Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shuxin Li | -- | 1351 | 2022-10-11 22:56:58 | | | |
| 2 | Sirius Huang | Meta information modification | 1351 | 2022-10-12 06:16:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Huang, E.; Li, S. Cellular Functions of Liver Kinase B1. Encyclopedia. Available online: https://encyclopedia.pub/entry/28949 (accessed on 02 March 2026).
Huang E, Li S. Cellular Functions of Liver Kinase B1. Encyclopedia. Available at: https://encyclopedia.pub/entry/28949. Accessed March 02, 2026.
Huang, En, Shuxin Li. "Cellular Functions of Liver Kinase B1" Encyclopedia, https://encyclopedia.pub/entry/28949 (accessed March 02, 2026).
Huang, E., & Li, S. (2022, October 11). Cellular Functions of Liver Kinase B1. In Encyclopedia. https://encyclopedia.pub/entry/28949
Huang, En and Shuxin Li. "Cellular Functions of Liver Kinase B1." Encyclopedia. Web. 11 October, 2022.
Copy Citation
The liver kinase B1 (LKB1), also known as serine/threonine kinase 11 (STK11) and Par-4 in C. elegans, has been identified as a master kinase of AMPKs and AMPK-related kinases. LKB1 plays a crucial role in cell growth, metabolism, polarity, and tumor suppression.
liver kinase B1
neuronal polarity
Neuronal regeneration
1. Introduction
The liver kinase B1 (LKB1), also known as serine/threonine kinase 11 (STK11, Par-4 in C. elegans), was first identified in its mutated form with loss of function in Peutz–Jeghers syndrome (PJS), a rare dominantly inherited autosomal disease. Germline inactivating mutations of LKB1 are associated with the pathogenesis of PJS characterized by multiple benign polyps in the gastrointestinal system and numerous metastatic malignancies [1]. Because patients with PJS have a significantly increased risk of developing cancer, LKB1 is recognized as a tumor suppressor. Heterozygous knockout mice with downregulated LKB1 develop tumors, primarily hepatocellular carcinoma, spontaneously and late in life [2]. LKB1 mutations are also linked to extraintestinal cancers, including lung cancer, breast cancer, and cervical carcinomas [3].
LKB1 is essential for controlling the metabolism, growth, and polarity of various types of cells [4], including neuronal polarity, axon formation, and axon extension. In response to stimulation by extracellular factors, including BDNF, NGF, Sema3A, netrin-1, Reelin, and Wnt, LKB1 acts as the downstream effector of cAMP/PKA and PI3 kinase, and is a major determinant for axon differentiation by regulating neuronal migration, axon initiation, and axon elongation in the CNS. LKB1 deletion or downregulation eliminates axon formation in vivo; its overexpression stimulates the formation of multiple axons. As a master upstream kinase of the multiple signals that control cell growth, LKB1 controls axon development largely by activating AMPK-related kinases, especially SAD-A, SAD-B, and NUAK1 kinases.
Given the critical roles of LKB1 in controlling axon genesis and elongation, this kinase could be important to mediating axon regeneration and neural repair in the adult nervous system after injury. Recent studies demonstrate that LKB1 upregulation promotes robust and long-distance regrowth of injured descending motor tracts into the caudal spinal cord in adult rodents with spinal cord injury (SCI) [5].
2. LKB1 Gene, Structure, and Overall Cellular Functions
The human LKB1 gene (23 kb) is composed of 10 exons on chromosome 19p13.3 [6]. LKB1 has various homologs in mouse, Drosophila, Xenopus (called XEEK1), and C. elegans (called PAR-4) [7][8][9][10]. Despite the unknown significance, LKB1 mRNA has several splice variants of 1302 bp and 444 bp in-frame deletion of exons 5–7 and part of exon 8, and a variant retaining intron [11][12]. Human LKB1 protein (436 amino acids, MW: ~50 kDa) consists of an N-terminal non-catalytic domain with two nuclear localization signals, a kinase domain at N-terminus (aa 49-309, close to AMPK members), and a C-terminal regulatory domain with conserved phosphorylation sites [4][12].
