Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | MARIA VRANCEANU | -- | 6930 | 2022-10-11 12:44:27 | | | |
| 2 | Catherine Yang | Meta information modification | 6930 | 2022-10-12 03:06:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vrânceanu, M.; Galimberti, D.; Banc, R.; Dragoş, O.; Cozma-Petruţ, A.; Hegheş, S.; Voştinaru, O.; Cuciureanu, M.; Stroia, C.M.; Miere, D.; et al. Plant-Derived Nutraceuticals. Encyclopedia. Available online: https://encyclopedia.pub/entry/28923 (accessed on 07 February 2026).
Vrânceanu M, Galimberti D, Banc R, Dragoş O, Cozma-Petruţ A, Hegheş S, et al. Plant-Derived Nutraceuticals. Encyclopedia. Available at: https://encyclopedia.pub/entry/28923. Accessed February 07, 2026.
Vrânceanu, Maria, Damiano Galimberti, Roxana Banc, Ovidiu Dragoş, Anamaria Cozma-Petruţ, Simona-Codruţa Hegheş, Oliviu Voştinaru, Magdalena Cuciureanu, Carmina Mariana Stroia, Doina Miere, et al. "Plant-Derived Nutraceuticals" Encyclopedia, https://encyclopedia.pub/entry/28923 (accessed February 07, 2026).
Vrânceanu, M., Galimberti, D., Banc, R., Dragoş, O., Cozma-Petruţ, A., Hegheş, S., Voştinaru, O., Cuciureanu, M., Stroia, C.M., Miere, D., & Filip, L. (2022, October 11). Plant-Derived Nutraceuticals. In Encyclopedia. https://encyclopedia.pub/entry/28923
Vrânceanu, Maria, et al. "Plant-Derived Nutraceuticals." Encyclopedia. Web. 11 October, 2022.
Copy Citation
The term nutraceutical combines the words nutrition and pharmaceutical and indicates those nutrient principles that are found within foods. These have beneficial health effects. Nutraceutical substances derive mainly from plants, food, and microbial sources.
nutraceuticals
gene expression
epigenetic therapy
cancer
1. Curcumin
Curcuma longa L. is an herbaceous plant, perennial and rhizomatous, which belongs to the family of Zingiberaceae, as ginger (Zingiber officinale Rosc.) also does. The root, which is the most important component of phytotherapeutic and nutritional interest, is constituted by a cylindrical, branched, aromatic rhizome of orange-yellow color. It is used in food as a spice, especially in traditional Indian, Middle Eastern, and Thai cuisine. The plant contains more than 100 chemical compounds, but the term curcumin generally refers to 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, a compound known as “curcumin I”. Two other best-known compounds are curcumin II (demethoxycurcumin, 1-(4-hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione) and curcumin III (bisdemethoxycurcumin, 1,7-bis(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione) [1]. The specific and well-known yellow curcumin color is due to “curcumin I” and the curcuminoids, bisdemethoxycurcumin and demethoxycurcumin, generally used as a natural dye in the food industry [2]. The principal essential oils of curcumin are turmerone (ar-turmerone), β-turmerone, α-turmerone, β-bisabolene, β-sesquiphellandrene, α-zingiberene, curcumol, and curcumenol [3].
Curcumin is famous for its antioxidant, anti-inflammatory, and anticancer properties and recently has been shown to act as an epigenetic modulator [4]. The role of curcumin as an epigenetic regulator includes histone modification by the regulation of histone acetyltransferase (HAT) and histone deacetylase (HDAC); DNA methylation by the inhibition of DNA methyltransferase (DNMT); microRNA modulation by the upregulation of tumor-suppressive miRNAs (miR-15a, miR-16, miR-22, miR-26a, miR-34a, miR-145, miR-146a, miR-200b, c, miR-203, and let-7) [5][6]; the downregulation of oncogenic miRNAs (miR-19a, b, miR-21, miR-27a, miR-130a, miR-186) [7]; and the activation of transcription factors, cytokines, and tumor suppressor genes [8]. DNA methylation is a great target in the treatment of acute myeloid leukemia (AML) as it is well known that the inactivation of genes due to DNA methylation has a major role in the development of AML. It has been shown that curcumin is able to downregulate DNMT1 expression in AML cell lines, in vitro and in vivo [9]. p65 and Sp1 expression, positive regulators of DNMT1, may be reduced by curcumin, which correlates with reductions in the binding of these transcription factors to the DNMT1 promoter in AML cell lines. These characteristics of curcumin make it a promising compound in the treatment of AML [10]. Due to the changes in DNA methylation, curcumin is a hypomethylating agent in breast, prostate, colon, and lung cancer.
Curcumin is able to target other, different cancer-related pathways, such as tumor suppressor genes, growth-signaling factors, transcription factors, apoptotic genes, oncoproteins, the biomarkers of inflammation, or protein kinases [11].
