Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ekaterina V Medvedeva | -- | 2125 | 2022-10-11 16:41:34 | | | |
| 2 | Sirius Huang | Meta information modification | 2125 | 2022-10-12 04:27:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kurenkova, A.D.; Romanova, I.A.; Kibirskiy, P.D.; Timashev, P.; Medvedeva, E.V. Autologous Chondrocytes from Different Sources. Encyclopedia. Available online: https://encyclopedia.pub/entry/28934 (accessed on 07 February 2026).
Kurenkova AD, Romanova IA, Kibirskiy PD, Timashev P, Medvedeva EV. Autologous Chondrocytes from Different Sources. Encyclopedia. Available at: https://encyclopedia.pub/entry/28934. Accessed February 07, 2026.
Kurenkova, Anastasiia D., Irina A. Romanova, Pavel D. Kibirskiy, Peter Timashev, Ekaterina V. Medvedeva. "Autologous Chondrocytes from Different Sources" Encyclopedia, https://encyclopedia.pub/entry/28934 (accessed February 07, 2026).
Kurenkova, A.D., Romanova, I.A., Kibirskiy, P.D., Timashev, P., & Medvedeva, E.V. (2022, October 11). Autologous Chondrocytes from Different Sources. In Encyclopedia. https://encyclopedia.pub/entry/28934
Kurenkova, Anastasiia D., et al. "Autologous Chondrocytes from Different Sources." Encyclopedia. Web. 11 October, 2022.
Copy Citation
Damaged hyaline cartilage gradually decreases joint function and growing pain significantly reduces the quality of a patient’s life. The clinically approved procedure of autologous chondrocyte implantation (ACI) for treating knee cartilage lesions has several limits. Various ACI modifications are being developed using autologous chondrocytes from alternative sources, such as the auricles, nose, and ribs.
osteoarthritis
articular cartilage
autologous chondrocytes
1. Introduction
Articular cartilage has a complex structural tissue consisting of several layers, which differ by their composition in the collagen-type content, collagen fiber orientation and cell morphology and density [1] (Figure 1b). Such a complex hierarchical structure is fundamental to the unique biomechanical properties of articular cartilage [1] and, simultaneously, a challenge for regenerative medicine. However, finding a solution is essential since degenerative joint diseases are widespread. Osteoarthritis (OA) is the most common joint disease, with progressive degeneration