LKB1 is an important serine/threonine kinase required for maintaining cell metabolism, energy homeostasis, and polarity by activating AMPK (Thr-172) and ~12 other AMPK-related kinases. Numerous highly conserved residues on LKB1 are phosphorylated either by auto-phosphorylation (Thr-185, Thr-189, Thr-336, and Ser-404) or by upstream kinases (Ser-31, Ser-325, Thr-366, and Ser-431). Activation of LKB1 requires the formation of a complex in the nucleus with its cofactors STE20-related kinase adaptor alpha (STRADɑ) and MO25 (also known as calcium-binding protein 39, Figure 1). After activation and translocation to the cytoplasm, LKB1 is phosphorylated at Ser431 by active cytoplasmic PKA, PKCζ, and S6 kinases, and stimulates the active transport of LKB1 to the cytoplasm [13]. Among the numerous phosphorylation sites, phosphorylation at Ser431 by PKA or p90RSK is especially important for controlling LKB1 functions, including cell cycle management, polarity formation, and axon specification. LKB1 activates various downstream signaling pathways, including AMPK, SAD (synapses of amphids defective kinase), NUAKs, and other kinases [14].
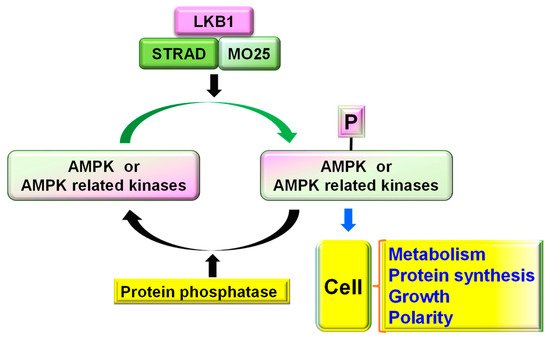
Figure 1. Schematic of LKB1–STRAD–MO25 complex and its major functions. LKB1–STRAD–MO25 complex phosphorylates AMPK and AMPK-related kinases, which can be dephosphorylated by protein phosphatases. Increased phosphorylation of AMPK and its related kinases controls various cellular functions, including cell energy metabolism, protein synthesis, growth, and polarity.
LKB1 is widely expressed in many embryonic and adult tissues, including the developing brain, with the highest level in the forebrain [15][16]. LKB1 is concentrated in the cell nucleus and is also present in the axons of cortical neurons. Its expression pattern is similar to that of its cofactors STARD and MO25. They usually form an LKB1–STARD–MO25 complex to function. Integration of LKB1 with various environmental signals activates downstream signaling and regulates the functions of various types of cells, including controlling glucose/lipid metabolism and cell polarity, and suppressing the growth of cancer cells. LKB1 is critical to the polarization of epithelial cells and axon formation of developing neurons by regulating the distribution of the Golgi apparatus in the cytoplasm. Its function in the adult nervous system is largely unknown although it is required for myelination of peripheral axons by Schwann cells [17][18][19]. LKB1 is also critical to the differentiation of neural crest stem cells [20] and polarization of epithelial cells in mammals [21][22].
3. LKB1 Forms Protein Complexes for Function in Neural Cells
LKB1 functions as a critical convergent signal downstream of numerous extracellular factors and transmembrane receptors. The binding of various extracellular molecules to their receptors and the intrinsic asymmetry of cytoplasmic components may activate the cAMP/PKA, Ras/ERK, and Ras/PI3K signaling pathways, alter the activities of downstream signals and regulate diverse cellular functions. Among the diverse signals downstream of these pathways, phosphorylating LKB1 at different sites is crucial to cell functions [23]. Particularly, neurotrophins interact with their receptor tyrosine kinases and stimulate neuronal growth by activating the cAMP/PKA, Ras/ERK, and Ras/PI3K/Akt signals [24][25], all of which could modulate LKB1 phosphorylation and activity (Figure 2) [26][27].
