You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesco Scavello | -- | 2136 | 2022-10-12 13:43:12 | | | |
| 2 | Conner Chen | + 7 word(s) | 2143 | 2022-10-13 10:05:44 | | | | |
| 3 | Conner Chen | -11 word(s) | 2132 | 2022-10-19 14:47:27 | | | | |
| 4 | Conner Chen | -6 word(s) | 2126 | 2022-10-21 02:39:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Scavello, F.; Amiche, M.; Ghia, J. Chromogranin A -Derived Peptides as Inflammatory Modulator Molecules. Encyclopedia. Available online: https://encyclopedia.pub/entry/29059 (accessed on 15 December 2025).
Scavello F, Amiche M, Ghia J. Chromogranin A -Derived Peptides as Inflammatory Modulator Molecules. Encyclopedia. Available at: https://encyclopedia.pub/entry/29059. Accessed December 15, 2025.
Scavello, Francesco, Mohamed Amiche, Jean-Eric Ghia. "Chromogranin A -Derived Peptides as Inflammatory Modulator Molecules" Encyclopedia, https://encyclopedia.pub/entry/29059 (accessed December 15, 2025).
Scavello, F., Amiche, M., & Ghia, J. (2022, October 12). Chromogranin A -Derived Peptides as Inflammatory Modulator Molecules. In Encyclopedia. https://encyclopedia.pub/entry/29059
Scavello, Francesco, et al. "Chromogranin A -Derived Peptides as Inflammatory Modulator Molecules." Encyclopedia. Web. 12 October, 2022.
Copy Citation
Chromogranin A (CgA) is a glyco-phosphoprotein discovered for the first time in the adrenal medulla but also produced in several cells. CgA can generate different derived antimicrobial peptides (AMPs) influencing numerous physiological processes. CgA-derived peptides modulate inflammation and represent an example of endogenous Multifunctional AMPs (MF-AMPs).
antimicrobial peptides
multifunctional antimicrobial peptides
chromogranin A-derived peptides
immunomodulators
1. CgA-Derived AMPs
Chromogranin A (CgA) is a glycoprotein with 431 residues and a molecular weight of 49 kDa belonging to the Granin family, discovered for the first time at the end of the 1960s in the granules of adrenomedullary chromaffin cells [1]. In the last few decades, CgA has been identified in immune cells [2][3][4], neurons [5], cardiomyocytes [6], keratinocytes and fibroblasts [7]. This protein may influence different physiological processes. Its role was reported in cardiac function and cardio-protection [8], catecholamine storage and feedback release [9] and the modulation of vascular function [10] but also in cellular recruitment and the modulation of immune response [1][4][11]. However, this prohormone produces, by proteolytic processing, active biological peptides, such as Vasostatins (Vs), Prochromacin, Chromacin, Pancreastatin, WE 14, Catestatin (Cts), Parastatin and Serpinin [12][13]. At the end of the 1990s and early 2000s, several CgA-derived peptides were discovered as antimicrobial peptides (AMPs) acting against several bacteria, fungi and yeast. The CgA-derived peptides have been found in biological fluids involved in host-defense responses, such as serum, saliva and neutrophils secretions, or against pathogens in the first barrier of the human body, such as the skin [2][3][7][14][15]. The CgA-derived peptides act as antimicrobial agents in the micro-molar range [2][15][16][17]. These concentrations are also reported in the biological fluid after stimulation with pathogen toxins or during infection [2][3][11][15]. Among the CgA-derived peptides, the Vs-I and Cts were first identified as antimicrobial agents; however, their antimicrobial domains were rapidly reported. Vs-I was initially identified as a vasoinhibitory agent [18]. For Vs-I, Lugardon characterized this peptide’s antimicrobial activities against many pathogens [2][16][19]. However, after the incubation of Vs-I with endoproteinase Glu-C, a digested sequence CgA47-66, called Chromofungin (Chr), was identified and found highly active against several fungi and yeasts [16]. It has a global hydrophobicity and amphipathic character, allowing a strong interaction with the membrane. Specifically, Chr possesses a positive charge of +3.5, showing an amphipathic helix in the C-terminal part in the sequence CgA53-66 and at the N-terminal domain, a hydrophobic sequence corresponding to CgA48-51 and a hydrophilic structure CgA53-46, respectively [16][20]. The Vs-I and Chr antimicrobial mechanism of action is explained through the specific interaction of peptides with ergosterol, one of the main components of yeast and fungal membranes, inducing increased pressure and penetration into the membrane [16][20] (Figure 1). Other data demonstrated that Chr could inhibit Calcineurin activity by interacting with Calmodulin [16] (Figure 1). Within microbial cells, Vs-I and Chr may interfere with the Calcium/Calmodulin/Calcineurin signaling pathway by blocking the pathway implicated in virulence and skeleton development of cell walls [21]. Cts was identified as a catecholamine release-inhibitory peptide [22]. Cts is a small 21-amino-acid cationic peptide with a positive net charge of +5 within the bovine sequence (bCgA344-364) possessing a C-terminal hydrophobic sequence. Taylor and colleagues identified a smaller peptide (CgA344-358) derived from Cts with a more substantial inhibitory effect on catecholamine release [23]. This peptide was called Cateslytin (Ctl) by Briolat et al. and is also characterized by its antimicrobial activities with potent effects compared to Cts [15]. Ctl is also a positively charged (+5) arginine-rich antimicrobial peptide and, in an aqueous solution, is a linear peptide with a disordered structure. However, when interacting with the membrane, Ctl acquires an α-helical form [24]. Other studies with a system mimicking bacterial membrane demonstrated that Ctl could convert its structure into antiparallel β-sheets precipitating against the negatively charged part of the membranes [25]. Then, Ctl induced an increased rigidity, permeability gradient and membrane pore formation in the domains containing ergosterol [25][26][27] (Figure 1).