1.1. Anticancer Activity and the Suppression of Carcinogenesis
One of the main mechanisms of the anticancer effects of curcumin is due to its interference in the cell cycle and reduction in cyclin-dependent kinase (CDK) expression that controls cell-cycle progression [12]. Curcumin is able to suppress the human epidermal growth factor receptor 2, a tyrosine kinase (HER2-TK), and in this manner inhibits breast cancer cell lines [13]. By administering curcumin, there is a decrease in the activation of the PI3K (phosphoinositide 3-kinase)/AKT (AKT serine/threonine kinase) signaling pathway, resulting in an anticancer effect via the negative modulation of this cell-signaling pathway [14].
Curcumin can modulate the activity of different transcription factors, inhibiting some of them, such as nuclear factor-κB (NF-κB), activated protein-1 (AP-1), signal transducer and activator of transcription (STAT) proteins, hypoxia-inducible factor-1 (HIF-1), Notch-1, early growth response-1 (Egr-1), and β-catenin, but activating others, such as NF-E2-related factor (Nrf2) [11][15]. Transcription factors play an important role in various stages of carcinogenesis, being involved in cell proliferation, cell survival, invasion, angiogenesis, and inflammation. Most of these factors are upregulated in most cancers [15]. It has been demonstrated that curcumin inhibits STAT3 phosphorylation, which is responsible for signaling carcinogenic pathways [16]. Furthermore, curcumin is a potent inhibitor of NF-κB, and this effect is correlated with cellular apoptotic response [17]. Likewise, curcumin stimulates the expression of pro-apoptotic Bax and inhibits the activation of Mcl-1 and Bcl-2 (apoptosis regulator) antiapoptotic agents, also altering the expression of apoptotic mechanisms associated with NF-κB proteins, p38 and p53 [18].
1.2. Inhibition of Angiogenesis
In some tumors, curcumin inhibits angiogenesis by suppressing angiogenic cytokines, such as IL-6, IL-23, and IL-1β [19], and it is a direct inhibitor of angiogenesis by downregulating transcription factors, such as NF-κB, and proangiogenesis factors, such as bFGF (basic fibroblast growth factor), VEGF (vascular endothelial growth factor), and MMPs (matrix metalloproteinases), all of them linked with tumorigenesis [20].
1.3. Anti-Inflammatory Properties
Curcumin is a highly pleiotropic molecule, able to interact with numerous molecular targets involved in the inflammatory process, hence the strong anti-inflammatory action both in the acute phase and in the chronic phase of inflammation. Due to its strong anti-inflammatory effects, in several studies, curcumin showed the ability to prevent the development of some types of cancer by reducing the production of COX-2, lipoxygenase 2, iNOS, and related cytokines, known as mediators of the inflammatory process [21].
Furthermore, curcuminoids are able to exert antioxidant action by blocking free-circulating radicals and inhibiting the formation of new ones [22]. Curcumin can also increase the antioxidant activity, in vitro and in vivo, of the enzymes SOD, CAT, GST, and GSR, and, in this manner, curcumin directly inhibits the formation of reactive species, including superoxide radicals, nitric oxide radicals, and hydrogen peroxide. On the other hand, curcumin also increases the activity of detoxifying enzymes by reducing xenobiotics, therefore protecting against carcinogenic processes [23]. In light of these facts, research is aimed at clarifying the beneficial effects of the combination of curcumin with various antineoplastic drugs so as to improve their clinical effects and reduce their toxicity [13][24].
Although curcumin has significant medicinal properties, its poor bioavailability has limited the success of in vivo epigenetic studies, only partly bypassed by the possibility of using high dosages of the active ingredient in relation to its very low toxicity. Recently, pharmaceutical research has led to the introduction in the market of molecules with better bioavailability (phytosome technology), also opening new therapeutic horizons in terms of preventive medicine and antiaging [25].
In order to overcome the main disadvantages related to the oral administration of curcumin, new strategies for its efficient delivery have been investigated. Among the curcumin formulation strategies used in order to enhance its absorption are lipid additions (such as turmeric oil, piperine, or turmeric oleoresin), the adsorption and dispersion of curcumin onto various matrices (such as γ-cyclodextrin or whey protein), and particle size reduction, but also modified structures of curcumin analogs and micellar and nanoparticle formulations of curcumin [26][27]. Unfortunately, some of these formulations claimed an enhanced bioavailability of curcumin only on the basis of increased solubility, without considering the solubility–permeability interplay in the gastrointestinal tract when using solubility-enabling formulations for oral lipophilic drugs [26][28]. The most important goals in the development of curcumin delivery systems are enhancing solubility, increasing bioavailability by enhancing small intestine permeation, preventing degradation in the intestinal environment, increasing content in the bloodstream, and increasing efficacy. Among the delivery systems that have shown promising results in this regard are micelles, liposomes, phospholipid complexes, nanoemulsions, microemulsions, emulsions, solid lipid nanoparticles, nanostructured lipid carriers, biopolymer nanoparticles, microgels, nanogels, etc. [26][27][29][30][31].
