Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | qiuyue qin | -- | 678 | 2022-10-12 13:43:41 | | | |
| 2 | Sirius Huang | + 725 word(s) | 1403 | 2022-10-13 03:01:06 | | | | |
| 3 | qiuyue qin | Meta information modification | 1403 | 2022-10-13 08:05:33 | | | | |
| 4 | Sirius Huang | Meta information modification | 1403 | 2022-10-13 09:59:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Qin, Q.; Liu, Y.; Yang, Z.; Aimaijiang, M.; Ma, R.; Yang, Y.; Zhang, Y.; Zhou, Y. Hypoxia-Inducible Factors in Osteogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/29060 (accessed on 03 March 2026).
Qin Q, Liu Y, Yang Z, Aimaijiang M, Ma R, Yang Y, et al. Hypoxia-Inducible Factors in Osteogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/29060. Accessed March 03, 2026.
Qin, Qiuyue, Yiping Liu, Zhen Yang, Maierhaba Aimaijiang, Rui Ma, Yixin Yang, Yidi Zhang, Yanmin Zhou. "Hypoxia-Inducible Factors in Osteogenesis" Encyclopedia, https://encyclopedia.pub/entry/29060 (accessed March 03, 2026).
Qin, Q., Liu, Y., Yang, Z., Aimaijiang, M., Ma, R., Yang, Y., Zhang, Y., & Zhou, Y. (2022, October 12). Hypoxia-Inducible Factors in Osteogenesis. In Encyclopedia. https://encyclopedia.pub/entry/29060
Qin, Qiuyue, et al. "Hypoxia-Inducible Factors in Osteogenesis." Encyclopedia. Web. 12 October, 2022.
Copy Citation
As central mediators of homeostasis, hypoxia-inducible transcription factors (HIFs) can allow cells to survive in a low-oxygen environment and are essential for the regulation of osteogenesis and skeletal repair.
hypoxia-inducible factors
HIF
osteogenesis
1. Hypoxia-Inducible Factors
Hypoxia-inducible factors (HIFs) are transcriptional activator complexes that perform a central role in the expression of oxygen-regulated genes. These genes are involved in the proliferation and apoptosis of cells, angiogenesis, erythropoiesis, energy metabolism, vasomotor function, and so on [1][2]. Thus, HIFs are essential for normal growth and development and also participate in the pathological processes, including tumor progression and tissue regeneration [3]. Heterodimeric transcription factors (HIFs) complex are composed of α-subunits (HIF-1α, HIF-2α, and HIF-3α) and the β-subunit (HIF-1β)/aryl hydrocarbon receptor nuclear translocator (ARNT). HIF-1β/ARNT is expressed stably in cells, whereas HIF-αs are degraded under the condition of normal oxygen bioavailability and accumulate rapidly in a hypoxic environment. HIF-1α, HIF-2α, and HIF-3α bind to HIF-1β to form HIF-1, HIF-2, and HIF-3, respectively. Thus, the stability of the HIF-1α subunit seems to determine HIF-1 formation. Similarly, the formation of HIF-2 is mainly determined by the abundance of the HIF-2α subunit.