of articular cartilage and subsequent slow joint space narrowing, pathological remodeling of all joint tissues, including bone, and chronic inflammatory processes accompanied by movement limitation [2][3][4]. Clinical remediation is urgently required, with a growing list of developing drug treatments for OA being unsuccessful in clinical trials [5].
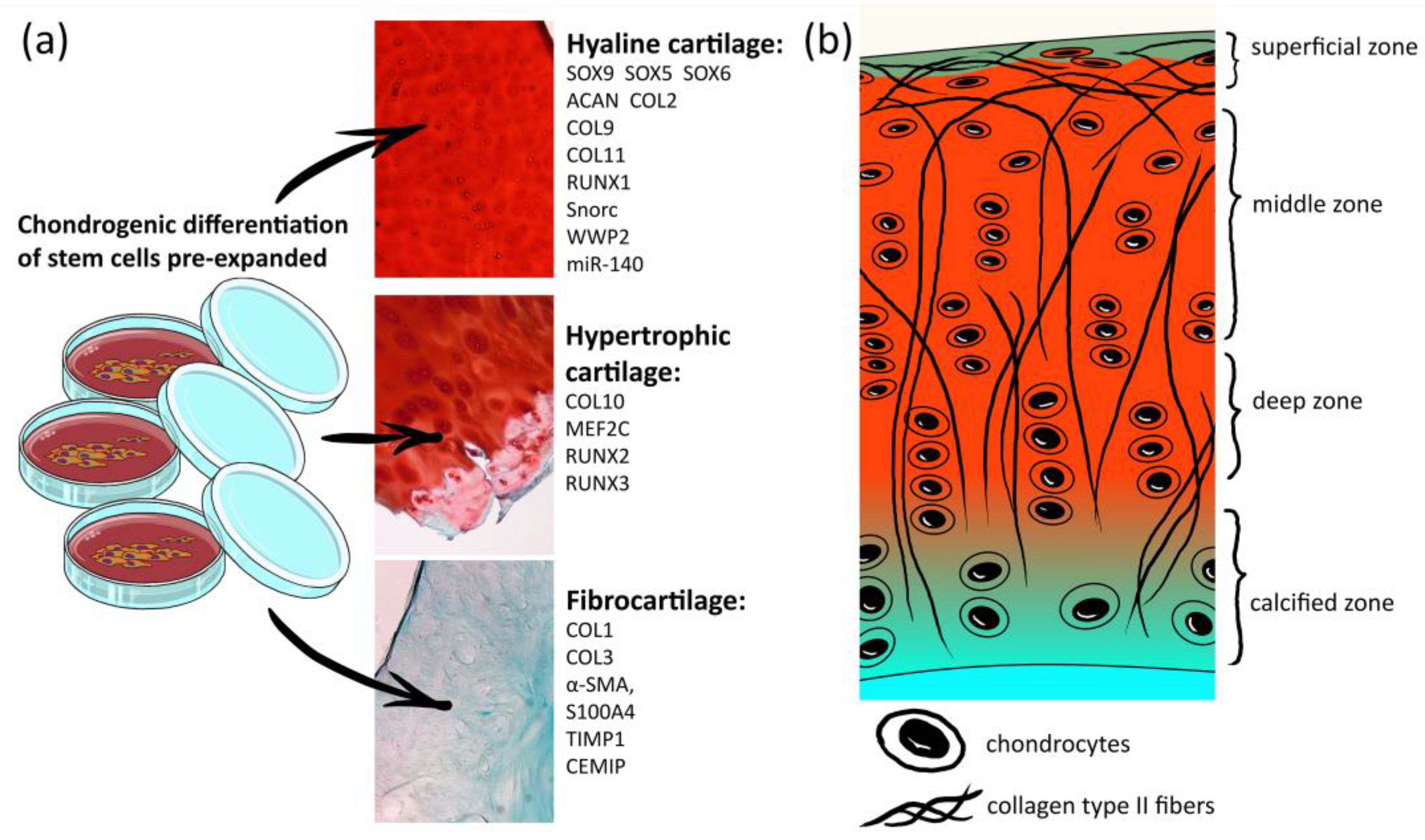
Figure 1. List of markers for hyaline cartilage chondrocytes, hypertrophic chondrocytes and fibrocartilage. (a) Histological images are photos of human knee articular cartilage sections stained by Safranin-O/Fast green; (b) scheme of the human articular cartilage structure.
2. Autologous Chondrocytes from Different Sources
Autologous chondrocyte implantation (ACI) and matrix-induced ACI (MACI) procedures are approved for clinical application. However, these therapies have significant drawbacks. In the late stages of OA, extraction of the autologous chondrocytes is no longer an available option. Another widespread problem is that the obtained chondrocytes are inclined to lose their chondrogenic features (de-differentiate) in monolayers [6].
2.1. Chondrons, the Functional Units of Cartilage Tissue
To avoid chondrocyte de-differentiation in vitro, it was suggested to isolate chondrons composed of chondrocytes surrounded by a pericellular matrix. A milieu of chondrocytes play an essential role in the maintenance of their chondrogenic state and utilization of chondrons could retard chondrocyte de-differentiation processes in vitro [7]. A recent study demonstrated that cultured chondrons expressed higher levels of proteoglycan and COL2 than chondrocyte culture [8]. Surprisingly, a mixture of chondrons and chondrocytes significantly elevated the production of ECM in culture even more than the chondrons alone. Implantation of alginate spheres, including chondrons and chondrocytes, accelerated the regeneration of injured knee cartilage in rabbits [8].
The extraction of chondrons from cartilage is not more difficult than chondrocyte isolation [8][9]. However, X-ray micro-computed tomography (μCT) methods showed that the morphology of chondrons is affected by OA progression [10]. The limitations of using autologous chondrons to repair a cartilage defect are the same as for autologous chondrocytes.
