classified in the following subsets: conventional dendritic cell (cDC), plasmacytoid dendritic cell (pDC), monocyte-derived dendritic cell (moDC), and Langerhans cells. DCs are primary antigen-presenting cells involved in interactions with T cells leading to the proliferation of Th1 or Th2 cell types. The role of DCs in inducing or preventing allergic inflammation involves different mechanisms of activity, ranging from antigen sampling and DC migration to complex interactions with other cells, infectious agents and allergens. DCs play a central role in allergic inflammation; they are the link between allergens and the environment, epithelial cells, and immune effector cells. A number of signals trigger the activation of quiescent and immature DCs into mature DCs, including: danger signals (TNF-α,IL-1β, type I IFN, ATP and UTP, heat-shock proteins-HSPs, necrotic cells), innate immunity maturation signals (TLRL, viral RNA and poly IC) Mycobacterial extracts, single strand RNA, CpG deoxy-oligonucleotides, bacterial DNA) adoptive immunity maturation signals (CD40LAg-Ab complex), and others (growth factors GM-CSF, cytokines IL-15, IL-17, TSLP) [32]. DCs internalize and process antigens and then display them on the surface, in conjunction with human leukocyte antigen molecules, allowing for the “presentation” to the lymphocytes, resulting in their activation. It has been confirmed that aberrant DC expression of surface receptors leads to Th2 polarization and significant changes in the expression of important molecules to interact with T cells, such as costimulatory molecules of the B7 family (CD80, CD86, PD-L2/B7-DC, ICOS-L), members of the TNF family (CD137/4-1BBL, CD134/OX40L, CD70) and chemokine receptors (CCR5, CCR7). DC function can be directly or indirectly regulated by inflammatory cells and their cytokines. Activated DCs actively produce and release numerous cytokines and chemokines during inflammatory responses. These products contribute to the recruitment of inflammatory cells to the site of inflammation, including differentiation of cells of the mononuclear phagocytic system into different subsets of tissue macrophages with additional specific functions leading to tissue damage and the development of chronic allergies independent of the role of DC cells in antigen presentation. Monocyte-derived DCs are key players in both innate and adaptive immunity, given their antibacterial capacity as well as their ability to stimulate CD4+ helper T-cells through antigen presentation, and induce (Th)1, Th2, Th17 and T regulatory cells. A few studies reported higher FcεRI expression on DCs in individuals suffering from atopic diseases, such as allergic rhinitis, AD, and asthma [33].
3. Oxidative Stress in Allergic Disorders
Oxidative stress occurs in many allergic and immunologic disorders, such as asthma, rhinitis, and and inflammatory skin diseases such as AD, urticaria, psoriasis and other skin diseases. Although ROS serve as cell signaling molecules for normal biologic processes, excessive exposure to ROS and nitrogen species can also provoke damage to multiple cellular organelles and processes [34]. ROS can react with proteins, lipids, and nucleic acids, leading to cell death. After reaction with lipids, the lipid structure is destroyed, permeability increases and cellular death occurs, leading to tissue damage and necrosis. Furthermore, arachidonic acid (AA) is a precursor of enzymatic and non-enzymatic oxidized products, such as prostaglandins, thromboxanes, leukotrienes, lipoxins, and isoprostanes, which play an important role in asthma. These products may exert signaling or damaging roles during physiological and pathological conditions, some of them being markers of oxidative stress linked to inflammation. ROS attack proteins to form carbonyls and can react with nitrogen species and tyrosine to form nitrotyrosine whereas reaction with DNA can form base pair derivatives, such as 8-oxo-2-deoxyguanosine [34][35]. DNA damage caused by ROS induces the release of 8-oxoguanine (8-oxoG) from damaged DNA. The damaged base is cleaved by 8-oxoguanine-DNA glycosylase-1 (OGG1); and 8-oxoG and OGG1 form a signaling complex that activates NF-kB and inflammation.