In mammalian cells, three HIF-α subunit isoforms (HIF-1α, HIF-2α, and HIF-3α) are encoded by three HIF-α genes: HIF1A, HIF2A, and HIF3A, respectively. When oxygen concentration drops to <5%, HIF-1α is stably expressed, enters the nucleus, dimerizes with HIF-1β, and binds to HIF-response elements (HRE) of targeted gene promoters [2]. When oxygen is abundant in cells (>5%), the Prolyl-4-hydroxylases (PHDs) bind to HIF-1α and hydroxylate the proline residues, which leads to the recruitment of the Von Hippel-Landau (VHL) tumor suppressor E3 ligase complex. Eventually, the proteasomal is poly-ubiquitylated and degraded [4]. In addition, factor inhibiting HIF (FIH) also restricts the binding of HIF-αs to transcriptional co-activators CBP/p300 through hydroxylating (N-terminal) asparaginyl residues when oxygen is abundant [5]. HIF-2α is regulated by oxygen in a similar manner to HIF-1α. In addition to intracellular oxygen tension, several growth factors can also regulate HIF-α subunits in a hypoxia-independent way [6]. HIF-1 has been studied more extensively than HIF-2, and HIF-1 and HIF-2 have overlapping and unique biological functions. It is reported that HIF-1α responds to acute hypoxia mainly, whereas HIF-2α is the prime subunit that responded to chronic exposure to low oxygen at high altitudes [7]. HIF-1α is generally expressed in cells and regulates downstream genes, including VEGF, GLUT-1, AK-3, ALD-A, PGK-1, PFK-L, and LDH-A through binding to HRE to regulate many metabolic enzymes [8] (Maxwell, 1999). The role of HIF-1 in promoting angiogenesis also benefits cancer development. HIF-2 regulates erythropoiesis and vascularization and is essential for embryonic development [4]. In addition, HIF-2 is also involved in the progression and metastasis of solid tumors [9]. Another HIF-α protein, HIF-3α, can bind to ARNTs to restrain HIF-1α- or HIF-2α-mediated transcription, but its transcriptional capacity is weaker than other HIFs [2][10]. HIF-3α is relatively unknown in terms of regulating the hypoxia response, and many studies have shown that HIF-3α may play a dual part as a hypoxia-inducible transcription factor in recent years [11]. The determination of genome-wide binding of the human HIF-3 and its role requires extensive scientific research (Figure 1).
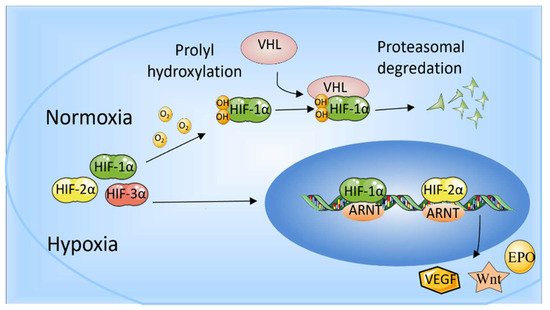
Figure 1. The role of HIF-1α and HIF-2α. When oxygen levels are low (hypoxia), HIF-1α and HIF-2α are protected from degradation and accumulate in the nucleus, where they bind to ARNT and bind to specific DNA fragments in hypoxia regulatory genes. At normal oxygen levels, oxygen regulates the degradation process by adding hydroxyl groups to HIF-αs. VHL recognizes and forms a complex that carries HIF-αs and degrades them in an oxygen-dependent manner.
2. Effect of HIFs on Bone
More recent studies have demonstrated the role of HIF-1 in bone growth and repair. Ref. [12] used spongy scaffolds that contained dimethyloxalylglycine (DMOG) in rat calvarial defects to imitate hypoxia to up-regulate HIF-1α, and found that angiogenesis was accelerated and bone regeneration was enhanced. Ref. [13] found that HIF-1α could facilitate osseointegration of tissue-engineered bone, dental implants, and new bone formation around implants, which was verified in a canine model. Another study has shown that expression of gingival HIF-1α protein in mice was apparently increased, and the ability of bone regeneration was enhanced at the onset of periodontitis resolution, after subcutaneous injection of 1,4-dihydrophenonthrolin-4-one-3-carboxylic acid (1, 4-DPCA/hydrogel), a hydrogel-formulated PHD inhibitor [14]. Gene ablation of phd2 in chondrocytes promotes endochondral osteogenesis through up-regulation of HIF-1α signaling, resulting in a significant growth of long bones and vertebrae [15]. In the process of bone regeneration, HIF-1α not only promotes angiogenesis but also regulates metabolic adaptations by inducing glycolysis transformation to promote cell survival [16]. Hence, HIF-1 serves an indispensable role in osteogenesis and bone restoration [12][17]. When osteoblasts and other associated cells sense reduced oxygen tension, intracellular HIF-1α is stably expressed to regulate the expression of the angiogenic and osteogenic genes [18]. Additionally, the mechanisms by which HIF-1 regulates downstream genes to promote osteogenesis and bone repair are quite complex. The role of HIF-1 in mediating downstream signaling to regulate bone mass in different animal models or cells is displayed in Table 1.