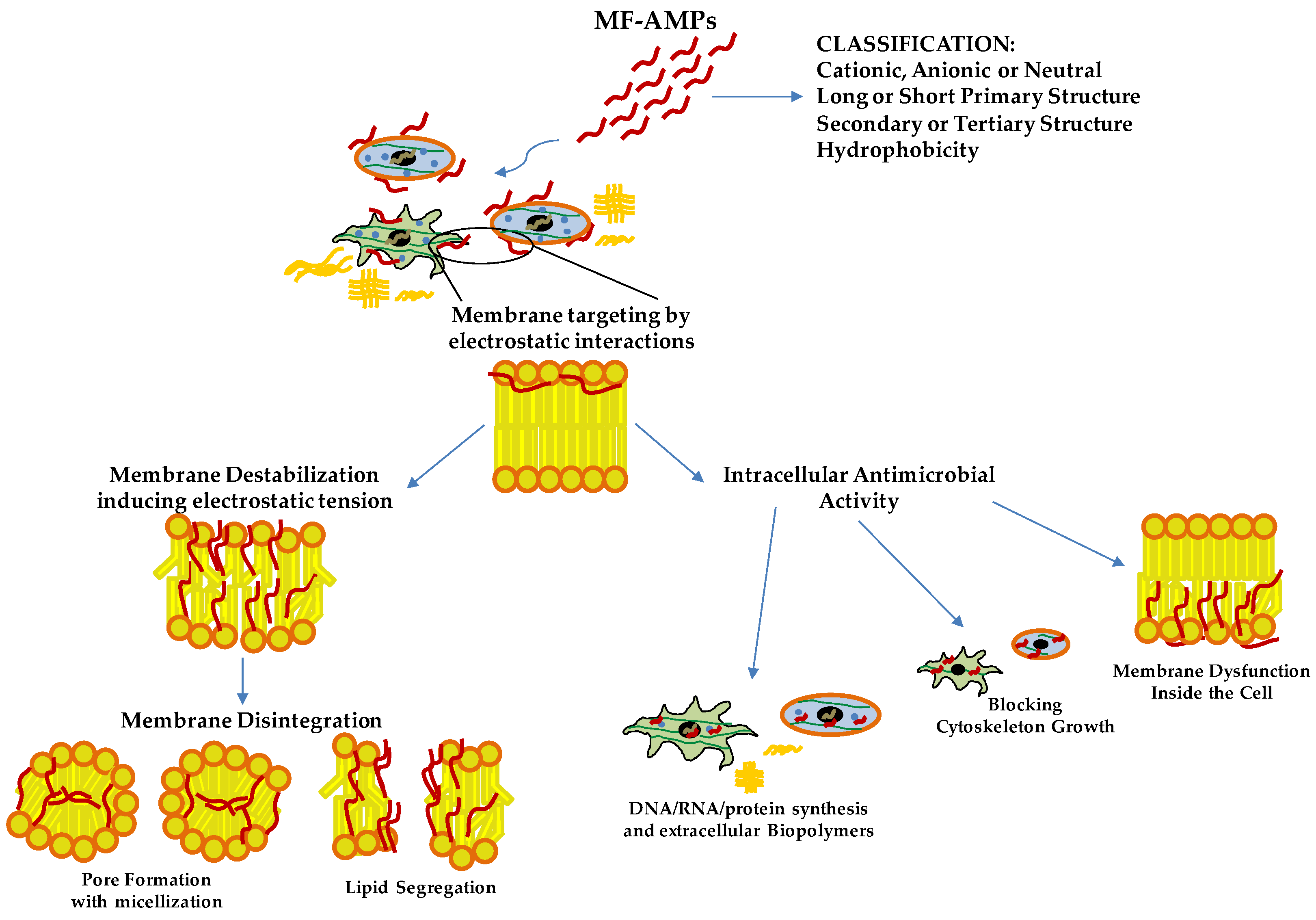
Figure 1. Antimicrobial Mechanisms of Action of Multifunctional Antimicrobial Peptides (MF-AMPs). Based on their biochemical characteristics, MF-AMPs can interact with the membrane lipids, inducing the pathogens’ instability and rigidity of the cell membrane. Upon reaching the minimum inhibitory concentration (MIC) values, they cause pore formation and cell lysis. Some MF-AMPs exert a cell-penetrating peptides-like function. They can penetrate the membrane, blocking pathogens’ growth by internal membrane dysfunction, cytoskeleton interaction and interference of biomolecules production. Deoxyribonucleic acid (DNA); ribonucleic acid (RNA).