An imbalance between ROS and antioxidants can lead to elevated oxidative stress levels. Inflammatory cells (mast cells, monocite/macrophages, eosinophils and neutrophils) and airway tissue cells (epithelium and smooth muscle) are the likely source of reactive radicals and produce endogenous ROS after their activation by a variety of stimuli. Given the location of mast cells at the host-environment interface, such as perivascular areas and mucous membranes where they encounter antigens and invading pathogens, mast cells play a key role in allergic inflammation, host defense, as well as in coordinating the early stages of certain allergies and autoimmune diseases. FceRI cross-linking in mast cell causes degranulation and releases wide range mediators, such as histamine, prostaglandin D2, certain cytokines, chemokines, and tryptase, followed by allergic inflammation. Moreover, FceRI cross-linking results in ROS production within mast cells. It is known that inflammatory cells, especially monocytes, macrophages and neutrophils, as defense cells of innate immunity, create high levels of ROS and inflammation via NADPH oxidase and myeloperoxidase (MPO) through a “respiratory burst”. Furthermore, key cells in allergic inflammation, such as mast cells, generate high levels of ROS in response to antigens; therefore, an imbalance between ROS and antioxidants occurs in allergic diseases. Apart from mast cells, eosinophils are the most dominant inflammatory cells in both allergic and non-allergic asthma, AD, and allergic rhinitis; and they have greater ability to synthesize ROS than neutrophils. In addition, the activity of NADPH oxidase is higher in eosinophils than in other phagocytes, although activated macrophages possess a powerful ROS production system [34][35]. Circulating inflammatory cells (peripheral blood monocytes and eosinophils) might also be a source of ROS and allergic inflammation. Monocytes are activated to secrete superoxide when IgE binds to membrane receptors. Thus, in atopic dermatitis, the inflammation process may be increased by oxidative stress. AD is a chronic, itchy skin disorder caused by a combination of genetic predisposition, immune dysregulation, and damage to the skin barrier. The source of oxidative stress may be irritants, environmental and food allergens, which bind to the AhR and induce the production of ROS, DNA damage and the production of inflammatory cytokines that cause skin inflammation. Increased oxidative stress in the skin leads to skin barrier dysfunction or immune dysregulation. Another source of oxidative stress may be skin microbes such as skin colonization with Staphylococcus aureus or defective host defense mechanisms involved in controlling bacterial infection. The presence of a bacterial pathogen stimulates the synthesis of IL-4 and IgE to cause dermal inflammation and therefore, itching and scratching. It seems that monocytes from patients with AD are primed to generate ROS and that oxidative stress and redox imbalance may develop or worsen AD by inducing pruritus or enhancing Th2 polarization. In addition, increased levels of IgE, cytokines IL-4, IL-4 receptor and IL-13, or altered cutaneous inflammation, such as mast cell chymase, are visible. Namely, by colonizing inflamed skin via TLR ligands, Staphylococcus aureus converts transient dermatitis mediated by Th2 cells into permanent and aggravated chronic inflammation [36]. Furthermore, mast cells generate mainly intracellular ROS following the aggregation of FcεRI, which may act as secondary messenger in the induction of several biological responses. Oxidative stress can activate nuclear factor kappa-B (NF-κB) pathways to activate gene expression and synthesis of antioxidant enzymes and proinflammatory cytokines, such as IL-6, IL-8, IL-9, and IL-33, which in turn enhances dermal inflammatory infiltrate and histamine release in the affected skin to worsen symptoms [37][38].
In psoriatic skin, reactive species are generated by keratinocytes and activated leukocytes, mostly neutrophils and macrophages, which play a key role in inducing psoriasis-like skin disease [36][37][38]. Lactoferrin released by specific neutrophil granules can promote neutrophil-endothelial cell adhesion and, as a source of iron, may promote the Fenton reaction with the generation of the hydroxyl radical (OH•) [36]. Psoriatic skin is characterized by an advanced state of lipid peroxidation [36] and depletion of intracellular GSH [36]. In psoriasis, ROS produced during the inflammatory process affects primarily the polyunsaturated fatty acid in a biological system, forming a lipid peroxidation product MDA, which serves as an important biological marker of lipid peroxidation. The increased levels of other reactive species, such as nitric oxide (NO), superoxide anion (O2•−) and hydrogen peroxide (H2O2) have been found in the skin of psoriatic patients [36][37][38]. Hydrogen peroxide (H2O2) and the superoxide anion (O2•−) can be generated by the action of the enzyme xanthine oxidase, which displays a higher activity in psoriatic epidermis [36][37][38]. Additionally, cytokines such as tumor necrosis factor alpha can contribute to H2O2 production [36]. This suggests that cellular redox status plays a pivotal role in healthy skin environment and that an imbalance between pro-oxidant and antioxidant mechanisms could result in skin diseases, including psoriasis.
