Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Magda Zanelli | -- | 1601 | 2022-10-12 20:49:42 | | | |
| 2 | Amina Yu | + 9 word(s) | 1610 | 2022-10-13 04:00:07 | | | | |
| 3 | Amina Yu | Meta information modification | 1610 | 2022-10-13 08:12:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Melli, B.; Gentile, P.; Nicoli, D.; Farnetti, E.; Croci, S.; Gozzi, F.; Bolletta, E.; Simone, L.D.; Sanguedolce, F.; Palicelli, A.; et al. Diagnosis of Primary Vitreoretinal Lymphoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/29072 (accessed on 07 February 2026).
Melli B, Gentile P, Nicoli D, Farnetti E, Croci S, Gozzi F, et al. Diagnosis of Primary Vitreoretinal Lymphoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/29072. Accessed February 07, 2026.
Melli, Beatrice, Pietro Gentile, Davide Nicoli, Enrico Farnetti, Stefania Croci, Fabrizio Gozzi, Elena Bolletta, Luca De Simone, Francesca Sanguedolce, Andrea Palicelli, et al. "Diagnosis of Primary Vitreoretinal Lymphoma" Encyclopedia, https://encyclopedia.pub/entry/29072 (accessed February 07, 2026).
Melli, B., Gentile, P., Nicoli, D., Farnetti, E., Croci, S., Gozzi, F., Bolletta, E., Simone, L.D., Sanguedolce, F., Palicelli, A., Zizzo, M., Ricci, S., Ilariucci, F., Rossi, C., Cavazza, A., Ascani, S., Cimino, L., & Zanelli, M. (2022, October 12). Diagnosis of Primary Vitreoretinal Lymphoma. In Encyclopedia. https://encyclopedia.pub/entry/29072
Melli, Beatrice, et al. "Diagnosis of Primary Vitreoretinal Lymphoma." Encyclopedia. Web. 12 October, 2022.
Copy Citation
Intraocular lymphomas (IOLs) include vitreoretinal lymphomas (VRLs) and primary uveal or choroidal lymphomas. VRLs are further subdivided into primary VRLs and secondary VRLs, the latter deriving from systemic lymphomas. Primary uveal or choroidal lymphomas are usually low-grade neoplasms and are frequently extranodal marginal zone lymphomas with very good outcomes, unlike primary vitreoretinal lymphomas (PVRLs) which are high-grade diseases with poor outcomes. Secondary IOLs derive from ocular involvement by systemic lymphomas through haematogenous spread. Systemic lymphomas mainly disseminate to the uvea, due to its rich blood flow. PVRL represents a diagnostic challenge for both clinicians and pathologists, and it is critical, for the patient’s life, to shorten the time between the onset of symptoms often mistaken for chronic uveitis and correct diagnosis. Different laboratory methods are in use to diagnose PVRL. The main employed techniques are described, highlighting the principal diagnostic issues with the different laboratory methods.
lymphoma
vitreoretinal
IL-10
1. Cytology plus Immunohistochemistry
Cytological examination of vitreous fluid obtained through vitrectomy is considered the mainstay for primary vitreoretinal lymphomas (PVRLs) diagnosis [1].
Decreasing the cut rate during vitrectomy is essential for obtaining a sufficient number of cells and further improving the diagnostic rate of cytological examination. Regarding this issue, Jiang et al. recommended a cut rate of 600 cpm or less during diagnostic vitrectomy to confirm vitreoretinal lymphomas (VRLs), because cell viability began to decrease at 600 cpm in the in vitro experiments [2].
The morphological assessment of the vitreous sample is complicated, requiring a pathologist with expertise in dealing with this kind of specimen.
The main difficulty is due to the small volume of vitreous sample, which often contains a low number of lymphoma cells. Lymphoma cells are known to be fragile and easily degenerate; therefore, the undiluted and refrigerated vitreous sample should be promptly transported within 1 h from the surgery room to the pathology laboratory and immediately processed.