2. CgA-Derived Peptides and Immune Cells Activities and Inflammation
The involvement of CgA and its derived peptides in innate immunity is well known for its antimicrobial activity. In addition, the role of these MF-AMPs is also reported in immune cells, conferring them a complex profile of immunity modulators. The role of CgA-derived peptides on immune cells was studied for the first time in 2009; in particular, the effects of Chr and Cts were evaluated on polymorphonuclear neutrophils. After the treatments with the peptides, Chr and Cts were observed inside the cells, demonstrating their ability to penetrate the mammalian membrane and the profile of cell-penetrating peptides (CPPs) [3]. Then, in the presence of extracellular calcium, the two peptides induced a transient calcium influx in the cells, binding Calmodulin-binding factors (W7 and CMZ) and activating iPLA2 [3]. In addition, the pharmacological block of these channels inhibited the calcium flux induced by Chr and Cts [3]. On the other hand, when extracellular calcium is absent, the peptides cannot induce calcium secretion [3]. Notably, the secretion of polymorphonuclear neutrophils treated with the two CgA-derived peptides induced the secretion of several important factors for innate immunity and inflammation, such as Lactotransferrin, Lysozyme, Neutrophil Gelatinase Associated Lipocalin and S100 calcium-binding protein A8/A9 [3]. Several studies reported the role of Vs-1 as an anti-atherogenesis and anti-inflammatory factor suppressing the adhesion of monocytes to endothelial cells by adhesion molecule down-regulation [28][29]. Xiong and coworkers reported the anti-inflammatory role of Vs-II (N-terminal fragment of CgA containing Vs-I; CgA1-113) in an apolipoprotein E-deficient (ApoE−/−) mice model fed with a high-fat diet developing atherosclerosis. In this study, Vs-II treatment reduced the occurrence of atherosclerotic plaque and attenuated lesions [28]. Furthermore, Vs-II significantly reduced the production of pro-inflammatory cytokines in aortic tissue, such as Tumor Necrosis Factor-α (TNF-α), Monocyte Chemoattractant Protein-1 (MCP-1) and Vascular Cell Adhesion Molecule-1 (VCAM-1) [28]. The same authors demonstrated, by several in vivo analyses, that these anti-inflammatory properties are based on the ability of Vs-II to reduce leukocytes adhesion on ApoE−/− mice arteries but also on the recruitment, transmigration and accumulation of M1 macrophages in the lesions [28]. In the same animal model, Sato et al. showed that Vs-I treatment reduces aortic atherosclerotic lesions development due to reductions in intra-plaque inflammation, macrophage infiltration and aortic smooth muscle cells proliferation and plasma glucose level [29]. From a cellular point of view, Vs-I suppressed the lipopolysaccharide (LPS)-induced production of chemokine MCP1 and vascular damage markers, such as VCAM-1 and E-selectin, in human endothelial cells [29]. At the same time, Vs-I was found to reduce M1 pro-inflammatory macrophages differentiation and IL6 release but also oxidized low-density lipoprotein (oxLDL)-induced foam cell formation of macrophages [29]. Of great clinical interest, Vs-I is expressed around Monckeberg’s medial calcific