2. Resveratrol
Resveratrol (3,5,4′-trihydroxystilbene) is a stilbenoid, a polyphenolic phytoalexin produced by some plants in response to injury or attack by pathogens, such as fungi or bacteria. Sources of resveratrol in food include grapes (Vitis vinifera L.), blueberries (Vaccinium corymbosum L.), raspberries (Rubus idaeus L.), mulberries (Morus alba Hort. ex Loudon L.), and peanuts (Arachis hypogaea L.). Resveratrol presents two geometric isomers: cis-(Z) and trans-(E). The trans form exposed to ultraviolet radiation can undergo isomerization to the cis form [32]. The cis form is dominant in prevalence and especially in= biological activity such as cell-cycle arrest, apoptosis, differentiation, and the anti-proliferation of cancer cells [33][34]. Originally, resveratrol was isolated by Takaoka in 1940, from the roots of white hellebore (Veratrum album L.), and in 1963, from knotweed (Polygonum cuspidatum Sieb. et Zucc) root. However, only in 1992 did resveratrol attract attention when its presence in wine was associated with the cardioprotective effects of this beverage. Polygonum cuspidatum Sieb. et Zucc. is one of the richest sources of resveratrol in nature and, for this reason, it has become a very important plant in modern herbal medicine [35][36].
2.1. Antioxidant and Anti-Inflammatory Activity
Resveratrol is able to exert powerful antioxidant and anti-inflammatory action. As an antioxidant, it has a superior activity to that of more known molecules, such as vitamin C and E, and is also more effective than flavonoids because it also acts upstream of the reaction, rendering copper inactive as a catalyst through its chelation [37].
In addition to the direct antioxidant effect, resveratrol also regulates the gene expression of the prooxidant and antioxidant enzymes: SOD1 and GPX1 are strengthened by resveratrol in a concentration-dependent manner. Therefore, the suppression of the expression of the prooxidant genes (via NADPH-oxidase) and the induction of antioxidant enzymes, such as SOD1 and GPX1, are important components of the antioxidant protective effect induced by resveratrol [38]. Resveratrol has been proven to be an effective scavenger of free radicals, including superoxide radical (O2−), hydrogen peroxide (H2O2), hydroxyl radical (OH−), nitric oxide (NO), and nitrogen dioxide (NO2) [39][40].
However, direct scavenger activities are relatively scarce, also due to the reduced in vivo half-life of this molecule. The antioxidant properties of resveratrol in vivo, on the other hand, are due to its effect as a regulator of gene expression. Resveratrol induces the downregulation of NADPH-oxidase, with a consequent reduction in reactive oxygen species (ROS). Furthermore, by hyperstimulating tetrahydrobiopterin-GTP-cyclohydrolase, the expression of a variety of antioxidant enzymes is increased. Some of the genes regulating the effect of resveratrol are mediated by Nrf2 [41].
2.2. Resveratrol and Cells Apoptosis
The role of resveratrol as a modulator of cell apoptosis is fundamental. The cellular apoptosis promoted by resveratrol can be mediated by multiple mechanisms, such as the upregulation of cyclin-dependent kinase inhibitors; the activation of mitochondria and cascade of caspases, apoptosis-inducing cytokines, and related receptors; the downregulation of cell survival proteins (e.g., survivin, XIAP (X-linked inhibitor of apoptosis protein), cIAPs, cFLIP, Bcl-XL, Bcl-2); and the inhibition of cell survival kinases (e.g., MAPK, AKT/phosphoinositide 3-kinase (PI3K), PKC, EGFR kinase) and transcription survival factors (e.g., NF-κB, AP-1, HIF-1α, and signal transducer and transcription activator (STAT3)). The induction of one of these pathways by resveratrol leads to cell death [42][43].
Resveratrol regulates proteins involved in DNA and cell-cycle synthesis, such as p53 and Rb/E2F, CDK, and their inhibitors. Resveratrol influences the activity of transcription factors involved in proliferation and stress response, such as NF-κB, AP1, and EGR1. One part of these events is mediated by MAPK and tyrosine kinase, for example, SRC, and leads to the modulation of survival and apoptotic factors (e.g., members of the Bcl-2 family, inhibitors of apoptosis) as well as to the modulation of enzymes involved in carcinogenesis (e.g., cyclooxygenase (COX), nitric oxide synthase (NOS), phase I and II enzymes) [42]. Finally, resveratrol helps regulate the activity and expression of co-transcription factors such as p300 and SIRT1 [44].
Resveratrol also promotes the activation of sirtuins [45] (Figure 1) in synergy with melatonin. The fact that melatonin and resveratrol are present in various foods implies possible synergistic effects, suggesting combined use to promote health and longevity [46].