Prior steroid treatments, performed in clinical suspicion of chronic uveitis, may not only delay diagnosis for a transient beneficial effect but compromise the morphological evaluation due to degenerative changes of lymphoma cells [3].
Diffuse large B cell lymphomas (DLBCLs) cells are large-sized atypical elements with large irregular nuclei, evident nucleolus, and scanty cytoplasm (Figure 1 and Figure 2).

Figure 1. Cytology of vitreous sample with a discrete number of atypical lymphoid cells with a high nuclear–cytoplasmic ratio (haematoxylin and eosin, 200× magnification; original image from Dr M. Zanelli).

Figure 2. Immunohistochemistry performed on cytological sample of vitreous fluid: CD20 highlights the B cell phenotype of the majority of atypical cells (immunostaining; 200× magnification; original image from Dr M. Zanelli).
However, the morphological assessment of vitreous fluid can be complicated by the presence of a background rich in inflammatory cells (T lymphocytes and histiocytes) admixed with only rare lymphoma B cells (Figure 3, Figure 4 and Figure 6).

Figure 3. Cytology of vitreous sample with only rare large lymphoid cells and a discrete number of small lymphocytes (blue arrow pointing toward a large lymphoid cell; red circle highlighting small lymphoid cells) (haematoxylin and eosin, 200× magnification; original image from Dr M. Zanelli).

Figure 4. Immunohistochemistry performed on cytological sample of vitreous fluid showing only sparse CD20-positive atypical B cells (immunostaining; 200× magnification; original image from Dr M. Zanelli).

Figure 5. Immunohistochemistry performed on cytological sample of vitreous fluid showing numerous small-sized reactive CD3-positive T cells (immunostaining; 200× magnification; original image from Dr M. Zanelli).
Immunohistochemistry with B cell (CD20) and T cell markers (CD3) is often used in adjunct to cytology. However, the paucity and fragility of lymphoma cells often reduce the diagnostic power of immunophenotyping analysis. For the above-mentioned reasons, the sensitivity of cytology is variable (31–87.5%) [4][5] and further tests are needed in the case of a negative cytological result.
2. IL-10 and IL-6
The levels of both IL-10 and IL-6 may be measured in vitreous fluid using enzyme-linked immunosorbent assays (ELISA), multiplex bead-based assays, and cytometric bead array assays. IL-10 is a growth and differentiation factor for B lymphocytes, whereas IL-6 is produced by different types of cells, including inflammatory cells [6][7][8]. Unlike IL-6, which is a marker of inflammatory diseases, IL-10 is high in the intraocular fluid of PVRL patients, and an IL-10: IL-6 ratio over 1.0 is highly suggestive of lymphoma [1][9].
Recently, the ‘Interleukin Score for intraOcular Lymphoma Diagnosis’, or ISOLD, was developed [9]. Aqueous or vitreous IL-10 and IL-6 levels are inserted into a mathematical formula, resulting in a probability score for PVRL diagnosis. In the recent consensus recommendation paper for PVRL diagnosis by Carbonell et al., the IL-10 level or the IL-10:IL-6 ratio are considered useful parts of the diagnostic repertoire for PVRL diagnosis [1].
However, it needs to be underlined that cytokine production may be influenced by previous steroid or immunosuppressive therapies or certain systemic diseases, and hence, the diagnostic power of IL-10 to IL-6 ratio can be reduced [10]. Taking these limitations into consideration, cytokine analysis is currently considered a valuable adjunctive tool for screening patients suspected of PVRL.
3. Clonality Analyses
The majority of PVRLs are aggressive B cell lymphomas, mainly DLBCL, and a minority of cases are T cell lymphomas.