sclerosis in human radial arteries [29]. Additionally, the immunomodulatory role of Chr has been reported in a mice model of ulcerative colitis induced by dextran sulfate sodium administration [30][31]. This model decreased Chr expression [31]. Furthermore, Chr treatment, by intracolonic administration, significantly reduced the inflammation and severity of colitis. The anti-inflammatory effects were due to the differentiation of macrophages into M2 anti-inflammatory clones with the consequent reduction of released IL-18 and the increased expression of M2 markers [30][31]. Using the same animal model, Kapoor and colleagues demonstrated that intrarectal Chr treatment reduced colitis severity and inflammation [32]. In parallel, this was associated with a significant decrease in the expression of CD11c, CD40, CD80, CD86 IL6 and IL12p40 in the inflamed colonic mucosa, mesenteric lymph nodes and spleen [32]. In addition, Chr reduces in CD11c positive cells the expression of CD80, CD86 and NF-κB in the spleen and colon, respectively [32]. All these in vivo data demonstrated that Chr has protective properties against intestinal inflammation and exerts the role of immunomodulator for intestinal macrophages and dendritic cells (DCs). These effects were also demonstrated in vitro with macrophages showing that Chr increased the production of anti-inflammatory factors and the M2 differentiation [30]. At the same time, Chr treatment significantly reduced the expression of M1 macrophage markers and the activation of the NF-κB pathway [31]. Additionally, in this case, in vitro experiments with M1 macrophages demonstrated that this peptide could decrease cellular migration, proinflammatory cytokines production and release, and NF-κB phosphorylation [31]. Furthermore, treatment with Chr or a conditioned medium of Chr-treated macrophages M2 induced epithelial cell proliferation and migration but also decreased oxidative stress and pro-inflammatory cytokine production [30]. In addition, the Chr treatment of naïve bone marrow-derived CD11c positive DCs reduced the LPS-induced expression of CD40, CD80, CD86 IL-6 and IL-12p40 [32]. These results were also confirmed in intestinal tissue isolated from patients with ulcerative colitis, demonstrating that Chr expression was down-regulated in these patients compared to healthy controls [31]. Indeed, the mRNA levels of Chr were positively correlated with the mRNA expression of M2 macrophages activation markers and negatively to the expression of collagen, IL-8 and IL-18, but also with M1 activation markers (TLR-4 expression and NF-kB activation) and consequent pro-inflammatory cytokines production [30][31]. In these patients, the reduction of Chr level is also associated with a negative linear relationship with CD11c and CD86 [32]. Moreover, another study confirmed the anti-inflammatory effects of Chr on monocytes. Treatment with this peptide significantly inhibited the transcription of pro-inflammatory factors, such as NF-kB and AP-1, in these cells [33]. Furthermore, Rabbi and colleagues showed that Cts reduced intestinal inflammation and the onset of colitis lesions by a Stat-3 activation [34]. At the same time, the markers of M1 macrophage activation