
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kevin Zhai | + 495 word(s) | 495 | 2020-11-05 05:20:42 | | | |
| 2 | Camila Xu | + 2 word(s) | 497 | 2020-11-06 02:55:08 | | |
Video Upload Options
Curcumin, a natural polyphenolic compound derived from the South Asian turmeric plant (Curcuma longa), has well-characterized antioxidant, anti-inflammatory, anti-protein-aggregate, and anticancer properties.
1. Introduction
Curcumin ((1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione) is derived from the rhizome of the turmeric plant (Curcuma longa). Turmeric is native to South Asia (e.g., India, China, and Indonesia) and is cultivated for its traditional medicinal properties [1].
Curcumin exists as a yellow-orange solid, with a molecular weight of 368 g/mol and a melting point of 183 °C. Chemically, it is a polyphenolic compound with two aromatic rings, each with one hydroxy and one methoxy substituent (Figure 1). A seven-carbon chain with two α-β unsaturated carbonyl groups (which are subject to tautomerization) links the rings [2].
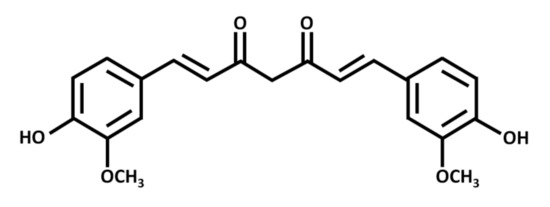
Figure 1. Chemical structure of curcumin, a polyphenolic chemical constituent of turmeric with antioxidant, anti-inflammatory, and anticancer effects. Curcumin is a beta-diketone compound containing two substituted aromatic rings linked by a seven-carbon chain. Each aromatic ring has one hydroxy and one methoxy group.
Commercially available curcumin contains three primary components, namely diferuloylmethane (the most abundant and active component of turmeric, at 82%) and its derivatives demethoxycurcumin (15%) and bisdemethoxycurcumin (3%); these are collectively referred to as “curcuminoids” [3][4]. Curcuminoids have the potential to treat various diseases through the modulation of molecular signaling targets, including transcription factors (e.g., NF-κB, AP-1, ß-catenin, and peroxisome proliferator-activated receptor), enzymes (e.g., COX-2, 5-LOX, and iNOS), and pro-inflammatory cytokines (e.g., TNF-α, IL-1, and IL-6) [3][5][6][7][8][9][10].
2. Discussion
The biological and physiological properties of curcumin have been extensively examined and reviewed in the context of neurodegenerative and inflammatory diseases, such as Alzheimer’s disease and dementia. Curcumin exhibits notable antioxidant activities through iron chelation, inhibition of lipid peroxidation, and scavenging of reactive oxygen species (ROS) [11]. The compound also has anti-inflammatory properties in acute and chronic inflammation [12]. Beyond these functions, curcumin is widely examined as an anticancer agent. Orally administered curcumin—alone and in combination with conventional chemotherapeutics—has been clinically trialed in pancreatic, breast, and prostate cancer patients [13][14][15][16]. Curcumin also demonstrates oncologic effects by itself on murine glioblastoma xenografts and in combination with the chemotherapeutic 5-fluorouracil on colorectal cancer cells [8][17][18][19].
Although curcumin has many positive effects on health, it is sometimes criticized for its low bioavailability—a result of its low absorption and rapid metabolism by the body. However, its bioavailability can be enhanced through combination with adjuvants. Indeed, piperine, a component of black pepper, is suitable for this purpose [20][21][22]. Notably, while curcumin’s anticancer properties are frequently reported, its membrane transport mechanisms remain mostly uncharacterized. Molecular dynamics simulations and solid-state nuclear magnetic resonance experiments indicate that curcumin can insert itself into plasma membranes [23][24]. However, specific transport mechanisms for the nervous system and NB cells are unclear at this time.