The determination of clonality when evaluating IGH and TCR gene rearrangements is considered a valuable adjunct for lymphoma diagnosis. PCR analysis targeting rearranged IG genes gives multiple amplicons in the case of polyclonal cells, such as in inflammatory conditions (Figure 6), and a single amplicon if the cells are monoclonal and neoplastic, such as in lymphomas (Figure 7).

Figure 6. Fragment analysis by capillary electrophoresis: case analysed in clinical suspicion of PVRL, not confirmed by clonality analysis showing a polyclonal pattern suggestive of an inflammatory condition (previously unpublished image).
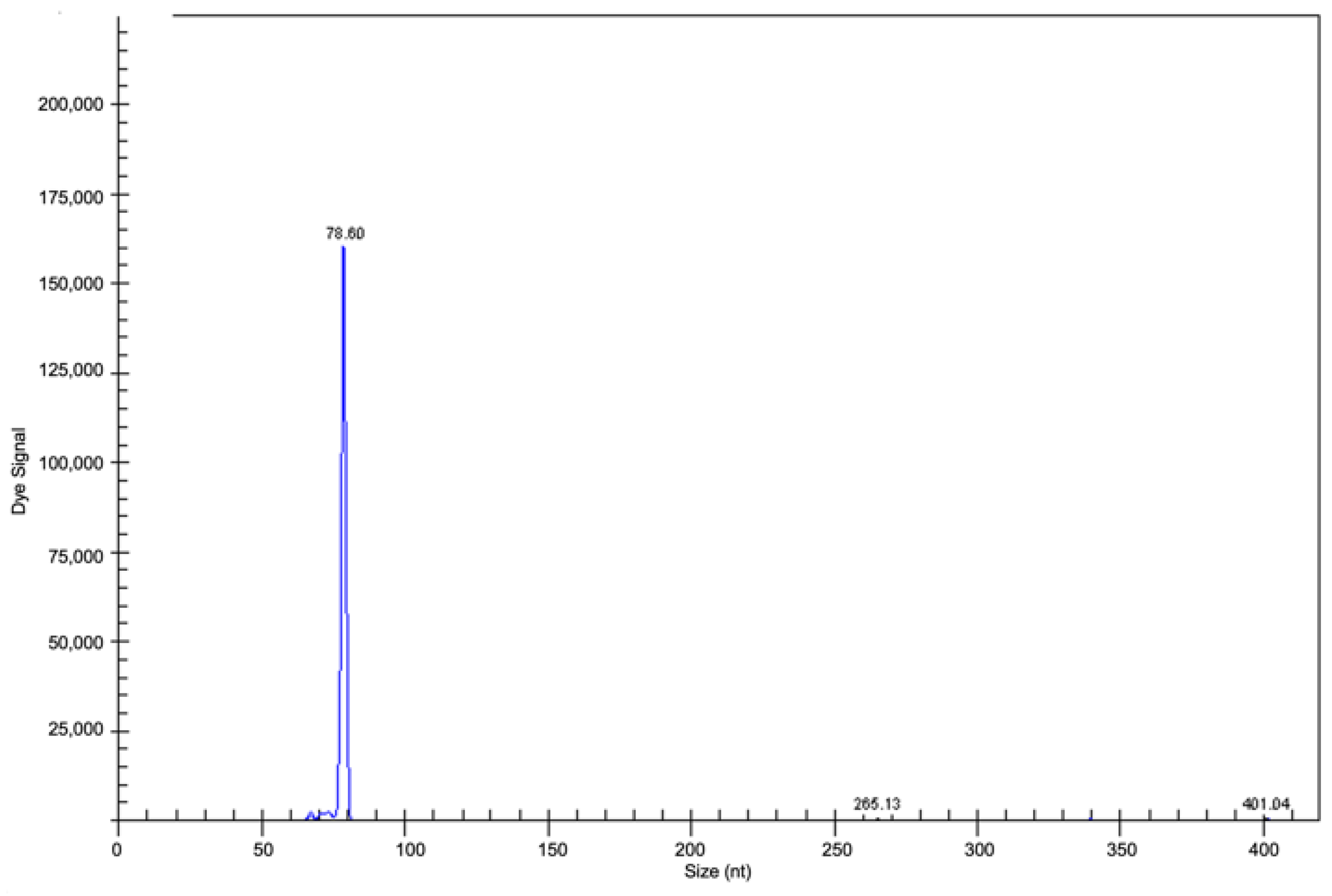
Figure 7. Fragment analysis by capillary electrophoresis: clonal gene rearrangement in CDR3 in the range of positivity 70–100 nt in a PVRL case (previously unpublished image).