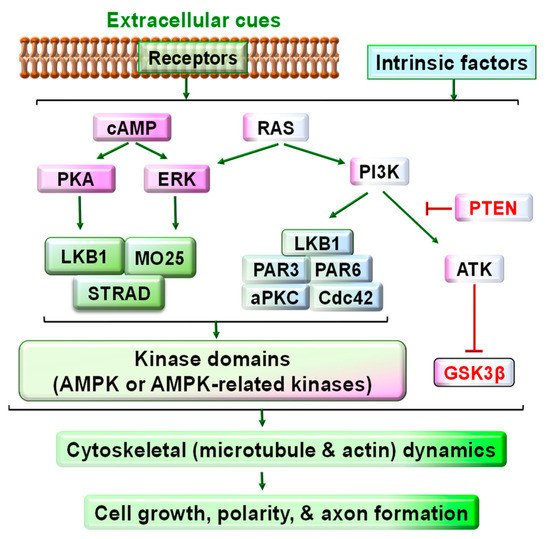
Figure 2. Schematic of the signaling pathways upstream and downstream of LKB1 in developmental neurons. Various extracellular cues and intracellular intrinsic factors activate many signaling pathways, including cAMP-PKA, cAMP/RAS-Erk, and RAS-PI3 kinase signaling. Downstream of these pathways, LKB1 is critical to convergent signaling for regulating cell growth. LKB1 usually forms a complex with STRAD and MO25 for LKB1 stability and activation. LKB1 may also form a complex with other proteins, including PAR3/PAR6/aPKC/Cdc42. As an intracellular master kinase, LKB1 is crucial for controlling cell growth, polarity, and axon formation and elongation during development by phosphorylating AMPK and AMPK-related kinases and regulating their activities accordingly. AMPK and AMPK-related kinases are associated with the cytoskeletal (such as microtubule and actin) dynamics and cell growth. Other LKB1-independent intracellular pathways, such as PI3K-Akt-mTOR signaling, may also modulate cell growth by diverse molecular mechanisms.
LKB1 monomer is primarily located in the nucleus and is largely inactive, but it usually forms a heterotrimeric complex with STRADα and MO25, which stabilize LKB1 and activate its kinase activity. The function and localization of LKB1 are highly associated with its binding to STRAD, and this binding is essential for phosphorylating AMPK-related enzymes by LKB1 [4]. The binding of LKB1 to STRAD alters LKB1 conformation and enhances its activity 100-fold [28]. The removal of endogenous STRADα by small interfering RNA abolishes the LKB1-induced G1 phase arrest [29]. LKB1 and STRADα have a reciprocal protein-stabilizing relationship in vivo and STRADα maintains LKB1 protein levels specifically by cytoplasmic compartmentalization [30]. The ubiquitously expressed scaffolding MO25 (mouse protein 25) is the third component of the LKB1–STRAD–MO25 complex in a similar stoichiometry, and it interacts with STRADα C-terminal and further stabilizes the binding of LKB1 to STRADα [28][31]. Upon interaction with STRAD and MO25 in the nucleus, LKB1 is translocated to the cytoplasm from the nucleus for functioning [12][29].
Atypical PKC (aPKC) complexes, including PKCζ/Par6/Par3 and PKCζ/Par6/Par3/CDC-42, probably also activate LKB1 in neurons and other types of cells. The aPKC complexes are required for proteoglycan-induced axon growth inhibition [32]. Out of several components in the aPKC complex, PKCζ is a member of the aPKC subfamily, phosphorylates LKB1 at Ser-428/431, and mediates the activation of AMPK in endothelial cells [33][34]. Activating LAR receptors by CSPG application reduces the activities of PKCζ and LKB1, indicating that PKCζ-mediated LKB1 suppression also mediates axon growth inhibition by the scar-scoured inhibitor CSPGs [35]. Other signaling proteins may also interact with LKB1 and modulate its relocation and activity. For example, the interactions between LKB1 and PTEN have been shown to promote LKB1 relocation to the cytoplasm in cancer cells [36].
References
- Hemminki, A.; Markie, D.; Tomlinson, I.; Avizienyte, E.; Roth, S.; Loukola, A.; Bignell, G.; Warren, W.; Aminoff, M.; Hoglund, P.; et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 1998, 391, 184–187.
- Nakau, M.; Miyoshi, H.; Seldin, M.F.; Imamura, M.; Oshima, M.; Taketo, M.M. Hepatocellular carcinoma caused by loss of heterozygosity in Lkb1 gene knockout mice. Cancer Res. 2002, 62, 4549–4553.
- Gan, R.Y.; Li, H.B. Recent progress on liver kinase B1 (LKB1): Expression, regulation, downstream signaling and cancer suppressive function. Int. J. Mol. Sci. 2014, 15, 16698–16718.