2.2. Chondroprogenitors from Superficial Zone of Articular Cartilage
One way to avoid chondrocyte de-differentiation during expansion is to use chondrogenic progenitors, which are cells with an incomplete differentiation cycle but a higher ability to proliferate. A population of endogenous, slowly dividing progenitor cells was discovered in the superficial zone of adult articular cartilage [11][12]. Recently, it was confirmed that these articular cartilage progenitor cells (ACPCs) in the superficial zone were responsible for the turnover of articular cartilage tissue in the postnatal period in mice [12][13].
Since ACPC cultures have not been scrupulously investigated yet, there are no unique cell markers for the selective isolation protocol, especially for human ACPCs. Different unsuccessful efforts have been made [14][15] to set apart the ACPCs and mature chondrocytes; however, the method of ACPC isolation, which is predominant in publications but has low specificity, relies on adhesion to a fibronectin coating (owing to the high cell expression of the fibronectin receptors integrin-α5 and -β1) [11].
To determine the optimal chondrogenic conditions, bovine ACPCs were cultured as pellets (a high-cell-density scaffold-free culture compressed by centrifugation) for 21 days in the presence of known chondrogenic factors, such as transforming growth factor-β (TGFβ) 1 to 3, bone morphogenetic proteins (BMP) 2 and 9, synthetic glucocorticoid dexamethasone, C-natriuretic peptide, dibutyryl-cAMP, concanavalin-A, ethanol and chelerythrine chloride [16]. Histological analysis, immunohistochemical (IHC) labeling, polymerase chain reaction (PCR) and atomic force microscopy measurements revealed BMP9 as a promising chondrogenic factor for ACPC differentiation in vitro. However, the concentration of BMP9 in the culture medium should not exceed 100 ng/mL to avoid the production of the hypertrophic marker COL10 [16].
Replacing the routinely used growth supplement fetal bovine serum (FBS) with human platelet lysate in the culturing protocol led to faster doublings and increased expression of ACAN and COL2, as well as COL10 and COL1, in ACPC monolayer cultures [17]. Compared to monolayers, the technique of ACPC expansion on commercial macroporous gelatin-coated microcarrier beads in the presence of TGFβ1 resulted in a significant enhancement in ACPC proliferation, maintaining the ability to produce an ECM abundant with COL2 and glycosaminoglycans (GAGs) [18].
The articular cartilage chondrocytes are well-specialized cells that are sensitive to mechanical stimuli, which are a complex combination of compression, stretching, shear stress and hydrostatic pressure. Mechanical loading increased SOX9 IHC labeling in the superficial zone of bovine cartilage cultured as cylindrical osteochondral plugs [19]. The application of bioreactor-generated joint-like movements demonstrated the mechanosensitivity of ACPC cultures [20]. Intermittent hydrostatic pressure (IHP) applied to ACPCs seeded within alginate beads for four weeks stimulated more significant production of cartilage ECM components and the expression of chondrocyte-related genes compared to cultures of adipose-derived stem cells (ADSCs) or even chondrocytes [21]. Mechanical stimulation is assumed to be more relevant to chondroprogenitors than chondrocyte cultures.
During in vivo studies, a construct consisting of goat ACPCs seeded on a COL1/COL3 membrane (Chondro-Gide®) was transplanted into a knee defect. Analysis showed acceptable integration with the surrounding cartilage and COL2-positive staining of neo-tissue. However, no difference was found in the final results between ACPC- and chondrocyte-treated defects [22]. During a clinical trial, human CD146-positive cells collected from the upper zone of articular cartilage were differentiated to a chondrogenic lineage in a culture medium containing TGFβ3 and BMP4 [23] and surgically implanted on a COL1/COL3 membrane into knee cartilage defects. Subsequent magnetic resonance imaging (MRI) and arthroscopy showed complete attachment of the construct and filling of the defect, with histology and IHC estimation of neo-tissue confirming a good repair [23]. However, the significant regeneration was believed to have resulted from the young age of the patients treated (the average age was 25 years).
2.3. Non-Articular Autologous Chondrocytes
To exclude the donor-site morbidity of a joint during autologous articular chondrocyte (AC) isolation, it is suggested that non-articular chondrocytes be used for joint defect treatment. The efficacy of replacing hyaline cartilage cells with non-articular chondrocytes remains debatable.