Airway hyperresponsiveness is closely related to the redox and immunological status of asthma patients. The oxygen stress level in asthma is increased because of inflammatory cells in vivo, and cigarette smoke (CS) or particulate matter (PM), which are a major component of air pollution. It has been suggested that ROS play an important role in pathogenesis of airway inflammatory diseases [37], especially increased concentrations of NO●, H2O2 and 8-isoprostane. Some radicals such as O2•−, NO●, and halides interact to form highly reactive species such as peroxynitrite and HOBr, which in turn cause nitration and bromination of protein tyrosine residues. Hypohalous acids, strong oxidants and important factors in host defense systems are produced by activated neutrophils, monocytes, eosinophils, and possibly macrophages (HOCl) and activated eosinophils (HOBr). HOCl and HOBr are formed by the reactions of H2O2 with Cl− or Br− catalyzed by the heme enzymes, myeloperoxidase (MPO) and eosinophil peroxidase (EPO). Both HOCl and HOBr readily react with biological molecules, including amino acids, proteins, antioxidants (including thiols), carbohydrates, lipids, and DNA. These oxidants also destroy ciliary functions of the respiratory epithelium and decrease surfactant activity while increasing mucus secretion, activity of cytokines and proteases, neutrophil chemotaxis and alveolar permeability and smooth muscle contractility. Oxidative stress may also reduce glutathione (GSH) levels and cause the inactivation of antioxidant enzymes such as superoxide dismutase, with a consequent increase in apoptosis, shedding of airway epithelial cells and airway remodeling [34][35][36][37][38]. Based on the above, the use of antioxidants, such as polyphenolic/flavonoid components of propolis, may be crucial in inhibiting the production of ROS and activation of mast cells, eosinophils, macrophages and other cells including their cytokines involved in allergic inflammation.
References
- Haque, T.T.; Frischmeyer-Guerrerio, P.A. The Role of TGFβ and Other Cytokines in Regulating Mast Cell Functions in Allergic Inflammation. Int. J. Mol. Sci. 2022, 23, 10864.
- González-de-Olano, D.; Álvarez-Twose, I. Mast Cells as Key Players in Allergy and Inflammation. J. Investig. Allergol. Clin. Immunol. 2018, 28, 365–378.
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765.
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14.
- Miyake, K.; Karasuyama, H. Emerging roles of basophils in allergic inflammation. Allergol. Int. 2017, 66, 382–391.
- Strzelak, A.; Ratajczak, A.; Adamiec, A.; Feleszko, W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. Int. J. Environ. Res. Public Health 2018, 15, 1033.
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218.
- Diny, N.L.; Rose, N.R.; Čiháková, D. Eosinophils in Autoimmune Diseases. Front. Immunol. 2017, 8, 484.
- Ghosh, S.; Hoselton, S.A.; Dorsam, G.P.; Schuh, J.M. Eosinophils in Fungus-Associated Allergic Pulmonary Disease. Front. Pharmaco. 2013, 4, 8.
- Barthel, S.R.; Johansson, M.W.; McNamee, D.M.; Mosher, D.F. Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J. Leukocyte Biol. 2007, 83, 1–12.
- Siracusa, M.C.; Kim, B.S.; Spergel, J.M.; Artis, D. Basophils and allergic inflammation. J. Allergy Clin. Immunol. 2013, 132, 789–801.
- Taunk, S.T.; Cardet, J.C.; Ledford, D.K. Clinical implications of asthma endotypes and phenotypes. Allergy Asthma Proc. 2022, 43, 375–382.
- Babina, M.; Motakis, E.; Zuberbier, T. Mast cell transcriptome elucidation: What are the implications for allergic disease in the clinic and where do we go next? Expert Rev. Clin. Immunol. 2014, 10, 977–980.
- Numata, T.; Harada, K.; Nakae, S. Roles of Mast Cells in Cutaneous Diseases. Front. Immunol. 2022, 13, 923495.
- Theoharides, T.C.; Cochrane, D.E. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J. Neuroimmunol. 2004, 146, 1–12.
- Berghi, N.O.; Dumitru, M.; Vrinceanu, D.; Ciuluvica, R.C.; Simioniuc-Petrescu, A.; Caragheorgheopol, R.; Tucureanu, C.; Sfrent-Cornateanu, R.; Giurcaneanu, C. Relationship between chemokines and T lymphocytes in the context of respiratory allergies (Review). Exp. Ther. Med. 2020, 20, 2352–2360.
- Lezmi, G.; Abou Taam, R.; Dietrich, C.; Chatenoud, L.; De Blic, J.; Leite-de-Moraes, M. Circulating IL-17-producing mucosal-associated invariant T cells (MAIT) are associated with symptoms in children with asthma. Clin. Immunol. 2018, 188, 7–11.
