Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
作为稳态的中心介质,缺氧诱导的转录因子(HIFs)可以使细胞在低氧环境中存活,并且对于骨形成和骨骼修复的调节至关重要。
- hypoxia-inducible factors
- HIF
- osteogenesis
1. Hypoxia-Inducible Factors
Hypoxia-inducible factors (HIFs) are transcriptional activator complexes that perform a central role in the expression of oxygen-regulated genes. These genes are involved in the proliferation and apoptosis of cells, angiogenesis, erythropoiesis, energy metabolism, vasomotor function, and so on [15,16]. Thus, HIFs are essential for normal growth and development and also participate in the pathological processes, including tumor progression and tissue regeneration [17]. Heterodimeric transcription factors (HIFs) complex are composed of α-subunits (HIF-1α, HIF-2α, and HIF-3α) and the β-subunit (HIF-1β)/aryl hydrocarbon receptor nuclear translocator (ARNT). HIF-1β/ARNT is expressed stably in cells, whereas HIF-αs are degraded under the condition of normal oxygen bioavailability and accumulate rapidly in a hypoxic environment. HIF-1α, HIF-2α, and HIF-3α bind to HIF-1β to form HIF-1, HIF-2, and HIF-3, respectively. Thus, the stability of the HIF-1α subunit seems to determine HIF-1 formation. Similarly, the formation of HIF-2 is mainly determined by the abundance of the HIF-2α subunit.
In mammalian cells, three HIF-α subunit isoforms (HIF-1α, HIF-2α, and HIF-3α) are encoded by three HIF-α genes: HIF1A, HIF2A, and HIF3A, respectively. When oxygen concentration drops to <5%, HIF-1α is stably expressed, enters the nucleus, dimerizes with HIF-1β, and binds to HIF-response elements (HRE) of targeted gene promoters [16]. When oxygen is abundant in cells (>5%), the Prolyl-4-hydroxylases (PHDs) bind to HIF-1α and hydroxylate the proline residues, which leads to the recruitment of the Von Hippel-Landau (VHL) tumor suppressor E3 ligase complex. Eventually, the proteasomal is poly-ubiquitylated and degraded [18]. In addition, factor inhibiting HIF (FIH) also restricts the binding of HIF-αs to transcriptional co-activators CBP/p300 through hydroxylating (N-terminal) asparaginyl residues when oxygen is abundant [19]. HIF-2α is regulated by oxygen in a similar manner to HIF-1α. In addition to intracellular oxygen tension, several growth factors can also regulate HIF-α subunits in a hypoxia-independent way [20]. HIF-1 has been studied more extensively than HIF-2, and HIF-1 and HIF-2 have overlapping and unique biological functions. It is reported that HIF-1α responds to acute hypoxia mainly, whereas HIF-2α is the prime subunit that responded to chronic exposure to low oxygen at high altitudes [21]. HIF-1α is generally expressed in cells and regulates downstream genes, including VEGF, GLUT-1, AK-3, ALD-A, PGK-1, PFK-L, and LDH-A through binding to HRE to regulate many metabolic enzymes [22] (Maxwell, 1999). The role of HIF-1 in promoting angiogenesis also benefits cancer development. HIF-2 regulates erythropoiesis and vascularization and is essential for embryonic development [18]. In addition, HIF-2 is also involved in the progression and metastasis of solid tumors [23]. Another HIF-α protein, HIF-3α, can bind to ARNTs to restrain HIF-1α- or HIF-2α-mediated transcription, but its transcriptional capacity is weaker than other HIFs [16,24]. HIF-3α is relatively unknown in terms of regulating the hypoxia response, and many studies have shown that HIF-3α may play a dual part as a hypoxia-inducible transcription factor in recent years [25]. The determination of genome-wide binding of the human HIF-3 and its role requires extensive scientific research (Figure 1).
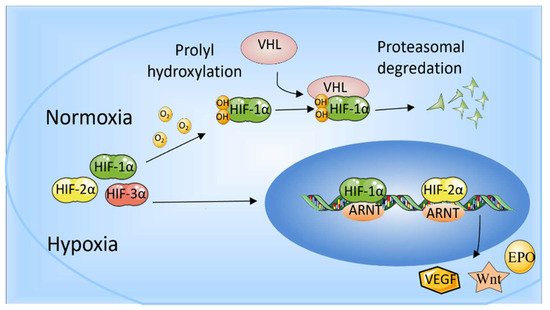
Figure 1. The role of HIF-1α and HIF-2α. When oxygen levels are low (hypoxia), HIF-1α and HIF-2α are protected from degradation and accumulate in the nucleus, where they bind to ARNT and bind to specific DNA fragments in hypoxia regulatory genes. At normal oxygen levels, oxygen regulates the degradation process by adding hydroxyl groups to HIF-αs. VHL recognizes and forms a complex that carries HIF-αs and degrades them in an oxygen-dependent manner.