The accuracy of PVRL diagnosis is improved by the molecular analysis of DNA obtained by PCR, particularly in samples with low cellularity, poorly preserved neoplastic cells, or a prevalence of non-neoplastic T lymphocytes, in which cytology may give a negative result [10].
However, in vitreous samples, there is the potential risk of false negative or false positive results, even by clonality tests.
False negative results may occur because of the high frequency in PVRL of somatic hypermutation, potentially abrogating primer binding [11].
False positive results may be due to the detection of pseudoclonal/oligoclonal B cells by PCR due to the low cellularity of vitreous sample; this event may occur even in benign/inflammatory conditions, making the diagnosis of PVRL even more difficult [12][13].
4. MYD88 Mutation Analysis
The MYD88 gene is on chromosome 3p22.2. The MYD88 protein, the gene product, is involved in signalling within the immune system. It is a cell membrane-associated protein acting as an adaptor molecule involved in Toll-like receptors (TLRs) and the interleukin-1 receptor (IL-1R) signalling pathway. Following a TLR stimulus, MYD88 activation causes intracellular signalling cascades, such as nuclear factor (NF)-kB activation, favouring the survival of tumour cells [14][15][16][17].
The change of adenine by guanine in the DNA sequence of MYD88 results in the substitution in MYD88 protein of the amino acid lysine by proline at position 265; this determines the activation of B cells in various diseases [18][19].
DLBCLs arising in immune privileged sites are frequently associated with MYD88 mutation, predominantly L265P. MYD88-L265P mutation is commonly associated with DLBCLs of activated B cell (ABC) phenotypes, such as PCNSL, in which the mutation is detected in approximately 75% of cases [20].
Bonzheim et al. retrospectively evaluated the frequency of MYD88 mutation in PVRLs, analysing 75 vitrectomy specimens of 69 patients, and identified MYD88 mutations in 69% of cases [21].
The high frequency of MYD88 mutations, mainly L265P, identified in PVRL further supports the concept that PVRL and PCNSL represent the same disease [21]. Narasimhan et al. suggested that MYD88 mutation analysis has a high diagnostic profile in terms of sensitivity, specificity, and accuracy and that detection of MYD88 mutation significantly improves the diagnostic yield of vitrectomy samples [17][21].
Real-time PCR is a variation of the standard PCR technique commonly used to quantify DNA or RNA in a sample. Using sequence-specific primers, the number of copies of a DNA or RNA sequence can be determined. By measuring the amount of amplified product at each stage during the PCR cycle, quantification is possible. The threshold of the real-time PCR reaction is the level of signal that reflects a statistically significant increase over the calculated baseline signal, as shown in the detection of MYD88 L265P mutation (Figure 8).

Figure 8. Real-time PCR analysis for MYD88 L265P mutation. Real-time PCR cycler with 2 channels (green, yellow); test performed through CORBETT/QIAGEN ROTOR-GENE RG-6000 REAL-TIME PCR. (A) Wild-type sample with one-channel amplifications; (B) mutated sample with two-channel amplification; (C) amplification of reference (previously unpublished image).
Several studies report that the PCR-based IgH rearrangement assay has some limitations, requiring a larger quantity of cells and having a higher limit of detection (10–20% of clonal B cell population) compared to MYD88 mutation analysis, which may detect 5% or less of mutant cells [16][17][22][23][24].