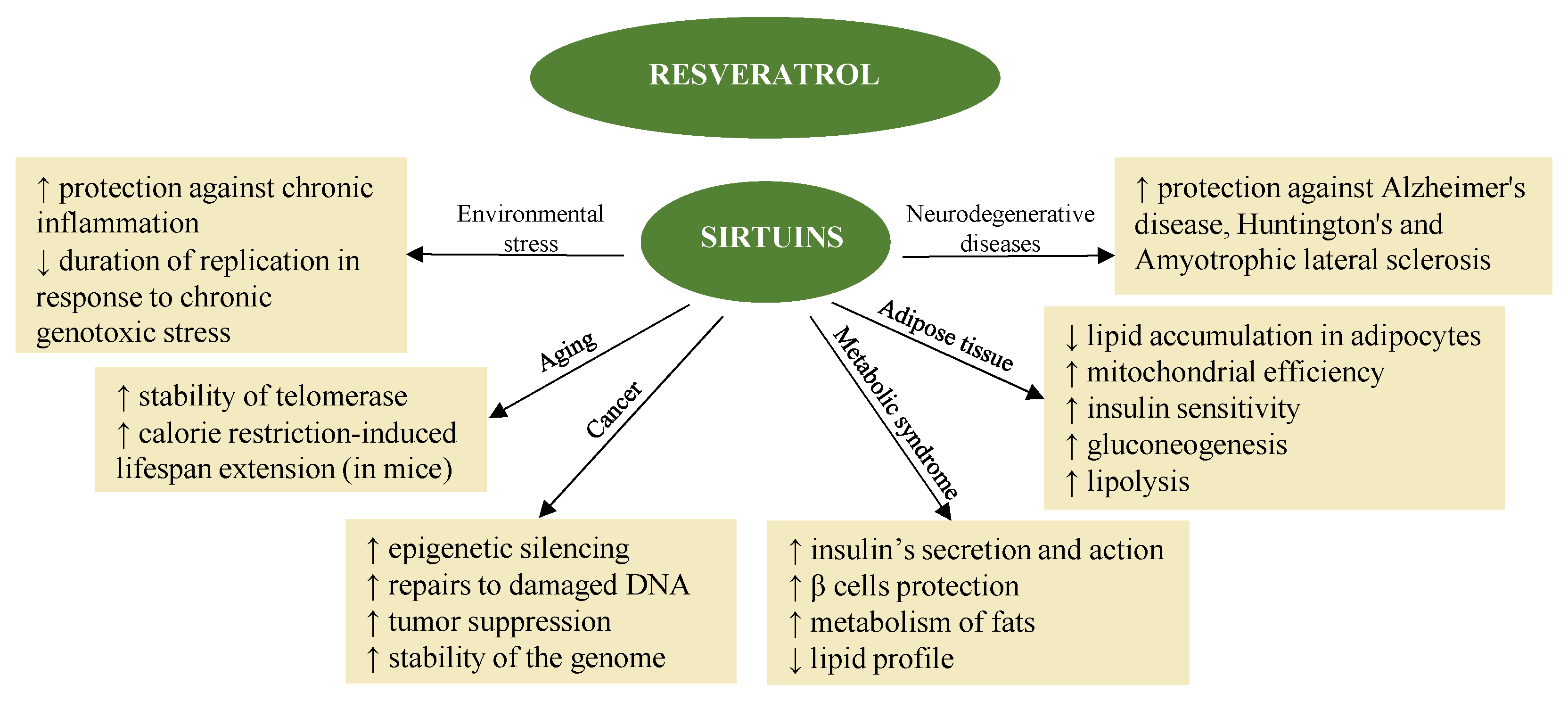
Figure 1. Resveratrol exerts different effects by activating sirtuin [47].
The mechanism of epigenetic action in the case of resveratrol also suggests its indication in the treatment of neurodegenerative diseases [48][49]. In fact, the neuroprotective and neurotrophic effects induced by resveratrol have been the subject of multiple studies, both in vitro and in vivo, and make it again a dietary epidrug in the adjuvant treatment and prevention of these diseases [49]. Resveratrol induces autophagy, directly inhibiting the mTOR pathway through interaction with the ATP-binding pocket of mTOR (it direct competes with ATP) [50]. Likewise, it induces the death of tumor cells, also thanks to the inhibition of the mTORC1 pathway [51].
Resveratrol bioavailability is increased by gastric juices, so it is recommended to take it with meals [52]. In addition, the circadian rhythm and the type of meal may influence bioavailability [53][54]. Therefore, in order to increase the bioavailability of resveratrol, the best time to administer it turned out to be in the morning [54]. There are wide margins of safety and non-toxicity. Lower doses have a beneficial effect, while higher doses (2 g/day or more) can be associated with a number of side effects, such as diarrhea, nausea, abdominal pain, hypersensitivity, or frontal headache [53][55]. The best dose range, for an actual clinical benefit in vivo, is between 250 mg and 500 mg/day [56].
One of the strategies that may improve the pharmacokinetics and bioavailability of resveratrol is the synergism with other phytochemicals, such as piperine. Thus, the co-administration of resveratrol and piperine has improved the bioavailability of resveratrol by inhibiting its rapid metabolism [57][58]. The use of polydatin, a compound that is extracted from the roots of the Polygonum cuspidatum Sieb. et Zucc. plant and differs from resveratrol by the presence of one molecule of glucose—which makes the compound more water-soluble and, consequently, more bioavailable than resveratrol—has also been discussed [55]. It has also been considered to increase the bioavailability of orally administrated resveratrol by using alternative routes of administration, such as inhalers and transdermal, buccal, and nasal–brain routes, obtaining promising results [59].