and the colonic levels of pro-inflammatory cytokines, such as IL-6, IL-1β and TNF-α, were significantly decreased by Cts treatment [35]. However, Cts did not influence M2 macrophage markers [35]. Furthermore, these anti-inflammatory effects were confirmed in cellular experiments using macrophages isolated from the peritoneal cavity and the bone marrow, demonstrating that in vitro treatment with Cts significantly decreased the production of the pro-inflammatory cytokines and phosphorylation of Stat-3 [34]. In addition, peritoneal macrophages isolated from naïve mice and treated with Cts and LPS displayed a reduction in the expression and production of pro-inflammatory cytokines blocking the activation of M1 macrophages [35]. In addition, the genetic deletion of Cts induces hypertensive conditions and left ventricular hypertrophy accompanied by significant macrophage infiltration in cardiac tissue and adrenal gland [36]. In this context, the absence of Cts induced an increased level of pro-inflammatory cytokines TNF-α, C-C motif chemokine ligand (CCL)-2, 3, C-X-C motif chemokine ligand (CXCL)-1 and catecholamines but also an elevated inflammation in heart with the up-regulation of cardiac genes, such as Tnfa, Ifng, Emr1, Itgam, Itgax, Nos2a, IL12b, Ccl2 and Cxcl1 [36]. It is of great interest that the intraperitoneal administration of Cts reversed this phenotype. In addition, macrophage depletion blocked the onset of hypertension in Cts-knockout (KO) mice [36]. Furthermore, bone-marrow transfer of KO animals in wild-type (WT) counterparts induced hypertension and cardiac inflammation, while opposite conditions showed the opposite phenotype [36]. All these data strongly suggest that the anti-hypertensive effects of Cts are partially mediated by an immunosuppressive action of this peptide on macrophages [36]. The role of Cts as an immunomodulator was also explored in the context of atherosclerosis and vascular injury. In fact, in vitro treatment with Cts on endothelial cells significantly reduces the release of TNF-α and vascular damage markers, such as ICAM-1 and VCAM-1, after LPS exposure [37]. At the same time, Cts treatment suppresses inflammatory responses and oxidizes the low-density lipoprotein-induced foam cell formation of human macrophages [37]. Kojima and colleagues demonstrated that Cts injection to ApoE−/− mice significantly reduces macrophage infiltration and the consequent atherosclerotic lesions onset in the aorta but also suppresses aortic smooth muscle cells proliferation and collagen deposition in atheromatous plaques [37]. Furthermore, in vitro experiments with human aortic smooth muscle cells showed that Cts treatment can block collagen-1 and fibronectin expression and migration, proliferation and apoptotic process [37]. Of significant clinical impact, coronary artery disease patients displayed a substantial reduction of plasmatic levels of Cts but an increased expression in coronary atheromatous plaques [37]. In an acute pulmonary embolism in vivo model and cellular experiments with human pulmonary artery endothelial cells, Cts treatment was found to abolish thrombin-induced inflammation blocking TLR-4 expression and p38 phosphorylation, decreasing the consequent acute pulmonary embolism [38]. In conclusion, Vs-I and Chr than Cts are key attenuators of inflammation in different tissue and pathological conditions by reducing immune cell infiltration and inflammatory activation (Figure 2).