Table 1. HIF-1 functions through regulating different signals in different animal models or cells.
| HIFs | Mouse Models /Cells |
Signaling Pathway |
Effects | Ref. |
|---|---|---|---|---|
| HIF-1 | EC-specific loss-of-function mice (Hif1aiΔEC) | HIF-1/VEGF | An increased number of type H vessels and enhanced endochondral angiogenesis and osteogenesis | [19][20] |
| Mature osteoblasts | HIF-1/VEGF | Contribute to the coordination of vascularization, ossification and matrix resorption in endochondral bone development | [2][10] | |
| The mouse model of hindlimb ischemia | HIF-1/VEGF | HIF-1 activation in myeloid cells promotes angiogenesis | [21] | |
| HIF-1α-deficient embryos | HIF-1/EPO | Affect embryonic development | [22] | |
| rat calvaria bone defect model | HIF-1/EPO | Promote osteogenesis and accelerate bone repair | [23] | |
| MC3T3-E1 | HIF-1/Wnt | Promote osteoblast proliferation | [24] | |
| BMSCs in osteonecrosis of the femoral head | HIF-1/β-Catenin | Reduce cellular apoptosis, lower empty lacunae rate, enhance bone formation, and stronger trabecular bone | [25] | |
| PDGFRα + Sca-1+(PαS) MSC | SHIP-1 | SHIP-1 maintains the stable expression of HIF-1α in Pαs MSC under hypoxia, and reduced the expression of HIF-1α inhibits the proliferation of SHIP- 1KOPαs MSC | [26] | |
| periapical lesions in mice | HIF-1/NF-κB | Attenuate periapical bone loss, inhibit osteoclasts | [27] | |
| MSCs | HIF-1/CXCR4 and CXCR7 | Promote MSCs migration and survival capacity | [28] | |
| 10-wk-old osteoclast-specific HIF-1α conditional knockout mice | HIF-1/AMPK | Maintain osteoclast-induced resorption of calcified cartilage matrix | [29] |
The role of HIF-2 in osteogenesis is less understood compared to HIF-1 [30]. HIF-1 and HIF-2 have overlapping and opposite effects on the regulation of bone formation. Studies have suggested that HIF-2α can up-regulate the expression of VEGFA, COL10A1, and MMP13, and is a central transactivator of some key genes for endochondral ossification. When HIF-2α expression is reduced, chondrocyte hypertrophy, matrix degradation and vascularization, and other subsequent steps are impaired. Additionally, HIF-2α plays a more critical role in pathological endochondral ossification than in physiological endochondral ossification [31]. Studies have also shown that HIF-2 could up-regulate the expression of Sox9 to affect the differentiation of osteoblasts and regulate osteogenesis negatively, target Twist2 to down-regulate Runt-related transcription factor 2 (Runx2) and osteocalcin, and inhibit osteoblastic differentiation [32][33]. Moreover, HIF-2 might mediate the crosstalk between osteoblasts and osteoclasts by targeting RANKL in osteoprogenitor cells [32][34]. The up-regulation of HIF-2α expression in osteoblasts and osteoclasts is a novel intrinsic mediator of age-related bone loss [35]. In addition, studies have shown that HIF-2α, as a direct transcriptional target of NF-κB, destroys cartilage by regulating key catabolic genes in osteoarthritis (OA) [36][37]. However, HIF-1α inhibits the NF-κB-HIF-2α pathway to prevent cartilage degradation [38]. Bouaziz et al. demonstrated that HIF-1α interacts with β-catenin, which inhibits transcription factor 4-β-catenin transcriptional activity, suggesting that HIF-1α plays an important role in articular cartilage homeostasis and growth plate chondrocytes [39]. In the context of angiogenesis, HIF has been reported to control further up-regulation of hypoxia miR-424 in endothelial cells (ECs). This, in turn, contributes to HIF protein stabilization to adapt to low oxygen conditions and induces angiogenesis [40][41]. The role of HIF-2 in regulating bone homeostasis by mediating downstream signals in different animal models or cells is shown in Table 2.