2.3.1. Nasal Chondrocytes
Both articular and nasal tissues are permanent hyaline cartilage throughout life but have different origins during embryonic development. Nevertheless, in adults, nasal cartilage tissue has a similar composition and structure to joint cartilage, but the calcified zone is not present in nasal cartilage [24]. The monolayer of adult nasal chondrocytes (NCs) has three-times faster proliferative activity and promising chondrogenic capacities [25]. In an animal defect model, NCs within engineered grafts could generate hyaline cartilage markers even more than ACs [26].
Engineered autologous human cartilage grafts obtained from a nasal septum biopsy specimen were implanted into femoral full-thickness cartilage defects in a first-in-human trial, including ten patients [27]. IHC characterization of the non-used portions of the engineered cartilage constructs indicated positivity for hyaline cartilage markers, slight positivity for COL1 and negativity for COL10. Twenty-four months after the surgery, histological analysis of the biopsy at the engineered graft implantation site indicated heterogeneity in the repair tissue without the typical hyaline cartilage organization. However, the high patient self-assessment scores and MRI analyses of the defect filling allowed the authors to claim a satisfactory clinical outcome [27].
A pre-clinical study from 2021 showed that nasal-chondrocyte-based tissue-engineered cartilage (NTEC) implanted into osteochondral cartilage defects of sheep prevented the elevation of synovial fluid volumes and decreased concentrations of pro-inflammatory cytokines induced by knee OA. Autologous NTEC graft implantation into a human was evaluated in two patients with advanced OA. Fourteen months after the surgery, the patients confirmed improvements in pain, knee-joint function and quality of life. Accordingly, the authors declared that future controlled trials on other joints would be initiated [28].
2.3.2. Costal Chondrocytes
Costal hyaline cartilage is another chondrocyte source for ACI and MACI therapies, allowing for potential complications following additional joint surgery interventions to be avoided. Moreover, autologous costal cartilage is already widely used as a source of grafts in plastic surgery, demonstrating the procedure’s safety.
During culturing without growth factors, costal-derived chondrocytes (CCs) proliferate 9-fold faster than ACs. However, both the ACs and CCs gradually lose their phenotype following the first four passages [29]. The addition of fibroblast growth factor 2 (FGF2) in combination with commercial mesenchymal stem cell growth mediumTM (MSCGM) induced stromal cell features with a significant content of COL2 and ACAN, with trace amounts of COL1. At the same time, FGF2 and DMEM drove CCs to transition to fibroblast-like cells [30]. In a rabbit osteochondral defect model, CCs expanded in the FGF2-containing MSCGM, resulting in good defect healing [30].
A clinical test of autologous CC pellets with fibrin glue on seven patients with full-thickness articular cartilage lesions included CCs expanded in MSCGM with FGF2 before implantation [31][32]. The results showed significant improvements in all clinical scores during the 5-year follow-up period confirmed by MRI scans. Nevertheless, hypertrophy in implants or incomplete defect filling was observed in two patients [31]. According to recently published data, co-culturing CCs with synovial membrane-derived mesenchymal stem cells (SM-MSCs) could prevent hypertrophy and maintain the chondrogenic phenotype of CCs in cartilage repair [33].
2.3.3. Growth Plate Chondrocytes
The epiphyseal/growth plate hyaline cartilage cells are responsible for the elongation of long bones. The age of growth plate closure for humans is 17–25 years old [34]. Cell harvesting could damage the immature growth plate and disrupt bone growth [35] and it severely limits the use of growth plate chondrocytes for autologous transplantation. However, porcine chondrocytes from proliferating (middle) zones of growth plate cartilage have been isolated and characterized in vitro [36] as a more viable, proliferative and preserved chondrogenic phenotype that lasts longer during passages compared to ACs [36].
It has been previously assumed that an avascular hyaline cartilage nature provides “immune privilege”, inferring the potential of using the growth plate as a donor source in the case of allogeneic (cadavers or amputated material) or xenogeneic transplantation. This notion was disproved by researchers [37] when the rabbit model showed that, in contrast to allogeneic transplantation, xenogeneic implants in joint cartilage provoked a marked innate and adaptive immune response [37].
For obvious reasons, at present, studies about the application of growth plate chondrocytes to articular cartilage defect treatment are scarce.