5. Flow Cytometry
Flow cytometry is a technique used to identify phenotype cells by fluorescent antibodies or dyes. The use of flow cytometry in the diagnosis of lymphoma of B cell origin is based on the criteria that detecting the expression of either immunoglobulin kappa (IGK) or lambda (IGL) light chains may be suggestive of clonality.
In 1997, Davis et al. used flow cytometry for the first time in VRL diagnosis, with good results compared to cytology alone [5][25].
References
- Carbonell, D.; Mahajan, S.; Chee, S.P.; Sobolewska, B.; Agrawal, R.; Bülow, T.; Gupta, V.; Jones, N.P.; Accorinti, M.; Agarwal, M.; et al. Consensus Recommendations for the Diagnosis of Vitreoretinal Lymphoma. Ocul. Immunol. Inflamm. 2021, 29, 507–520.
- Jiang, T.; Zhao, Z.; Chang, Q. Evaluation of cytologic specimens obtained during experimental vitreous biopsy using B-cell lymphoma line. Eur. J. Ophthalmol. 2014, 24, 911–917.
- Pulido, J.S.; Johnston, P.B.; Nowakowski, G.S.; Castellino, A.; Raja, H. The diagnosis and treatment of primary vitreoretinal lymphoma: A review. Int. J. Retin. Vitr. 2018, 4, 4–22.
- Balikov, D.A.; Hu, K.; Liu, C.J.; Betz, B.L.; Chinnaiyan, A.M.; Devisetty, L.V.; Venneti, S.; Tomlins, S.A.; Cani, A.K.; Rao, R.C. Comparative Molecular Analysis of Primary Central Nervous System Lymphomas and Matched Vitreoretinal Lymphomas by Vitreous Liquid Biopsy. Int. J. Mol. Sci. 2021, 22, 9992.
- Davis, J.L. Intraocular lymphoma: A clinical perspective. Eye 2013, 27, 153–162.
- Frenkel, S.; Pe’Er, J.; Kaufman, R.; Maly, B.; Habot-Wilner, Z. The importance of cytokines analysis in the diagnosis of vitreoretinal lymphoma. Acta Ophthalmol. 2020, 98, e668–e673.
- Rousset, F.; Garcia, E.; Defrance, T.; Perrone, C.; Vezzio, N.; Hsu, D.H.; Kastelein, R.; Moore, K.W.; Banchereau, J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 1890–1893.
- Murray, P.I.; Hoekzema, R.; van Haren, M.A.; de Hon, F.D.; Kijlstra, A. Aqueus humor interleukin-6 levels in uveitis. Inv. Ophthalmol. Vis. Sci. 1990, 31, 917–920.
- Costopoulos, M.; Touitou, V.; Golmard, J.L.; Darugar, A.; Fisson, S.; Bonnemye, P.; Le Lez, M.L.; Soussain, C.; Cassoux, N.; Lamy, T.; et al. ISOLD: A new highly sensitive interleukin score for intraocular lymphoma diagnosis. Ophthalmology 2016, 123, 1626–1628.
- Wang, Y.; Shen, D.; Wang, V.M.; Sen, H.N.; Chan, C.C. Molecular biomarkers for the diagnosis of primary vitreoretinal lymphoma. Int. J. Mol. Sci. 2011, 12, 5684–5697.
- Sobolewska, B.; Chee, S.P.; Zaguia, F.; Goldstein, D.A.; Smith, J.R.; Fend, F.; Mochizuki, M.; Zierhut, M. Vitreoretinal Lymphoma. Cancers 2021, 13, 3921.
- Fend, F.; Ferreri, A.J.M.; Coupland, S. How we diagnose and treat vitreoretinal lymphoma. Br. J. Haematol. 2016, 173, 680–692.
- Ponzoni, M. Look into my eyes, please. Blood 2015, 126, 4.