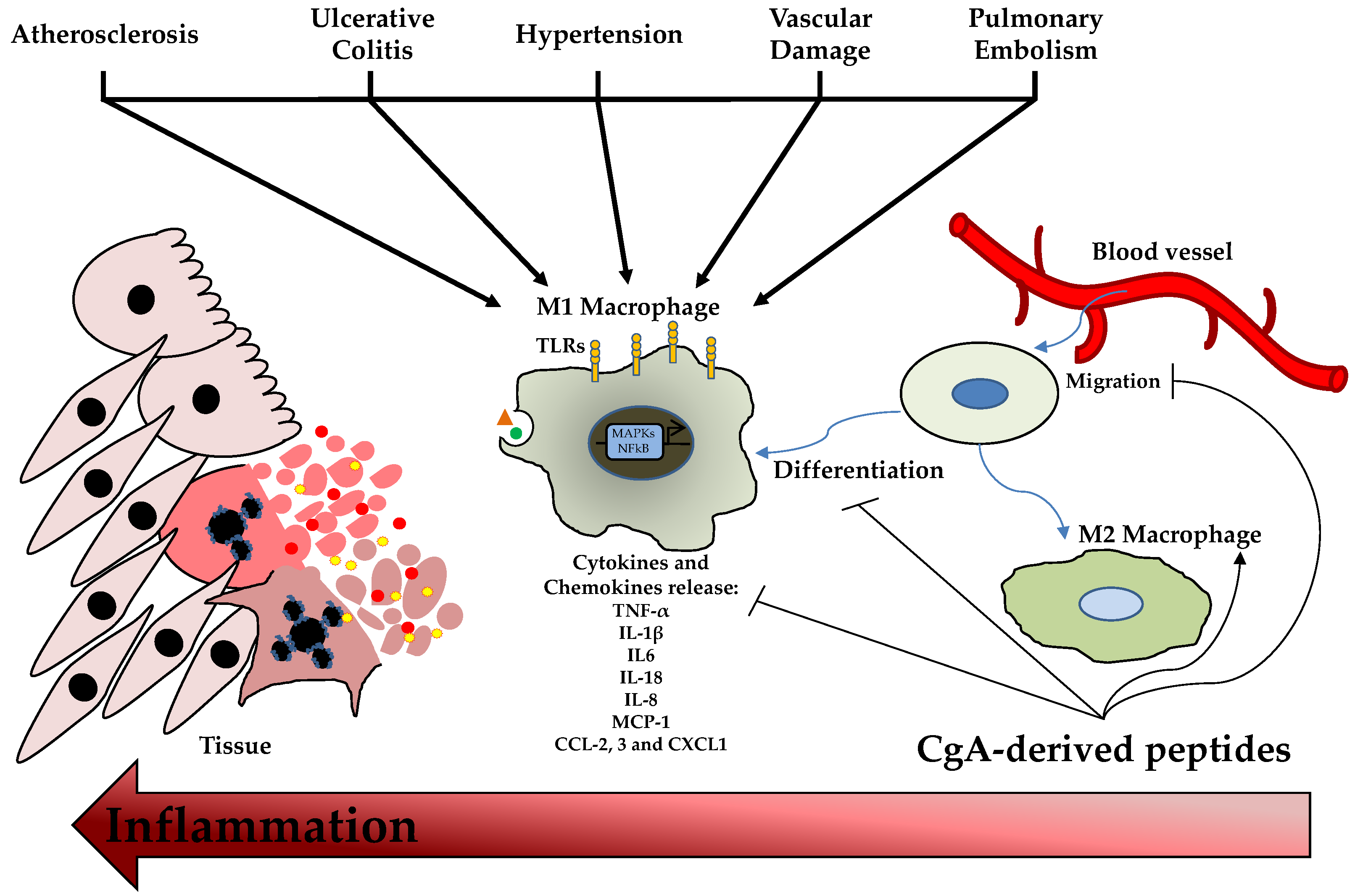
Figure 2. ChromgraninA (CgA)-derived Peptides and Inflammation. Damage or stress stimuli induce the migration, proliferation and activation of macrophages with consequent pro-inflammatory cytokines and chemokines release. This phenomenon generates cell death and inflammation. CgA-derived peptides can reduce M1 polarization and promote M2 anti-inflammatory macrophages differentiation. C-C motif chemokine ligand (CCL); C-X-C motif chemokine ligand (CXCL); interleukin (IL); monocyte chemoattractant protein (MCP); toll-like receptor (TLR); tumor necrosis factor (TNF).
References
- Helle, K.B.; Metz-Boutigue, M.H.; Cerra, M.C.; Angelone, T. Chromogranins: From discovery to current times. Pflugers Arch. 2018, 470, 143–154.
- Lugardon, K.; Raffner, R.; Goumon, Y.; Corti, A.; Delmas, A.; Bulet, P.; Aunis, D.; Metz-Boutigue, M.H. Antibacterial and antifungal activities of vasostatin-1, the N-terminal fragment of chromogranin A. J. Biol. Chem. 2000, 275, 10745–10753.
- Zhang, D.; Shooshtarizadeh, P.; Laventie, B.J.; Colin, D.A.; Chich, J.F.; Vidic, J.; de Barry, J.; Chasserot-Golaz, S.; Delalande, F.; Van Dorsselaer, A.; et al. Two chromogranin a-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated calcium independent phospholipase A2. PLoS ONE 2009, 4, e4501.
- Eissa, N.; Hussein, H.; Kermarrec, L.; Ali, A.Y.; Marshall, A.; Metz-Boutigue, M.H.; Hendy, G.N.; Bernstein, C.N.; Ghia, J.E. Chromogranin-A Regulates Macrophage Function and the Apoptotic Pathway in Murine DSS colitis. J. Mol. Med. 2018, 96, 183–198.
- Laguerre, F.; Anouar, Y.; Montero-Hadjadje, M. Chromogranin A in the early steps of the neurosecretory pathway. IUBMB Life 2020, 72, 524–532.
- Pasqua, T.; Corti, A.; Gentile, S.; Pochini, L.; Bianco, M.; Metz-Boutigue, M.H.; Cerra, M.C.; Tota, B.; Angelone, T. Full-length human chromogranin-A cardioactivity: Myocardial, coronary, and stimulus-induced processing evidence in normotensive and hypertensive male rat hearts. Endocrinology 2013, 154, 3353–3365.
- Radek, K.A.; Lopez-Garcia, B.; Hupe, M.; Niesman, I.R.; Elias, P.M.; Taupenot, L.; Mahata, S.K.; O’Connor, D.T.; Gallo, R.L. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J. Investig. Dermatol. 2008, 128, 1525–1534.
- Penna, C.; Tullio, F.; Perrelli, M.G.; Mancardi, D.; Pagliaro, P. Cardioprotection against ischemia/reperfusion injury and chromogranin A-derived peptides. Curr. Med. Chem. 2012, 19, 4074–4085.
- Kim, T.; Loh, Y.P. Chromogranin A: A surprising link between granule biogenesis and hypertension. J. Clin. Investig. 2005, 115, 1711–1713.
- Pasqua, T.; Rocca, C.; Spena, A.; Angelone, T.; Cerra, M.C. Modulation of the coronary tone in the expanding scenario of Chromogranin-A and its derived peptides. Future Med. Chem. 2019, 11, 1501–1511.
- Eissa, N.; Hussein, H.; Hendy, G.N.; Bernstein, C.N.; Ghia, J.E. Chromogranin-A and its derived peptides and their pharmacological effects during intestinal inflammation. Biochem. Pharmacol. 2018, 152, 315–326.