Other measures that may improve the pharmacokinetics of resveratrol, and therefore bioavailability, have focused on innovative delivery systems, such as nanoemulsions, nanosuspensions, dendrimers, liposomes and nanoliposomes, solid lipid nanoparticles, and polymeric nanoparticles [57][58][60].
3. Sulforaphane, Indole-3-Carbinol, and 3,3′-Diindolylmethane
The consumption of cruciferous vegetables, such as broccoli (Brassica oleracea var. italica Plenck), cabbage (Brassica oleracea var. capitata L.), brussels sprouts (Brassica oleracea var. gemmifera Zenker), cauliflower (Brassica oleracea var. botrytis L.), and kale (Brassica oleracea var. viridis DC. L.) has been associated with anticancer and antioxidant effects. Considerable evidence shows that glucosinolates (GLSs) are the main phytochemicals in cruciferous vegetables that contribute to their health effects [61]. GLSs are relatively inactive and necessitate hydrolysis by plant endogenous myrosinase (MYR) to deliver a variety of bioactive compounds, such as isothiocyanates (ITCs) and indoles. Neutral pH conditions are favorable for the formation of ITCs [62][63]. GLSs and the enzyme MYR are stored in different compartments of plant cells, requiring plant tissue to be damaged for cellular breakdown to occur and MYR to be released and act on GLSs. Therefore, the processing of cruciferous vegetables (i.e., by mastication, cutting, chopping) has an important impact on the bioavailability of GLSs and their hydrolysis products [64][65].
Concerning the assimilation by the body of the GLS hydrolysis products, absorbed ITCs are conjugated to glutathione, with the involvement of glutathione-S-transferase (GST) enzymes, and metabolized via the mercapturic acid pathway [63]. The polymorphisms of genes coding for GST may have an important effect on ITC metabolism, leading to interindividual variations in the benefits from exposure to these compounds. For instance, individuals carrying deletions in both GST M1 and GST T1 genes may show a more rapid elimination of ITCs, requiring a high intake of cruciferous vegetables in order to capitalize on their positive health effects [66]. As for the metabolism of indoles, molecules such as indole-3-carbinol (I3C) principally undergo oxidative metabolization to indole-3-carboxaldehyde and indole-3-carboxylic acid. The quantification of ITC and indole metabolites in human urine and plasma may serve as an approach to characterize the intake of bioactive compounds from cruciferous vegetables [67][68]. Indeed, it has been demonstrated that the urinary elimination of mercapturic acids after the consumption of cooked cruciferous vegetables accounts for a maximum of 20% of the ingested GLSs. If the vegetables are consumed in the raw form, the rate can reach 88% [62].
To date, the most extensively studied ITCs and indoles are SFN and I3C, respectively. SFN is the precursor of glucoraphanin, the main GLS in broccoli, accounting for about 80% of the total yield [69]. Glucobrassicin is also an important GLS in broccoli [68]. The cleavage of glucobrassicin by MYR generates predominantly I3C. In the acidic conditions at the gastric level, I3C further forms a mixture of dimers, linear and cyclic trimers, and higher oligomers, with 3,3′-diindolylmethane (DIM) being the major condensation product [67][70]. Between 20 and 40% of the ingested I3C is converted to DIM [71]. In fact, several studies have suggested that the health effects of I3C can be mainly attributed to DIM [70][72]. I3C, as well as its acid condensation products, are absorbed at the intestinal level and then distributed into several well-perfused tissues, where they exhibit their biological activities [73].
Currently, SFN, I3C, and DIM are considered promising cancer chemopreventive compounds. I3C is also recognized to have biological properties such as the inhibition of inflammation and angiogenesis, decreases in proliferation, and the promotion of tumor cell death [74].
3.1. Chemopreventive Activity and Epigenetic Role
There is much evidence to connect the chemopreventive properties of I3C, DIM, and SFN with epigenetic mechanisms [75]. Several studies suggest that, at least in part, the chemopreventive effects of I3C are due to the downregulation of class I HDAC isoenzymes (HDAC1, HDAC2, HDAC3, and HDAC8) by DIM. Decreased HDAC expression leads to the increased expression of the pro-apoptotic Bcl-2 (B-cell lymphoma 2)-associated X (Bax) protein, CDKNs p21, and p27 followed by the arrest of the cell cycle and increased rate of apoptosis. For this reason, HDAC inhibition may be a novel epigenetic mechanism for cancer prevention by DIM [76].
SFN may target the aberrant hypermethylation status by downregulating the expression of DNMT1 and DNMT3a in breast cancer cells [77].
Cyclin D2 is a major regulator of the cell cycle and its hypermethylation is correlated with prostate cancer progression. SFN is capable of decreasing the expression of DNMT1 and DNMT3b and epigenetically modulating cyclin D2 expression, acting as a prostate cancer chemopreventive agent [78].