2.3.4. Chondrocytes from the Auricle of an Ear
In contrast to joint cartilage, auricular cartilage is covered by a perichondrium, contains elastic fibers and COL1 and does not show a defined zonality in its structure. Auricular chondrocyte (AUC) yields per tissue volume are high and sufficient for cell therapy realization. De-differentiation has been reported to proceed more rapidly in AUCs than in ACs, which was closely related to their proliferative activity [38][39].
Wong et al. (2018) suggested converting the de-differentiated monolayer AUCs to hyaline cartilage using ECM [40]. The data demonstrated that the COL-coated culture dish promoted hyaline cartilage markers and suppressed auricular elastic cartilage markers (such as COL1 and elastin) in rabbit AUCs compared to cultivation on plastic. Three months after implantation into an osteochondral defect, AUCs cultured in COL2 scaffolds exhibited successful integration with the articular cartilage, abundant proteoglycan syntheses and intense staining for COL2 with traces of elastin staining [40]. It was noted that the AUCs without the COL2 scaffold did not result in compatible healing outcomes.
Wang et al. (2018) suggested an original cell-free approach combining AUC sheets and the microfracture technique [41]. Rabbit AUC sheets were gently decellularized, retaining the native architecture and ECM composition. Decellularized AUC sheets tested in vitro showed stimulation of potential migrating BM-MSCs, increasing SOX9 production and decreasing COL10 production detected by RT-PCR and Western blots [41]. Agreeable results for the application of decellularized AUC sheets in combination with microfracture to osteochondral knee defect regeneration in rabbits were confirmed by macroscopic observation, μCT imaging and histological analysis 12 weeks after the surgery [41].
Goat AUC sheets differentiated in a chondrogenic medium supplemented with TGFβ1, insulin-like growth factor 1 (IGF1) and dexamethasone indicated a gradual increase in hyaline-cartilage-specific ECM analyzed by histological and IHC staining [39]. However, after subcutaneous implantation of AUC sheets in nude mice, a threat of AUC sheet tissue ossification was demonstrated [39].
The clinically available growth factors FGF2 and IGF1 and the peptide hormone insulin were proposed as optimal for AUC expansion in monolayer cultures [42]. Their effect on the differentiation of three-dimensional (3D) cultures of AUCs was investigated [43]. Histological, biochemical and biomechanical analyses showed that IGF1 stimulated the chondrogenesis of AUC 3D constructs in vitro better than the other molecules tested. However, the chondrogenic effect was not confirmed after subcutaneous transplantation into nude mice [43].
Easy access to the auricle cartilage makes this cell source too attractive for regenerative medicine to give up trying to develop a strategy involving AUCs in cartilage defect regeneration.
References
- Gahunia, H.K.; Pritzker, K.P.H. Structure and Function of Articular Cartilage. In Articular Cartilage of the Knee; Springer: New York, NY, USA, 2020; pp. 3–70. ISBN 9781493975877.
- Felson, D.T.; Neogi, T. Osteoarthritis: Is It a Disease of Cartilage or of Bone? Arthritis Rheum. 2004, 50, 341–344.
- Karsdal, M.A.; Michaelis, M.; Ladel, C.; Siebuhr, A.S.; Bihlet, A.R.; Andersen, J.R.; Guehring, H.; Christiansen, C.; Bay-Jensen, A.C.; Kraus, V.B. Disease-Modifying Treatments for Osteoarthritis (DMOADs) of the Knee and Hip: Lessons Learned from Failures and Opportunities for the Future. Osteoarthr. Cartil. 2016, 24, 2013–2021.
- Ashford, S.; Williard, J. Osteoarthritis. Nurse Pract. 2014, 39, 1–8.
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular Fibrocartilage—Why Does Hyaline Cartilage Fail to Repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305.
- Caron, M.M.J.; Emans, P.J.; Coolsen, M.M.E.; Voss, L.; Surtel, D.A.M.; Cremers, A.; van Rhijn, L.W.; Welting, T.J.M. Redifferentiation of Dedifferentiated Human Articular Chondrocytes: Comparison of 2D and 3D Cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178.
- Shah, S.S.; Mithoefer, K. Scientific Developments and Clinical Applications Utilizing Chondrons and Chondrocytes with Matrix for Cartilage Repair. Cartilage 2021, 13, 1195S–1205S.