- Grydziuszko, E.; Phelps, A.; Bruton, K.; Jordana, M.; Koenig, J.F.E. Heterogeneity, subsets, and plasticity of T follicular helper cells in allergy. J. Allergy Clin. Immunol. 2022, in press.
- Chapoval, S.; Dasgupta, P.; Dorsey, N.J.; Keegan, A.D. Regulation of the T helper cell type 2 (Th2)/T regulatory cell (Treg) balance by IL-4 and STAT6. J. Leukocyte Biol. 2010, 87, 1011–1018.
- Noval Rivas, M.; Chatila, T.A. Regulatory T cells in allergic diseases. J. Allergy Clin. Immunol. 2016, 138, 639–652.
- Arman, M.; Payne, H.; Ponomaryov, T.; Brill, A. Role of Platelets in Inflammation. In The Non-Thrombotic Role of Platelets in Health and Disease; Kerrigan, S.W., Moran, N., Eds.; IntechOpen: Rijeka, Croatia, 2015.
- Turkalj, M.; Banic, I. The Role of Platelets in Allergic Inflammation and Asthma. In Asthma-Biological Evidences; IntechOpen: Rijeka, Croatia, 2019.
- Page, C.; Pitchford, S. Platelets and allergic inflammation. Clin. Exp. Allergy 2014, 44, 901–913.
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present High Mobility Group Box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb Haemost. 2014, 12, 2074–2088.
- Lambrecht, B.N.; Hammad, H. Allergens and the airway epithelium response: Gateway to allergic sensitization. J. Allergy Clin. Immuno. 2014, 134, 499–507.
- Ordovas-Montanes, J.; Dwyer, D.F.; Nyquist, S.K.; Buchheit, K.M.; Vukovic, M.; Deb, C.; Shalek, A.K. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature 2018, 560, 649–654.
- Goleva, E.; Berdyshev, E.; Leung, D.Y. Epithelial barrier repair and prevention of allergy. J. Clin. Investig. 2019, 129, 1463–1474.
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167.
- Asosingh, K.; Weiss, K.; Queisser, K.; Wanner, N.; Yin, M.; Aronica, M.; Erzurum, S. Endothelial cells in the innate response to allergens and initiation of atopic asthma. J. Clin. Investig. 2018, 2, 3116–3128.
- Van Linthout, S.; Miteva, K.; Tschope, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 2014, 102, 258–269.
- Smith and Levi-Schaffer (Robinson DS (ed): Immunological Mechanisms in Asthma and Allergic Diseases. Chem. Immunol. 2000, 78, 81–92.
- Humeniuk, P.; Dubiela, P.; Hoffmann-Sommergruber, K. Dendritic Cells and Their Role in Allergy: Uptake, Proteolytic Processing and Presentation of Allergens. Int. J. Mol. Sci. 2017, 11, 1491.
- Platzer, B.; Baker, K.; Vera, M.P.; Singer, K.; Panduro, M.; Lexmond, W.S.; Fiebiger, E. Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites. Mucosal Immunol. 2014, 8, 516–532.
- Bowler, R.P.; Crapo, J.D. Oxidative stress in allergic respiratory diseases. J. Allergy Clin. Immunol. 2002, 110, 349–356.
- van Rijt, L.S.; Utsch, L.; Lutter, R.; van Ree, R. Oxidative Stress: Promoter of Allergic Sensitization to Protease Allergens? Int. J. Mol. Sci. 2017, 18, 1112.
- Khan, A.Q.; Agha, M.V.; Sheikhan, K.S.A.M.; Younis, S.M.; Tamimi, M.A.; Alam, M.; Ahmad, A.; Uddin, S.; Buddenkotte, J.; Steinhoff, M. Targeting deregulated oxidative stress in skin inflammatory diseases: An update on clin-ical importance. Biomed. Pharmacother. 2022, 154, 113601.
- Choi, S.M.; Lee, P.H.; An, M.H.; Yun-Gi, L.; Park, S.; Baek, A.R.; Jang, A.S. N-acetylcysteine decreases lung inflammation and fibrosis by modulating ROS and Nrf2 in mice model exposed to particulate matter. Immunopharmacol. Immunotoxicol. 2022, 11, 1–6.
- Chelombitko, M.A.; Fedorov, A.V.; Ilyinskaya, O.P.; Zinovkin, R.A.; Chernyak, B.V. Role of reactive oxygen species in mast cell degranulation. Biochemistry 2016, 81, 1564–1577.
More
Information
Subjects:
Allergy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
18 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