Table 2. HIF-2 functions through regulating different signals in different animal models or cells.
| HIFs | Mouse Models /Cells |
Signaling Pathway |
Effects | Ref. |
|---|---|---|---|---|
| HIF-2 | Mature osteoblasts | HIF-2/VEGF | Contribute to the coordination of vascularization, ossification and matrix resorption in endochondral bone development | [42] |
| HIF-2α-ablated mice | HIF-2/EPO | Affect adult EPO synthesis | [43] | |
| N1511 mouse chondrocytes | HIF-2/Fas | Mediate chondrocyte apoptosis and regulates autophagy in maturing chondrocytes | [44][45] | |
| Human CD14+ monocytes | -- | Modulate osteoclast differentiation and formation | [46] | |
| Male mice | HIF-2/p16 and p21 | Act as a senescence-related intrinsic factor in age-related dysfunction of bone homeostasis | [35] | |
| Murine experimental OA models | NF-κB-HIF-2α pathway | Promote OA development | [36] |
References
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472.
- Yellowley, C.E.; Genetos, D.C. Hypoxia Signaling in the Skeleton: Implications for Bone Health. Curr. Osteoporos. Rep. 2019, 17, 26–35.
- Park, K.M.; Gerecht, S. Hypoxia-inducible hydrogels. Nat. Commun. 2014, 5, 4075.
- Pangou, E.; Befani, C.; Mylonis, I.; Samiotaki, M.; Panayotou, G.; Simos, G.; Liakos, P. HIF-2α phosphorylation by CK1δ pro motes erythropoietin secretion in liver cancer cells under hypoxia. J. Cell Sci. 2016, 129, 4213–4226.
- Liang, K.; Ding, X.-Q.; Lin, C.; Kang, Y.J. Featured Article: Hypoxia-inducible factor-1α dependent nuclear entry of factor inhibiting HIF-1. Exp. Biol. Med. 2015, 240, 1446–1451.
- Zelzer, E.; Levy, Y.; Kahana, C.; Shilo, B.; Rubinstein, M.; Cohen, B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J. 1998, 17, 5085–5094.
- Rauner, M.; Franke, K.; Murray, M.; Singh, R.P.; Hiram-Bab, S.; Platzbecker, U.; Gassmann, M.; Socolovsky, M.; Neumann, D.; Gabet, Y.; et al. Increased EPO Levels Are Associated With Bone Loss in Mice Lacking PHD2 in EPO-Producing Cells. J. Bone Miner. Res. 2016, 31, 1877–1887.
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.-W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275.
- Gkotinakou, I.-M.; Befani, C.; Simos, G.; Liakos, P. ERK1/2 phosphorylates HIF-2α and regulates its activity by controlling its CRM1-dependent nuclear shuttling. J. Cell Sci. 2019, 132, jcs225698.
- Suzuki, N.; Gradin, K.; Poellinger, L.; Yamamoto, M. Regulation of hypoxia-inducible gene expression after HIF activation. Exp. Cell Res. 2017, 356, 182–186.
- Tolonen, J.-P.; Heikkilä, M.; Malinen, M.; Lee, H.-M.; Palvimo, J.J.; Wei, G.-H.; Myllyharju, J. A long hypoxia-inducible factor 3 isoform 2 is a transcription activator that regulates erythropoietin. Cell. Mol. Life Sci. 2020, 77, 3627–3642.
- Jahangir, S.; Hosseini, S.; Mostafaei, F.; Sayahpour, F.A.; Eslaminejad, M.B. 3D-porous β-tricalcium phosphate–alginate–gelatin scaffold with DMOG delivery promotes angiogenesis and bone formation in rat calvarial defects. J. Mater. Sci. Mater. Med. 2018, 30, 1.
- Zou, D.; He, J.; Zhang, K.; Dai, J.; Zhang, W.; Wang, S.; Zhou, J.; Huang, Y.; Zhang, Z.; Jiang, X. The Bone-Forming Effects of HIF-1α-Transduced BMSCs Promote Osseointegration with Dental Implant in Canine Mandible. PLoS ONE 2012, 7, e32355.
- Nagai, K.; Ideguchi, H.; Kajikawa, T.; Li, X.; Chavakis, T.; Cheng, J.; Messersmith, P.B.; Heber-Katz, E.; Hajishengallis, G. An injectable hydrogel-formulated inhibitor of prolyl-4-hydroxylase promotes T regulatory cell recruitment and enhances alveolar bone regeneration during resolution of experimental periodontitis. FASEB J. 2020, 34, 13726–13740.