- Duan, W.; Zhao, Y.; Ren, X.; Zhao, R.; Li, Q.; Sun, Z.; Song, W.; Yang, Y.; Li, P.; Wei, X. Combination of Chondrocytes and Chondrons Improves Extracellular Matrix Production to Promote the Repairs of Defective Knee Cartilage in Rabbits. J. Orthop. Transl. 2021, 28, 47–54.
- Uzieliene, I.; Denkovskij, J.; Bernotiene, E.; Kalvaityte, U.; Vaiciuleviciute, R.; Ramos, Y.F.M.; Mobasheri, A. Protocol for the Isolation of Intact Chondrons from Healthy and Osteoarthritic Human Articular Cartilage. In Chondrocytes. Method and Protocols; Haqqi, T.M., Lefebvre, V., Eds.; Methods in Molecular Biology Series; Springer: New York, NY, USA, 2021; Volume 2245, pp. 13–22. ISBN 978-1-0716-1118-0.
- Kestilä, I.; Thevenot, J.; Finnilä, M.A.; Karhula, S.S.; Hadjab, I.; Kauppinen, S.; Garon, M.; Quenneville, E.; Haapea, M.; Rieppo, L.; et al. In Vitro Method for 3D Morphometry of Human Articular Cartilage Chondrons Based on Micro-Computed Tomography. Osteoarthr. Cartil. 2018, 26, 1118–1126.
- Dowthwaite, G.P.; Bishop, J.C.; Redman, S.N.; Khan, I.M.; Rooney, P.; Evans, D.J.R.; Haughton, L.; Bayram, Z.; Boyer, S.; Thomson, B.; et al. The Surface of Articular Cartilage Contains a Progenitor Cell Population. J. Cell Sci. 2004, 117, 889–897.
- Kozhemyakina, E.; Zhang, M.; Ionescu, A.; Ayturk, U.M.; Ono, N.; Kobayashi, A.; Kronenberg, H.; Warman, M.L.; Lassar, A.B. Identification of a Prg4-Expressing Articular Cartilage Progenitor Cell Population in Mice. Arthritis Rheumatol. 2015, 67, 1261–1273.
- Li, L.; Newton, P.T.; Bouderlique, T.; Sejnohova, M.; Zikmund, T.; Kozhemyakina, E.; Xie, M.; Krivanek, J.; Kaiser, J.; Qian, H.; et al. Superficial Cells Are Self-Renewing Chondrocyte Progenitors, Which Form the Articular Cartilage in Juvenile Mice. FASEB J. 2017, 31, 1067–1084.
- Rikkers, M.; Korpershoek, J.V.; Levato, R.; Malda, J.; Vonk, L.A. The Clinical Potential of Articular Cartilage-Derived Progenitor Cells: A Systematic Review. NPJ Regen. Med. 2022, 7, 2.
- Zieba, J.T.; Chen, Y.-T.; Lee, B.H.; Bae, Y. Notch Signaling in Skeletal Development, Homeostasis and Pathogenesis. Biomolecules 2020, 10, 332.
- Morgan, B.J.; Bauza-Mayol, G.; Gardner, O.F.W.; Zhang, Y.; Levato, R.; Archer, C.W.; van Weeren, R.; Malda, J.; Conlan, R.S.; Francis, L.W.; et al. Bone Morphogenetic Protein-9 Is a Potent Chondrogenic and Morphogenic Factor for Articular Cartilage Chondroprogenitors. Stem Cells Dev. 2020, 29, 882–894.
- Kachroo, U.; Zachariah, S.M.; Thambaiah, A.; Tabasum, A.; Livingston, A.; Rebekah, G.; Srivastava, A.; Vinod, E. Comparison of Human Platelet Lysate versus Fetal Bovine Serum for Expansion of Human Articular Cartilage-Derived Chondroprogenitors. Cartilage 2021, 13, 107S–116S.
- Melero-Martin, J.M.; Dowling, M.-A.; Smith, M.; Al-Rubeai, M. Expansion of Chondroprogenitor Cells on Macroporous Microcarriers as an Alternative to Conventional Monolayer Systems. Biomaterials 2006, 27, 2970–2979.
- Santos, S.; Richard, K.; Fisher, M.C.; Dealy, C.N.; Pierce, D.M. Chondrocytes Respond Both Anabolically and Catabolically to Impact Loading Generally Considered Non-Injurious. J. Mech. Behav. Biomed. Mater. 2021, 115, 104252.