- Helle, K.B.; Angeletti, R.H. Chromogranin A: A multipurpose prohormone? Acta Physiol. Scand. 1994, 152, 1–10.
- Koshimizu, H.; Cawley, N.X.; Kim, T.; Yergey, A.L.; Loh, Y.P. Serpinin: A novel chromogranin A-derived, secreted peptide up-regulates protease nexin-1 expression and granule biogenesis in endocrine cells. Mol. Endocrinol. 2011, 25, 732–744.
- Mizuhashi, F.; Koide, K.; Toya, S.; Takahashi, M.; Mizuhashi, R.; Shimomura, H. Levels of the antimicrobial proteins lactoferrin and chromogranin in the saliva of individuals with oral dryness. J. Prosthet. Dent. 2015, 113, 35–38.
- Briolat, J.; Wu, S.D.; Mahata, S.K.; Gonthier, B.; Bagnard, D.; Chasserot-Golaz, S.; Helle, K.B.; Aunis, D.; Metz-Boutigue, M.H. New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell. Mol. Life Sci. 2005, 62, 377–385.
- Lugardon, K.; Chasserot-Golaz, S.; Kieffer, A.E.; Maget-Dana, R.; Nullans, G.; Kieffer, B.; Aunis, D.; Metz-Boutigue, M.H. Structural and biological characterization of chromofungin, the antifungal chromogranin A-(47-66)-derived peptide. J. Biol. Chem. 2001, 276, 35875–35882.
- Lugardon, K.; Chasserot-Golaz, S.; Kieffer, A.E.; Maget-Dana, R.; Nullans, G.; Kieffer, B.; Aunis, D.; Metz-Boutigue, M.H. Structural and biological characterization of chromofungin, the antifungal chromogranin A (47-66)-derived peptide. Ann. N. Y. Acad. Sci. 2002, 971, 359–361.
- Aardal, S.; Helle, K.B.; Elsayed, S.; Reed, R.K.; Serck-Hanssen, G. Vasostatins, comprising the N-terminal domain of chromogranin A, suppress tension in isolated human blood vessel segments. J. Neuroendocrinol. 1993, 5, 405–412.
- Metz-Boutigue, M.H.; Goumon, Y.; Strub, J.M.; Lugardon, K.; Aunis, D. Antimicrobial chromogranins and proenkephalin-A-derived peptides: Antibacterial and antifungal activities of chromogranins and proenkephalin-A-derived peptides. Ann. N. Y. Acad. Sci. 2003, 992, 168–178.
- Metz-Boutigue, M.H.; Kieffer, A.E.; Goumon, Y.; Aunis, D. Innate immunity: Involvement of new neuropeptides. Trends Microbiol. 2003, 11, 585–592.
- Park, H.S.; Lee, S.C.; Cardenas, M.E.; Heitman, J. Calcium-Calmodulin-Calcineurin Signaling: A Globally Conserved Virulence Cascade in Eukaryotic Microbial Pathogens. Cell Host Microbe 2019, 26, 453–462.
- Mahata, S.K.; O’Connor, D.T.; Mahata, M.; Yoo, S.H.; Taupenot, L.; Wu, H.; Gill, B.M.; Parmer, R.J. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J. Clin. Investig. 1997, 100, 1623–1633.
- Taylor, C.V.; Taupenot, L.; Mahata, S.K.; Mahata, M.; Wu, H.; Yasothornsrikul, S.; Toneff, T.; Caporale, C.; Jiang, Q.; Parmer, R.J.; et al. Formation of the catecholamine release-inhibitory peptide catestatin from chromogranin A. Determination of proteolytic cleavage sites in hormone storage granules. J. Biol. Chem. 2000, 275, 22905–22915.
- Jean-Francois, F.; Khemtemourian, L.; Odaert, B.; Castano, S.; Grelard, A.; Manigand, C.; Bathany, K.; Metz-Boutigue, M.H.; Dufourc, E.J. Variability in secondary structure of the antimicrobial peptide Cateslytin in powder, solution, DPC micelles and at the air-water interface. Eur. Biophys. J. 2007, 36, 1019–1027.
- Jean-Francois, F.; Castano, S.; Desbat, B.; Odaert, B.; Roux, M.; Metz-Boutigue, M.H.; Dufourc, E.J. Aggregation of cateslytin beta-sheets on negatively charged lipids promotes rigid membrane domains. A new mode of action for antimicrobial peptides? Biochemistry 2008, 47, 6394–6402.