2. Effect of HIFs on Bone
最近的研究表明HIF-1在骨骼生长和修复中的作用。参考文献[26]使用大鼠颅骨缺陷中含有二甲基噁酰甘氨酸(DMOG)的海绵支架来模仿缺氧以上调HIF-1α,发现血管生成加速,骨再生增强。参考文献[27]发现HIF-1α可以促进组织工程骨,牙科植入物和植入物周围的新骨形成的骨整合,这在犬类模型中得到了验证。另一项研究表明,皮下注射1,4-二氢苯甲醚-4-酮-3-羧酸(1,4-DPCA/水凝胶),一种水凝胶配方的PHD抑制剂,小鼠牙龈HIF-1α蛋白的表达明显增加,并且在牙周炎消退开始时骨再生能力增强[28]。软骨细胞中phd2的基因消融通过HIF-1α信号传导的上调促进软骨内骨成骨,导致长骨和椎骨的显着生长[29]。在骨再生过程中,HIF-1α不仅促进血管生成,还通过诱导糖酵解转化来调节代谢适应,以促进细胞存活[30]。因此,HIF-1在成骨和骨骼修复中起着不可或缺的作用[26,31]。当成骨细胞和其他相关细胞感觉到氧张力降低时,细胞内HIF-1α稳定表达以调节血管生成和成骨基因的表达[32]。此外,HIF-1调节下游基因以促进成骨和骨修复的机制相当复杂。HIF-1在介导下游信号以调节不同动物模型或细胞中的骨量中的作用如表1所示。
表 1.HIF-1通过调节不同动物模型或细胞中的不同信号起作用。
| 高频 | 小鼠模型 /细胞 |
信号 通路 |
影响 | 裁判。 |
|---|---|---|---|---|
| 高频-1 | EC特异性功能丧失小鼠(Hif1a断续器) | 高夫-1/聚四氟乙烯 | H型血管数量增加,软骨内血管生成和成骨增强 | [3,33] |
| 成熟成骨细胞 | 高夫-1/聚四氟乙烯 | 有助于协调软骨内骨发育中的血管形成、骨化和基质再吸收 | [16,24] | |
| 后肢缺血的小鼠模型 | 高夫-1/聚四氟乙烯 | 骨髓细胞中的HIF-1激活促进血管生成 | [34] | |
| 高磷脂-1α缺陷胚胎 | 海夫-1/欧洲专利局 | 影响胚胎发育 | [35] | |
| 大鼠颅骨缺损模型 | 海夫-1/欧洲专利局 | 促进成骨,加速骨修复 | [4] | |
| 中控 3T3-E1 | 高频-1/断续器 | 促进成骨细胞增殖 | [36] | |
| 骨髓增生干细胞治疗股骨头坏死 | HIF-1/β-连环蛋白 | 减少细胞凋亡,降低空隙率,增强骨形成,更强壮的小梁骨 | [37] | |
| 相位分布图α + 血清-1+(PαS) MSC | 飞船-1 | 在缺氧状态下,SHIP-1保持HIF-1α在Pαs间量子力学中的稳定表达,降低HIF-1α的表达抑制了海博-1KOPαs间质干细胞的增殖 | [38] | |
| 小鼠根尖周病变 | 高夫-1/NF-κB | 减少根尖周骨质流失,抑制破骨细胞 | [39] | |
| 间充质干细胞 | 希夫-1/断续器4和断续器7 | 促进间充质干细胞的迁移和生存能力 | [40] | |
| 10-wk-old破骨细胞特异性HIF-1α条件性敲除小鼠 | 高频-1/安培 | 维持破骨细胞诱导的钙化软骨基质的再吸收 | [41] |
与HIF-1相比,HIF-2在成骨中的作用尚不清楚[42]。HIF-1和HIF-2对骨形成的调节具有重叠和相反的作用。研究表明,HIF-2α可以上调VEGFA,COL10A1和MMP13的表达,并且是一些用于软骨内骨化的关键基因的中枢反式激活剂。当HIF-2α表达降低时,软骨细胞肥大,基质降解和血管形成以及其他后续步骤受损。此外,与生理性软骨内骨化相比,HIF-2α在病理性软骨内骨化中的作用更为关键[43]。研究还表明,HIF-2可以上调Sox9的表达以影响成骨细胞的分化并消极地调节成骨,靶标Twist2可以下调Runt相关转录因子2(Runx2)和骨钙素,并抑制成骨细胞分化[44,45]。此外,HIF-2可能通过在骨祖细胞中靶向RANKL来介导成骨细胞和破骨细胞之间的串扰[44,46]。成骨细胞和破骨细胞中HIF-2α表达的上调是年龄相关性骨质流失的一种新的内在介质[47]。此外,研究表明,HIF-2α作为NF-κB的直接转录靶标,通过调节骨关节炎(OA)中的关键分解代谢基因来破坏软骨[48,49]。然而,HIF-1α抑制NF-κB-HIF-2α途径以防止软骨降解[50]。Bouaziz等人证明,HIF-1α与β-连环蛋白相互作用,后者抑制转录因子4-β-连环蛋白转录活性,提示HIF-1α在关节软骨稳态和生长板软骨细胞中起重要作用[51]。在血管生成的背景下,据报道,HIF可以控制内皮细胞(EC)中缺氧miR-424的进一步上调。这反过来又有助于HIF蛋白稳定以适应低氧条件并诱导血管生成[52,53]。HIF-2通过介导不同动物模型或细胞中的下游信号来调节骨稳态的作用如表2所示。
表 2.HIF-2通过调节不同动物模型或细胞中的不同信号起作用。
This entry is adapted from the peer-reviewed paper 10.3390/ijms231911201
This entry is offline, you can click here to edit this entry!