Despite these shortcomings, curcumin is a major component of numerous spices and foods, and is well tolerated in relatively high concentrations as part of daily diets without side effects. As such, it has high potential as a putative chemopreventive or therapeutic agent [25].
References
- Govindarajan, V.S. Turmeric--chemistry, technology, and quality. Crit. Rev. Food Sci. Nutr. 1980, 12, 199–301. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Rohani, S.; Gillies, E.R. Curcumin, a promising anti-cancer therapeutic: A review of its chemical properties, bioactivity and approaches to cancer cell delivery. RSC Adv. 2014, 4.
- Bharti, A.C.; Donato, N.; Singh, S.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood 2003, 101, 1053–1062. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398.
- Bharti, A.C.; Aggarwal, B.B. Chemopreventive agents induce suppression of nuclear factor-kappaB leading to chemosensitization. Ann. N. Y. Acad. Sci. 2002, 973, 392–395. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Bueso-Ramos, C.; Chatterjee, D.; Pantazis, P.; Aggarwal, B.B. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene 2001, 20, 7597–7609. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef]
- Buhrmann, C.; Kraehe, P.; Lueders, C.; Shayan, P.; Goel, A.; Shakibaei, M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: Potential role of EMT. PLoS ONE 2014, 9, e107514. [Google Scholar] [CrossRef]
- Buhrmann, C.; Shayan, P.; Banik, K.; Kunnumakkara, A.B.; Kubatka, P.; Koklesova, L.; Shakibaei, M. Targeting NF-κB Signaling by Calebin A, a Compound of Turmeric, in Multicellular Tumor Microenvironment: Potential Role of Apoptosis Induction in CRC Cells. Biomedicines 2020, 8, 236.
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Srimal, R.C.; Dhawan, B.N. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J. Pharm. Pharm. 1973, 25, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. 2010, 9, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Mahammedi, H.; Planchat, E.; Pouget, M.; Durando, X.; Cure, H.; Guy, L.; Van-Praagh, I.; Savareux, L.; Atger, M.; Bayet-Robert, M.; et al. The New Combination Docetaxel, Prednisone and Curcumin in Patients with Castration-Resistant Prostate Cancer: A Pilot Phase II Study. Oncology 2016, 90, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Varghese, E.; Samuel, S.M.; Abotaleb, M.; Cheema, S.; Mamtani, R.; Busselberg, D. The “Yin and Yang” of Natural Compounds in Anticancer Therapy of Triple-Negative Breast Cancers. Cancers 2018, 10, 346.
- Perry, M.C.; Demeule, M.; Regina, A.; Moumdjian, R.; Beliveau, R. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol. Nutr. Food Res. 2010, 54, 1192–1201. [Google Scholar] [CrossRef]
- Shakibaei, M.; Mobasheri, A.; Lueders, C.; Busch, F.; Shayan, P.; Goel, A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-kappaB and Src protein kinase signaling pathways. PLoS ONE 2013, 8, e57218. [Google Scholar] [CrossRef]
- Shakibaei, M.; Buhrmann, C.; Kraehe, P.; Shayan, P.; Lueders, C.; Goel, A. Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS ONE 2014, 9, e85397.
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; He, B.; Zhang, C.; Rodriguez, E.; Hage, D.S.; Moreau, R. Piperine potentiates curcumin-mediated repression of mTORC1 signaling in human intestinal epithelial cells: Implications for the inhibition of protein synthesis and TNFα signaling. J. Nutr. Biochem. 2018, 57, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [CrossRef]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.E.; Lee, D.K.; Ramamoorthy, A. Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: The case of the antioxidant curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Xiang, N.; Mondal, J.; Zhu, X.; Narsimhan, G. Characterization of Interactions between Curcumin and Different Types of Lipid Bilayers by Molecular Dynamics Simulation. J. Phys. Chem. B 2018, 122, 2341–2354. [Google Scholar] [CrossRef]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2008, 17, 1411–1417.