- Jansen, M.; Ten Klooster, J.P.; Offerhaus, G.J.; Clevers, H. LKB1 and AMPK family signaling: The intimate link between cell polarity and energy metabolism. Physiol. Rev. 2009, 89, 777–798.
- Ohtake, Y.; Sami, A.; Jiang, X.; Horiuchi, M.; Slattery, K.; Ma, L.; Smith, G.M.; Selzer, M.E.; Muramatsu, S.I.; Li, S. Promoting Axon Regeneration in Adult CNS by Targeting Liver Kinase B1. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 102–117.
- Mehenni, H.; Gehrig, C.; Nezu, J.; Oku, A.; Shimane, M.; Rossier, C.; Guex, N.; Blouin, J.L.; Scott, H.S.; Antonarakis, S.E. Loss of LKB1 kinase activity in Peutz-Jeghers syndrome, and evidence for allelic and locus heterogeneity. Am. J. Hum. Genet. 1998, 63, 1641–1650.
- Smith, D.P.; Spicer, J.; Smith, A.; Swift, S.; Ashworth, A. The mouse Peutz-Jeghers syndrome gene Lkb1 encodes a nuclear protein kinase. Hum. Mol. Genet. 1999, 8, 1479–1485.
- Su, J.Y.; Erikson, E.; Maller, J.L. Cloning and characterization of a novel serine/threonine protein kinase expressed in early Xenopus embryos. J. Biol. Chem. 1996, 271, 14430–14437.
- Watts, J.L.; Morton, D.G.; Bestman, J.; Kemphues, K.J. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development 2000, 127, 1467–1475.
- Martin, S.G.; St Johnston, D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature 2003, 421, 379–384.
- Abed, A.A.; Gunther, K.; Kraus, C.; Hohenberger, W.; Ballhausen, W.G. Mutation screening at the RNA level of the STK11/LKB1 gene in Peutz-Jeghers syndrome reveals complex splicing abnormalities and a novel mRNA isoform (STK11 c.597(insertion mark)598insIVS4). Hum. Mutat. 2001, 18, 397–410.
- Alessi, D.R.; Sakamoto, K.; Bayascas, J.R. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 2006, 75, 137–163.
- Dorfman, J.; Macara, I.G. STRADalpha regulates LKB1 localization by blocking access to importin-alpha, and by association with Crm1 and exportin-7. Mol. Biol. Cell 2008, 19, 1614–1626.
- Shelly, M.; Poo, M.M. Role of LKB1-SAD/MARK pathway in neuronal polarization. Dev. Neurobiol. 2011, 71, 508–527.
- Barnes, A.P.; Lilley, B.N.; Pan, Y.A.; Plummer, L.J.; Powell, A.W.; Raines, A.N.; Sanes, J.R.; Polleux, F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 2007, 129, 549–563.
- Jenne, D.E.; Reimann, H.; Nezu, J.; Friedel, W.; Loff, S.; Jeschke, R.; Muller, O.; Back, W.; Zimmer, M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 1998, 18, 38–43.
- Pooya, S.; Liu, X.; Kumar, V.B.; Anderson, J.; Imai, F.; Zhang, W.; Ciraolo, G.; Ratner, N.; Setchell, K.D.; Yoshida, Y.; et al. The tumour suppressor LKB1 regulates myelination through mitochondrial metabolism. Nat. Commun. 2014, 5, 4993.
- Shen, Y.A.; Chen, Y.; Dao, D.Q.; Mayoral, S.R.; Wu, L.; Meijer, D.; Ullian, E.M.; Chan, J.R.; Lu, Q.R. Phosphorylation of LKB1/Par-4 establishes Schwann cell polarity to initiate and control myelin extent. Nat. Commun. 2014, 5, 4991.
- Beirowski, B.; Babetto, E.; Golden, J.P.; Chen, Y.J.; Yang, K.; Gross, R.W.; Patti, G.J.; Milbrandt, J. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat. Neurosci. 2014, 17, 1351–1361.