- Neumann, A.J.; Gardner, O.F.W.; Williams, R.; Alini, M.; Archer, C.W.; Stoddart, M.J. Human Articular Cartilage Progenitor Cells Are Responsive to Mechanical Stimulation and Adenoviral-Mediated Overexpression of Bone-Morphogenetic Protein 2. PLoS ONE 2015, 10, e0136229.
- Li, Y.; Zhou, J.; Yang, X.; Jiang, Y.; Gui, J. Intermittent Hydrostatic Pressure Maintains and Enhances the Chondrogenic Differentiation of Cartilage Progenitor Cells Cultivated in Alginate Beads. Dev. Growth Differ. 2016, 58, 180–193.
- Williams, R.; Khan, I.M.; Richardson, K.; Nelson, L.; McCarthy, H.E.; Analbelsi, T.; Singhrao, S.K.; Dowthwaite, G.P.; Jones, R.E.; Baird, D.M.; et al. Identification and Clonal Characterisation of a Progenitor Cell Sub-Population in Normal Human Articular Cartilage. PLoS ONE 2010, 5, e13246.
- Jiang, Y.; Cai, Y.; Zhang, W.; Yin, Z.; Hu, C.; Tong, T.; Lu, P.; Zhang, S.; Neculai, D.; Tuan, R.S.; et al. Human Cartilage-Derived Progenitor Cells From Committed Chondrocytes for Efficient Cartilage Repair and Regeneration. Stem Cells Transl. Med. 2016, 5, 733–744.
- Li, T.; Chen, S.; Pei, M. Contribution of Neural Crest-Derived Stem Cells and Nasal Chondrocytes to Articular Cartilage Regeneration. Cell. Mol. Life Sci. 2020, 77, 4847–4859.
- Kafienah, W.; Jakob, M.; Démarteau, O.; Frazer, A.; Barker, M.D.; Martin, I.; Hollander, A.P. Three-Dimensional Tissue Engineering of Hyaline Cartilage: Comparison of Adult Nasal and Articular Chondrocytes. Tissue Eng. 2002, 8, 817–826.
- Mumme, M.; Steinitz, A.; Nuss, K.M.; Klein, K.; Feliciano, S.; Kronen, P.; Jakob, M.; Von Rechenberg, B.; Martin, I.; Barbero, A.; et al. Regenerative Potential of Tissue-Engineered Nasal Chondrocytes in Goat Articular Cartilage Defects. Tissue Eng. Part A 2016, 22, 1286–1295.
- Mumme, M.; Barbero, A.; Miot, S.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Asnaghi, A.M.; Baumhoer, D.; Bieri, O.; Kretzschmar, M.; et al. Nasal Chondrocyte-Based Engineered Autologous Cartilage Tissue for Repair of Articular Cartilage Defects: An Observational First-in-Human Trial. Lancet 2016, 388, 1985–1994.
- Acevedo Rua, L.; Mumme, M.; Manferdini, C.; Darwiche, S.; Khalil, A.; Hilpert, M.; Buchner, D.A.; Lisignoli, G.; Occhetta, P.; von Rechenberg, B.; et al. Engineered Nasal Cartilage for the Repair of Osteoarthritic Knee Cartilage Defects. Sci. Transl. Med. 2021, 13, eaaz4499.
- Lee, J.; Lee, E.; Kim, H.; Son, Y. Comparison of Articular Cartilage with Costal Cartilage in Initial Cell Yield, Degree of Dedifferentiation during Expansion and Redifferentiation Capacity. Biotechnol. Appl. Biochem. 2007, 48, 149–158.
- Lee, J.; Lee, J.-Y.; Chae, B.-C.; Jang, J.; Lee, E.; Son, Y. Fully Dedifferentiated Chondrocytes Expanded in Specific Mesenchymal Stem Cell Growth Medium with FGF2 Obtains Mesenchymal Stem Cell Phenotype In Vitro but Retains Chondrocyte Phenotype In Vivo. Cell Transplant. 2017, 26, 1673–1687.