- Jean-Francois, F.; Elezgaray, J.; Berson, P.; Vacher, P.; Dufourc, E.J. Pore formation induced by an antimicrobial peptide: Electrostatic effects. Biophys. J. 2008, 95, 5748–5756.
- Jean-Francois, F.; Desbat, B.; Dufourc, E.J. Selectivity of cateslytin for fungi: The role of acidic lipid-ergosterol membrane fluidity in antimicrobial action. FASEB J. 2009, 23, 3692–3701.
- Xiong, W.; Wang, X.; Dai, D.; Zhang, B.; Lu, L.; Tao, R. The anti-inflammatory vasostatin-2 attenuates atherosclerosis in ApoE(−/−) mice and inhibits monocyte/macrophage recruitment. Thromb. Haemost. 2017, 117, 401–414.
- Sato, Y.; Watanabe, R.; Uchiyama, N.; Ozawa, N.; Takahashi, Y.; Shirai, R.; Sato, K.; Mori, Y.; Matsuyama, T.; Ishibashi-Ueda, H.; et al. Inhibitory effects of vasostatin-1 against atherogenesis. Clin. Sci. 2018, 132, 2493–2507.
- Eissa, N.; Hussein, H.; Kermarrec, L.; Grover, J.; Metz-Boutigue, M.E.; Bernstein, C.N.; Ghia, J.E. Chromofungin Ameliorates the Progression of Colitis by Regulating Alternatively Activated Macrophages. Front. Immunol. 2017, 8, 1131.
- Eissa, N.; Hussein, H.; Kermarrec, L.; Elgazzar, O.; Metz-Boutigue, M.H.; Bernstein, C.N.; Ghia, J.E. Chromofungin (CHR: CHGA47-66) is downregulated in persons with active ulcerative colitis and suppresses pro-inflammatory macrophage function through the inhibition of NF-kappaB signaling. Biochem. Pharmacol. 2017, 145, 102–113.
- Kapoor, K.; Eissa, N.; Tshikudi, D.; Bernstein, C.N.; Ghia, J.E. Impact of intrarectal chromofungin treatment on dendritic cells-related markers in different immune compartments in colonic inflammatory conditions. World J. Gastroenterol. 2021, 27, 8138–8155.
- Schneider, F.; Marban, C.; Ajob, G.; Helle, S.; Guillot, M.; Launoy, A.; Maestraggi, Q.; Scavello, F.; Rohr, O.; Metz-Boutigue, M.H. In Trauma Patients, the Occurrence of Early-Onset Nosocomial Infections Is Associated with Increased Plasma Concentrations of Chromogranin A. Shock 2018, 49, 522–528.
- Rabbi, M.F.; Labis, B.; Metz-Boutigue, M.H.; Bernstein, C.N.; Ghia, J.E. Catestatin decreases macrophage function in two mouse models of experimental colitis. Biochem. Pharmacol. 2014, 89, 386–398.
- Rabbi, M.F.; Eissa, N.; Munyaka, P.M.; Kermarrec, L.; Elgazzar, O.; Khafipour, E.; Bernstein, C.N.; Ghia, J.E. Reactivation of Intestinal Inflammation Is Suppressed by Catestatin in a Murine Model of Colitis via M1 Macrophages and Not the Gut Microbiota. Front. Immunol. 2017, 8, 985.
- Ying, W.; Tang, K.; Avolio, E.; Schilling, J.M.; Pasqua, T.; Liu, M.A.; Cheng, H.; Gao, H.; Zhang, J.; Mahata, S.; et al. Immunosuppression of Macrophages Underlies the Cardioprotective Effects of CST (Catestatin). Hypertension 2021, 77, 1670–1682.
- Kojima, M.; Ozawa, N.; Mori, Y.; Takahashi, Y.; Watanabe-Kominato, K.; Shirai, R.; Watanabe, R.; Sato, K.; Matsuyama, T.A.; Ishibashi-Ueda, H.; et al. Catestatin Prevents Macrophage-Driven Atherosclerosis but Not Arterial Injury-Induced Neointimal Hyperplasia. Thromb. Haemost. 2018, 118, 182–194.
- Chen, H.; Liu, D.; Ge, L.; Wang, T.; Ma, Z.; Han, Y.; Duan, Y.; Xu, X.; Liu, W.; Yuan, J.; et al. Catestatin prevents endothelial inflammation and promotes thrombus resolution in acute pulmonary embolism in mice. Biosci. Rep. 2019, 39, BSR20192236.
More
Information
Subjects:
Immunology; Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
975
Revisions:
4 times
(View History)
Update Date:
21 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