- Radu, A.G.; Torch, S.; Fauvelle, F.; Pernet-Gallay, K.; Lucas, A.; Blervaque, R.; Delmas, V.; Schlattner, U.; Lafanechere, L.; Hainaut, P.; et al. LKB1 specifies neural crest cell fates through pyruvate-alanine cycling. Sci. Adv. 2019, 5, eaau5106.
- Baas, A.F.; Kuipers, J.; van der Wel, N.N.; Batlle, E.; Koerten, H.K.; Peters, P.J.; Clevers, H.C. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 2004, 116, 457–466.
- Forcet, C.; Etienne-Manneville, S.; Gaude, H.; Fournier, L.; Debilly, S.; Salmi, M.; Baas, A.; Olschwang, S.; Clevers, H.; Billaud, M. Functional analysis of Peutz-Jeghers mutations reveals that the LKB1 C-terminal region exerts a crucial role in regulating both the AMPK pathway and the cell polarity. Hum. Mol. Genet. 2005, 14, 1283–1292.
- Winckler, B. BDNF instructs the kinase LKB1 to grow an axon. Cell 2007, 129, 459–460.
- Zhou, F.Q.; Snider, W.D. Intracellular control of developmental and regenerative axon growth. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1575–1592.
- Liu, K.; Tedeschi, A.; Park, K.K.; He, Z. Neuronal intrinsic mechanisms of axon regeneration. Annu. Rev. Neurosci. 2011, 34, 131–152.
- Esteve-Puig, R.; Canals, F.; Colome, N.; Merlino, G.; Recio, J.A. Uncoupling of the LKB1-AMPKalpha energy sensor pathway by growth factors and oncogenic BRAF. PLoS ONE 2009, 4, e4771.
- Frodin, M.; Gammeltoft, S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell Endocrinol. 1999, 151, 65–77.
- Hawley, S.A.; Boudeau, J.; Reid, J.L.; Mustard, K.J.; Udd, L.; Makela, T.P.; Alessi, D.R.; Hardie, D.G. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003, 2, 28.
- Baas, A.F.; Boudeau, J.; Sapkota, G.P.; Smit, L.; Medema, R.; Morrice, N.A.; Alessi, D.R.; Clevers, H.C. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 2003, 22, 3062–3072.
- Veleva-Rotse, B.O.; Smart, J.L.; Baas, A.F.; Edmonds, B.; Zhao, Z.M.; Brown, A.; Klug, L.R.; Hansen, K.; Reilly, G.; Gardner, A.P.; et al. STRAD pseudokinases regulate axogenesis and LKB1 stability. Neural Dev. 2014, 9, 5.
- Boudeau, J.; Baas, A.F.; Deak, M.; Morrice, N.A.; Kieloch, A.; Schutkowski, M.; Prescott, A.R.; Clevers, H.C.; Alessi, D.R. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003, 22, 5102–5114.
- Lee, S.I.; Zhang, W.; Ravi, M.; Weschenfelder, M.; Bastmeyer, M.; Levine, J.M. Atypical protein kinase C and Par3 are required for proteoglycan-induced axon growth inhibition. J. Neurosci. 2013, 33, 2541–2554.
- Xie, Z.; Dong, Y.; Scholz, R.; Neumann, D.; Zou, M.H. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 2008, 117, 952–962.
- Zhu, H.; Moriasi, C.M.; Zhang, M.; Zhao, Y.; Zou, M.H. Phosphorylation of serine 399 in LKB1 protein short form by protein kinase Czeta is required for its nucleocytoplasmic transport and consequent AMP-activated protein kinase (AMPK) activation. J. Biol. Chem. 2013, 288, 16495–16505.
- Ohtake, Y.; Wong, D.; Abdul-Muneer, P.M.; Selzer, M.E.; Li, S. Two PTP receptors mediate CSPG inhibition by convergent and divergent signaling pathways in neurons. Sci. Rep. 2016, 6, 37152.
- Mehenni, H.; Lin-Marq, N.; Buchet-Poyau, K.; Reymond, A.; Collart, M.A.; Picard, D.; Antonarakis, S.E. LKB1 interacts with and phosphorylates PTEN: A functional link between two proteins involved in cancer predisposing syndromes. Hum. Mol. Genet. 2005, 14, 2209–2219.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
715
Revisions:
2 times
(View History)
Update Date:
12 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