- Yoon, K.-H.; Park, J.-Y.; Lee, J.-Y.; Lee, E.; Lee, J.; Kim, S.-G. Costal Chondrocyte-Derived Pellet-Type Autologous Chondrocyte Implantation for Treatment of Articular Cartilage Defect. Am. J. Sport. Med. 2020, 48, 1236–1245.
- Yoon, K.-H.; Yoo, J.D.; Choi, C.-H.; Lee, J.; Lee, J.-Y.; Kim, S.-G.; Park, J.-Y. Costal Chondrocyte-Derived Pellet-Type Autologous Chondrocyte Implantation versus Microfracture for Repair of Articular Cartilage Defects: A Prospective Randomized Trial. Cartilage 2021, 13, 1092S–1104S.
- Ma, Y.; Zheng, K.; Pang, Y.; Xiang, F.; Gao, J.; Zhang, C.; Du, D. Anti-Hypertrophic Effect of Synovium-Derived Stromal Cells on Costal Chondrocytes Promotes Cartilage Repairs. J. Orthop. Transl. 2022, 32, 59–68.
- Kilborn, S.H.; Trudel, G.; Uhthoff, H. Review of Growth Plate Closure Compared with Age at Sexual Maturity and Lifespan in Laboratory Animals. J. Am. Assoc. Lab. Anim. Sci. 2002, 41, 21–26.
- Shim, K.S. Pubertal Growth and Epiphyseal Fusion. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 8–12.
- Liu, Z.-M.; Shen, P.-C.; Lu, C.-C.; Chou, S.-H.; Tien, Y.-C. Characterization of the Proliferating Layer Chondrocytes of Growth Plate for Cartilage Regeneration. Tissue Eng. Part A 2019, 25, 364–378.
- Arzi, B.; DuRaine, G.D.; Lee, C.A.; Huey, D.J.; Borjesson, D.L.; Murphy, B.G.; Hu, J.C.Y.; Baumgarth, N.; Athanasiou, K.A. Cartilage Immunoprivilege Depends on Donor Source and Lesion Location. Acta Biomater. 2015, 23, 72–81.
- Malicev, E.; Kregar-Velikonja, N.; Barlic, A.; Alibegović, A.; Drobnic, M. Comparison of Articular and Auricular Cartilage as a Cell Source for the Autologous Chondrocyte Implantation. J. Orthop. Res. 2009, 27, 943–948.
- Hou, M.; Bai, B.; Tian, B.; Ci, Z.; Liu, Y.; Zhou, G.; Cao, Y. Cartilage Regeneration Characteristics of Human and Goat Auricular Chondrocytes. Front. Bioeng. Biotechnol. 2021, 9, 766363.
- Wong, C.-C.; Chen, C.-H.; Chiu, L.-H.; Tsuang, Y.-H.; Bai, M.-Y.; Chung, R.-J.; Lin, Y.-H.; Hsieh, F.-J.; Chen, Y.-T.; Yang, T.-L. Facilitating In Vivo Articular Cartilage Repair by Tissue-Engineered Cartilage Grafts Produced From Auricular Chondrocytes. Am. J. Sport. Med. 2018, 46, 713–727.
- Wang, Z.; Li, Z.; Li, Z.; Wu, B.; Liu, Y.; Wu, W. Cartilaginous Extracellular Matrix Derived from Decellularized Chondrocyte Sheets for the Reconstruction of Osteochondral Defects in Rabbits. Acta Biomater. 2018, 81, 129–145.
- Takahashi, T.; Ogasawara, T.; Kishimoto, J.; Liu, G.; Asato, H.; Nakatsuka, T.; Uchinuma, E.; Nakamura, K.; Kawaguchi, H.; Takato, T.; et al. Synergistic Effects of FGF-2 with Insulin or IGF-I on the Proliferation of Human Auricular Chondrocytes. Cell Transplant. 2005, 14, 683–693.
- Okubo, R.; Asawa, Y.; Watanabe, M.; Nagata, S.; Nio, M.; Takato, T.; Hikita, A.; Hoshi, K. Proliferation Medium in Three-Dimensional Culture of Auricular Chondrocytes Promotes Effective Cartilage Regeneration in Vivo. Regen. Ther. 2019, 11, 306–315.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
826
Revisions:
2 times
(View History)
Update Date:
12 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