- Cheng, S.; Xing, W.; Pourteymoor, S.; Schulte, J.; Mohan, S. Conditional Deletion of Prolyl Hydroxylase Domain-Containing Protein 2 (Phd2) Gene Reveals Its Essential Role in Chondrocyte Function and Endochondral Bone Formation. Endocrinology 2016, 157, 127–140.
- Stegen, S.; Deprez, S.; Eelen, G.; Torrekens, S.; Van Looveren, R.; Goveia, J.; Ghesquière, B.; Carmeliet, P.; Carmeliet, G. Adequate hypoxia inducible factor 1α signaling is indispensable for bone regeneration. Bone 2016, 87, 176–186.
- Tan, L.; Zhang, Y.; Huang, Y.; Luo, Y.; Liu, Y. Preservation of alveolar ridge after tooth extraction with hypoxia-inducible factor-1α protein in a dog model. Exp. Ther. Med. 2019, 17, 2913–2920.
- Liu, W.; Li, L.; Rong, Y.; Qian, D.; Chen, J.; Zhou, Z.; Luo, Y.; Jiang, D.; Cheng, L.; Zhao, S.; et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020, 103, 196–212.
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328.
- Zhang, J.; Pan, J.; Jing, W. Motivating role of type H vessels in bone regeneration. Cell Prolif. 2020, 53, e12874.
- Ahn, G.-O.; Seita, J.; Hong, B.-J.; Kim, Y.-E.; Bok, S.; Lee, C.-J.; Kim, K.S.; Lee, J.C.; Leeper, N.J.; Cooke, J.P.; et al. Transcriptional activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells promotes angiogenesis through VEGF and S100A8. Proc. Natl. Acad. Sci. USA 2014, 111, 2698–2703.
- Yoon, D.; Pastore, Y.D.; Divoky, V.; Liu, E.; Mlodnicka, A.E.; Rainey, K.; Ponka, P.; Semenza, G.L.; Schumacher, A.; Prchal, J.T. Hypoxia-inducible Factor-1 Deficiency Results in Dysregulated Erythropoiesis Signaling and Iron Homeostasis in Mouse Development. J. Biol. Chem. 2006, 281, 25703–25711.
- Yu, Y.; Ma, L.; Zhang, H.; Sun, W.; Zheng, L.; Liu, C.; Miao, L. EPO could be regulated by HIF-1 and promote osteogenesis and accelerate bone repair. Artif. Cells Nanomed. Biotechnol. 2020, 48, 206–217.
- Li, C.-T.; Liu, J.-X.; Yu, B.; Liu, R.; Dong, C.; Li, S.-J. Notch signaling represses hypoxia-inducible factor-1α-induced activation of Wnt/β-catenin signaling in osteoblasts under cobalt-mimicked hypoxia. Mol. Med. Rep. 2016, 14, 689–696.
- Zhao, H.; Yeersheng, R.; Xia, Y.; Kang, P.; Wang, W. Hypoxia Enhanced Bone Regeneration Through the HIF-1α/β-Catenin Pathway in Femoral Head Osteonecrosis. Am. J. Med. Sci. 2021, 362, 78–91.
- So, E.; Sun, C.; Wu, K.Q.; Driesman, A.; Leggett, S.; Isaac, M.; Spangler, T.; Dubielecka-Szczerba, P.M.; Reginato, A.M.; Liang, O.D. Lipid phosphatase SHIP-1 regulates chondrocyte hypertrophy and skeletal development. J. Cell. Physiol. 2020, 235, 1425–1437.
- Hirai, K.; Furusho, H.; Hirota, K.; Sasaki, H. Activation of hypoxia-inducible factor 1 attenuates periapical inflammation and bone loss. Int. J. Oral Sci. 2018, 10, 12.
- Meng, S.-S.; Xu, X.-P.; Chang, W.; Lu, Z.-H.; Huang, L.-L.; Xu, J.-Y.; Liu, L.; Qiu, H.-B.; Yang, Y.; Guo, F.-M. LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning. Stem Cell Res. Ther. 2018, 9, 280.
- Tang, Y.; Hong, C.; Cai, Y.; Zhu, J.; Hu, X.; Tian, Y.; Song, X.; Song, Z.; Jiang, R.; Kang, F. HIF-1α Mediates Osteoclast-Induced Mandibular Condyle Growth via AMPK Signaling. J. Dent. Res. 2020, 99, 1377–1386.