- Ngo, V.N.; Young, R.M.; Schmitz, R.; Jhavar, S.; Xiao, W.; Lim, K.H.; Kohlhammer, H.; Xu, W.; Yang, Y.; Zhao, H.; et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011, 470, 115–121.
- Deguine, J.; Barton, G.M. MYD88: A central player in innate immune signaling. F1000 Prim. Rep. 2014, 6, 97.
- Raja, H.; Salomão, D.R.; Viswanatha, D.S.; Pulido, J.S. Prevalence of MYD88 L265P mutation in histologically proven, diffuse large B-cell vitreoretinal lymphoma. Retina 2016, 36, 624–628.
- Narasimhan, S.; Joshi, M.; Parameswaran, S.; Rishi, P.; Khetan, V.; Ganesan, S.; Biswas, J.; Sundaram, N.; Sreenivasan, J.; Verma, S.; et al. MYD88 L265P mutation in intraocular lymphoma: A potential diagnostic marker. Indian J. Ophthalmol. 2020, 68, 2160–2165.
- de Groen, R.A.L.; Schrader, A.M.R.; Kersten, M.J.; Pals, S.T.; Vermaat, J.S.P. MYD88 in the driver’s seat of B-cell lymphomagenesis: From molecular mechanisms to clinical implications. Haematologica 2019, 104, 2337–2348.
- Tsang, M.; Cleveland, J.; Rubenstein, J.L. On point in primary CNS lymphoma. Hematol. Oncol. 2020, 38, 640.
- Montesinos-Rongen, M.; Godlewka, E.; Brunn, A.; Wiestler, O.D.; Siebert, R.; Deckert, M. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 2011, 122, 791–792.
- Bonzheim, I.; Giese, S.; Deuter, C.; Süsskind, D.; Zierhut, M.; Waizel, M.; Szurman, P.; Federmann, B.; Schmidt, J.; Quintanilla-Martinez, L.; et al. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: A valuable tool to improve diagnostic yield of vitreous aspirates. Blood 2015, 126, 76–79.
- Sehgal, A.; Pulido, J.S.; Mashayekhi, A.; Milman, T.; Deák, G.G. Diagnosing Vitreoretinal Lymphomas: An Analysis of the Sensitivity of Existing Tools. Cancers 2022, 14, 598.
- Takhar, J.; Doan, T.; Gonzales, J.A. Vitreoretinal Lymphoma: A Literature Review and Introduction of a New Diagnostic. Method. Asia Pac. J. Ophthalmol. 2021, 10, 93–98.
- Takase, H.; Arai, A.; Iwasaki, Y.; Imai, A.; Nagao, T.; Kawagishi, M.; Ishida, T.; Mochizuki, M. Challenges in the diagnosis and management of vitreoretinal lymphoma—Clinical and basic approaches. Prog. Retin. Eye Res. 2022, 90, 101053.
- Davis, J.L.; Viciana, A.L.; Ruiz, P. Diagnosis of intraocular lymphoma by flow cytometry. Am. J. Ophthalmol. 1997, 124, 362–372.
- Missotten, T.; Tielemans, D.; Bromberg, J.E.; van Hagen, P.M.; van Lochem, E.G.; van Dongen, J.J.M.; Baarsma, G.S.; Langerak, A.W. Multicolor flowcytometric immunophenotyping is a valuable tool for detection of intraocular lymphoma. Ophthalmology. 2013, 120, 991–996.
- Tan, W.J.; Wang, M.M.; Ricciardi-Castagnoli, P.; Chan, A.S.Y.; Lim, T.S. Cytologic and molecular diagnostics for vitreoretinal lymphoma: Current approaches and emerging single-cell analyses. Front. Mol. Biosci. 2021, 7, 611017.
More
Information
Subjects:
Hematology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
731
Revisions:
3 times
(View History)
Update Date:
18 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