I3C and DIM modulate the expression of several miRNAs and lncRNAs [19][20]. Thus, DIM increases the expression of tumor suppressor microRNAs, such as let-7a-e, miRNA-15a, miRNA-16, miR-27b, miR-30e, miR-31, miR-34a, miR-124, miR 200 a, miR 200b, miR 200c, miR-219-5p, and miR-320, and decreases the expression of oncogenic miR19a, miR19b, miR92a-2, miR 106a, miR 181a, miR 181b, miR 210-3p, miR 221, and miR 495 [71][79][80].
3.2. Effect of Estrogen Analog and Anticarcinogenic in Mammary Tumor Cells
I3C is capable of arresting the growth of human tumor cells in the G1 phase of the reproductive cell cycle [81]. I3C is also a potent inducer of cytochrome P450 enzymes, including CYP1A1, CYP1A2, and CYP1B1 [82][83]. These phase I metabolizing enzymes are involved in the oxidative metabolism of estrogens. I3C and DIM can alter endogenous estrogen metabolism by increasing the 2-hydroxylation reaction, resulting in an increase in the 2-OH:16-OH ratio relative to the estrogen metabolites [84]. The metabolites of these hormones can inhibit or stimulate the onset of hormone-sensitive neoplasms [85]. Several studies have demonstrated that estrone 2 (2OHE1) tends to inhibit the growth of the neoplasm, whereas estrone 16 (16OHE1) promotes tumor growth [86].
The positive effects of I3C and DIM are related to the fact that both are capable of modifying the estradiol hydroxylation receptor site, resulting in the diminution of 16-α-hydroxyestrone production in favor of 2-hydroxyestrone. I3C and DIM are also involved in the stimulation of liver detoxifying enzyme production, capable of neutralizing and degrading the harmful metabolites of estrogens and xenoestrogens, assimilated as environmental or food pollutants [87][88].
3.3. Anticancer Activity
The SFN also exhibits anticancer action by controlling the progression of tumorigenesis. In non-small cell lung cancer (NSCLC), the SFN is able to attenuate the signaling pathway of EGFR, suggesting an anticancer mechanism of action [89]. As a whole, it has shown multiple effects, including the arrest of cell growth, differentiation, and apoptosis, as recently demonstrated in the case of prostate neoplasms [90].
SFN inhibits the proliferation, in vivo, of breast cancer cells, while in normal cells the effect is insignificant. Cancer cells are characterized by the high expression of telomerase. Treatment with SFN inhibits the catalytic subunit of human telomerase reverse transcriptase (hTERT) [91]. At the same time, scientific studies have shown interference in DNA methyltransferase (DNMT) activity, in particular DNMT1 and DNMT3a, which have been reduced in breast cancer cells treated with SFN, suggesting that this compound may be able to repress hTERT through specific epigenetic pathways. Furthermore, the downregulation of hTERT expression facilitates the induction of cell apoptosis in breast cancer cells, paving the way for approaches aimed at the SFN-mediated prevention of this neoplasia and as preventive nutraceuticals [92].
3.4. Anti-Inflammatory Activity
Inflammation is usually associated with chronic disease and cancer. It is well known that NF-κB is a major transcription factor involved in the regulation of the expression of many pro-inflammatory genes, such as COX-2 and iNOS. I3C and DIM exert anti-inflammatory effects by the downregulation of COX-2, iNOS, CXCL5, and IL-6 expression, which may be mediated by reductions in NF-κB activation [93].
In conclusion, the GLSs present in cruciferous vegetables have beneficial effects on general health and are also potential anticancer agents, due to their antioxidant and detoxifying properties and epigenetic mechanisms, including the modification of CpG (cytosine–phosphate–guanine) methylation, which occurs predominantly in cancer-related genes, the regulation of histone modification, and changes in miRNA expression [94][95].
The daily dosage of SFN demonstrated to provide beneficial health effects is around 20–40 mg [96]. Furthermore, the recommended daily dosage for I3C ranges between 200 mg and 900 mg per day and for DIM between 25 mg and 450 mg per day, respectively. The use over time must include both urinary and blood hormone monitoring, including, in the urine, the observation of the relationship between estrone 2 and estrone 16 and, at the hematic level, of the total estrone and estradiol and total and free testosterone and androstenedione, so as to constantly adapt the therapy. A diet rich in cruciferous vegetables seems to provide SFN, I3C, and DIM in sufficient amounts for the prevention of many types of cancer, including those that are hormone-related, such as breast, ovary, uterus, and prostate neoplasms [97][98][99]. In contrast, to achieve therapeutic concentrations of SFN, I3C, and DIM, the intake of these compounds in the form of dietary supplements seems to be required [71][100].
The exploitation of SFN by the nutraceutical industry has faced some challenges because this ITC shows high lipophilicity, low aqueous solubility, and poor stability due to sensitivity to oxygen, heat, and alkaline conditions. However, the use of nanotechnology has allowed the increase in the aqueous solubility and bioavailability of SFN through the development of formulations such as polymeric nanoparticles, magnetic nanoparticles, micelles, liposomes, and carbon dots [100].