- Kapitsinou, P.P.; Liu, Q.; Unger, T.L.; Rha, J.; Davidoff, O.; Keith, B.; Epstein, J.A.; Moores, S.L.; Erickson-Miller, C.L.; Haase, V.H. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood 2010, 116, 3039–3048.
- Saito, T.; Fukai, A.; Mabuchi, A.; Ikeda, T.; Yano, F.; Ohba, S.; Nishida, N.; Akune, T.; Yoshimura, N.; Nakagawa, T.; et al. Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat. Med. 2010, 16, 678–686.
- Lee, S.Y.; Park, K.H.; Yu, H.-G.; Kook, E.; Song, W.-H.; Lee, G.; Koh, J.-T.; Shin, H.-I.; Choi, J.-Y.; Huh, Y.H.; et al. Controlling hypoxia-inducible factor-2α is critical for maintaining bone homeostasis in mice. Bone Res. 2019, 7, 14.
- Merceron, C.; Ranganathan, K.; Wang, E.; Tata, Z.; Makkapati, S.; Khan, M.P.; Mangiavini, L.; Yao, Q.; Castellini, L.; Levi, B.; et al. Hypoxia-inducible factor 2α is a negative regulator of osteoblastogenesis and bone mass accrual. Bone Res. 2019, 7, 7.
- Hannah, S.S.; McFadden, S.; McNeilly, A.; McClean, C. “Take My Bone Away?” Hypoxia and bone: A narrative review. J. Cell. Physiol. 2021, 236, 721–740.
- Lee, S.Y.; Park, K.H.; Lee, G.; Kim, S.-J.; Song, W.-H.; Kwon, S.-H.; Koh, J.-T.; Huh, Y.H.; Ryu, J.-H. Hypoxia-inducible factor-2α mediates senescence-associated intrinsic mechanisms of age-related bone loss. Exp. Mol. Med. 2021, 53, 591–604.
- Yang, S.; Kim, J.; Ryu, J.-H.; Oh, H.; Chun, C.-H.; Kim, B.J.; Min, B.H.; Chun, J.-S. Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 2010, 16, 687–693.
- Saito, T.; Kawaguchi, H. HIF-2α as a possible therapeutic target of osteoarthritis. Osteoarthr. Cartil. 2010, 18, 1552–1556.
- Saito, T. NF-κB and HIF Signaling in Osteoarthritis. Encyclopedia of Bone Biology; Zaidi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 605–608. ISBN 9780128140826.
- Bouaziz, W.; Sigaux, J.; Modrowski, D.; Devignes, C.-S.; Funck-Brentano, T.; Richette, P.; Ea, H.-K.; Provot, S.; Cohen-Solal, M.; Haÿ, E. Interaction of HIF1α and β-catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proc. Natl. Acad. Sci. USA 2016, 113, 5453–5458.
- Fröhlich, L.F. Micrornas at the Interface between Osteogenesis and Angiogenesis as Targets for Bone Regeneration. Cells 2019, 8, 121.
- Ghosh, G.; Subramanian, I.V.; Adhikari, N.; Zhang, X.; Joshi, H.P.; Basi, D.; Chandrashekhar, Y.; Hall, J.L.; Roy, S.; Zeng, Y.; et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-α isoforms and promotes angiogenesis. J. Clin. Investig. 2010, 120, 4141–4154.
- Maes, C.; Carmeliet, G.; Schipani, E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat. Rev. Rheu matol. 2012, 8, 358–366.
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53.
- Zhang, F.-J.; Luo, W.; Lei, G.-H. Role of HIF-1α and HIF-2α in osteoarthritis. Jt. Bone Spine 2015, 82, 144–147.
- Bohensky, J.; Terkhorn, S.P.; Freeman, T.A.; Adams, C.S.; Garcia, J.A.; Shapiro, I.M.; Srinivas, V. Regulation of autophagy in human and murine cartilage: Hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Care Res. 2009, 60, 1406–1415.
- Knowles, H.J. Distinct roles for the hypoxia-inducible transcription factors HIF-1α and HIF-2α in human osteoclast formation and function. Sci. Rep. 2020, 10, 21072.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
997
Revisions:
4 times
(View History)
Update Date:
13 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