Likewise, the low thermal- and photostability of I3C and DIM represent important challenges for the nutraceutical application of these compounds. One approach to overcome this issue has been proposed by Luo et al. (2013), who showed that the encapsulation of I3C and DIM in zein/carboxymethyl chitosan nanoparticles can protect these bioactives against temperature- and light-induced degradation [101].
4. Astaxanthin
Astaxanthin (3,3′-dihydroxy-β, β′-carotene-4,4′-dione) (ASX) is a red-orange pigment, a xanthophyll carotenoid, and a member of the macro-family of carotenoids [102]. Synthesized in appropriate quantities by microalgae—Haematococcus lacustris (Gir.-Chantr.) Rostaf., Chromochloris zofingiensis (Donz) Fucikova and L. A. Lewis, Chlorococcum sp., and Phaffia rhodozyma M.W. Mill., Yoney. and Soneda—ASX enters the food chain through crustaceans and predatory fish such as salmon, in whose meat it can easily reach 5–10 mg/kg [103].
ASX has antioxidant potential, as well as anti-inflammatory and antineoplastic activities, acting as an antioxidant and reducing oxidative stress, thereby preventing protein and lipid oxidation and DNA damage. Having antioxidant action, it helps to maintain the functionality of tissues and systems, promoting better overall homeostasis [102].
ASX affects tumor growth in different types of cancers. Several studies have demonstrated that ASX is able to resensitize gemcitabine-resistant human pancreatic cancer cells to gemcitabine [104]. ASX increases DNMT3a expression at low concentrations, but at high concentrations decreases the expression of DNMT1, 3a, and 3b and attenuates NAD(P)H Quinone Dehydrogenase 1 (NQO1) expression via the Nrf2/KEAP1 pathway, reducing cell viability in prostate and skin cancer cells [105][106]. ASX has also the ability to reduce tumor growth in prostate cancer by increasing the expression of tumor suppressor microRNAs, miR-375 and miR-478b [107]. In breast cancer, ASX negatively affects cell viability [108][109], due to apoptotic and autophagic effects that allow it to kill the cancer cells without affecting normal cells [110].
In colorectal cancer (CRC), ASX has demonstrated anti-migratory and anti-invasive activity by increasing miR-29a-3p and miR-200a expression, suppressing MMP2 and ZEB1 expression, resulting in the repression of the epithelial–mesenchymal transition (EMT) of CRC cells [111]. Regarding lung cancer, NSCLC accounts for the majority of lung cancer-related deaths [112]. There are few studies to show the effects of ASX against NSCLC or other lung cancers in vivo. In vitro, ASX is able to reduce the viability of NSCLC cells in a dose-dependent manner [113][114]. Moreover, ASX enhances apoptosis and decreases cell proliferation. ASX is able to enhance the cytotoxicity of the drugs with clinical activity in NSCLS, such as erlotinib, a selective epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor. The co-administration of erlotinib and ASX has increased cytotoxicity and inhibited cell growth in NSCLC cells, associated with the downregulation of xeroderma pigmentosum complementation group C (XPC) expression [114]. The overexpression of thymidylate synthase (TS) usually causes resistance to antitumor treatment, especially pemetrexed used in advanced NSCLC forms. ASX treatment decreases TS expression, both alone and in combination with pemetrexed. Moreover, ASX administration together with mitomycin C significantly reduces Rad51 expression, which exhibits high levels in chemoresistant carcinoma [115][116].
All these in vitro findings suggest that ASX may improve the efficacy of standard treatments in lung cancer. Some studies have also suggested that ASX could be used to treat gastric cancer, based on its role in necroptotic signaling [117].
Despite its biological activities, ASX has very low bioavailability, similar to other carotenoids [116][118]. When astaxanthin is administered orally, the bioavailability varies between 10 and 50% of the given dose [119]. The very poor bioavailability of ASX is due to dissolution limitations in gastrointestinal fluids and also to the saturated capacity of incorporation into bile micelles, which limits its absorption [120][121]. Being a very lipophilic compound, it has extremely low water solubility, which prevents its dispersibility and causes a low absorption rate [116][122]. After ingestion, ASX mixes with bile acid, forming micelles in the small intestine, partially absorbed by intestinal mucosa cells, which will incorporate astaxanthin into chylomicrons [103]. After their release into the lymph within the systemic circulation, chylomicrons with ASX are digested by lipoprotein lipase, ASX is assimilated with lipoproteins and transported to tissues, and chylomicrons remnants are quickly removed by the liver and other tissues [103]. In nature, astaxanthin is predominantly found in the form of mono- and diesters, being, respectively, esterified with one or two units of fatty acids in hydroxyl groups, or in the form of carotenoproteins when conjugated with proteins [119].
In order to improve the water solubility, stability, and bioavailability of ASX, several delivery systems have been developed, such as complex coacervation, liposomes, emulsions and nanoemulsions, microparticles, and polymeric nanoparticles [123][124][125]. Since lipid-based nanoparticles, such as liposomes, solid-lipid nanoparticles, and niosomes have limitations, such as poor water solubility and permeability, instability, rapid metabolism, and poor oral bioavailability, polymeric nanoparticles have begun to be used to overcome these limitations. Thus, after encapsulation in polymeric nanoparticles made from biodegradable natural polymers, such as polysaccharides and proteins, water solubility, stability, and absorption in the human body were enhanced [122][125]. In terms of nanoemulsion-based delivery systems, they provide improved physical stability and increase water dispersibility and bioavailability [114].
5. Quercetin
Quercetin is a flavonoid belonging to the flavonols group, present in a large variety of fruits—apples (Malus domestica (Suckow) Borkh.), grapes (Vitis vinifera L.), olives (Olea europaea Hoffmanns. and Link L.), citrus fruits such as oranges (Citrus sinensis (L.) Osbeck), and raspberries (Rubus idaeus L.)—vegetables—tomatoes (Solanum lycopersicum L.), onions (Allium cepa L.), broccoli (Brassica oleracea var. italica Plenck), and capers (Capparis spinosa L.)—drinks (tea and red wine), and herbal extracts [126]. In nature, quercetin is not present in the isolated form but is the aglyconic component of some glycosides, including rutin and quercitrin. In this form it abounds, in particular, in extracts of horse chestnut (Aesculus hippocastanum L.), Gingko biloba L., marigold (Calendula officinalis L.), hawthorn (Crataegus monogyna Jacq.), chamomile (Matricaria recutita L. and Chamaemelum nobile L.), lovage (Levisticum officinale W. D. J. Koch), and St. John’s wort (Hypericum perforatum L.) [127][128].
Quercetin is capable of increasing the pro-apoptotic molecules BAX, caspase-3, caspase-9, and p53 and stimulating the mitochondrial apoptosis pathway, resulting in increased proapoptotic effects [129][130]. Another important feature of quercetin is related to the arrest of the cell cycle in the G1 phase by activating p21 and decreasing D1/Cd4 and E/Cdk2 ratios [131][132]. Several studies have demonstrated that quercetin can inhibit carcinogenesis and metastasis in cancer and is capable of stabilizing p53, a key molecule in cancer therapy involved in cell death and survival regulation [133].
In gastrointestinal cancer (GC), the genes encoding for the proteins urokinase plasminogen activator (uPA) and uPA receptor (uPAR) are strongly associated with this type of cancer, being a crucial pathway for tumor invasion. Quercetin has the ability to decrease the expression of these genes, strongly associated with the suppression of cell viability, migration, and invasion. Likewise, quercetin has antimetastatic effects in GC by interfering with uPA/uPAR systems, AMPKα, NF-kβ, ERK1/2, and PKC-δ regulation [134]. In patients with CRC carrying the KRAS mutant gene, quercetin decreases cell viability and increases apoptosis by AKT pathway repression and the activation of the c-Jun N-terminal kinase (JNK) pathway in mutant KRAS cells [135].
In prostate cancer, quercetin inhibits the expression of androgen receptor (AR) and AR-mediated PSA expression at the transcriptional level with the inhibition of tumor progression. Quercetin can suppress survival protein Akt and enhance prostate cancer apoptosis in a dose-dependent manner [136].
Quercetin decreases IGF1 levels and increases IGFBP3, which is associated with an increase in proapoptotic effects and a decrease in anti-apoptotic proteins BCL2 and BCL-XL [137].
Src is a non-receptor tyrosine kinase that is deregulated in many types of cancer. Quercetin has an anti-NSCLC effect in lung cancer by inhibiting the Src-mediated Fn14/NF-κB pathway [138].
The epigenetic mechanisms associated with quercetin are the suppression of Janus kinase 2 (JAK2) with the inhibition of the proliferation, invasion, and migration of cancer cells [139]. Quercetin can also enhance apoptosis through its DNA-demethylating activity. Quercetin has an inhibiting effect on class I HDAC expression in leukemia cells due to increased proteasomal degradation [140]. Quercetin turns out to be a valid nutraceutical that can help reduce the formation of free radicals and pro-inflammatory substances, proving to be a valuable aid for human health.
Quercetin has also been shown to modulate the expression of microRNAs in different types of cancer by increasing the expression of tumor-suppressive miR-let-7, miR-15a, miR-16, miR-16, miR-22, miR-26, miR-200b-3p, miR-142-3p, miR-146a, miR-217, and miR-330 and decreasing the expression of oncogenic miR-27a, miR-155, miR-21, miR-19b, miR-148c [141].
The bioavailability of quercetin is generally poor and characterized by high interindividual variability, which could explain the conflicting results on quercetin bioactivities reported in various studies [142][143]. Pharmacokinetic studies indicate a low absorption of quercetin, with less than 1% of quercetin being absorbed in humans following oral administration [142][144]. The absorption of quercetin is related to its solubility in the vehicle used for administration [142][145]. Thus, the low solubility of quercetin in water, gastric fluids, and small intestine fluids will limit its absorption in the body [144][146]. The absorption of quercetin depends on its chemical structure. Thus, while quercetin aglycone is absorbed in both the stomach and small intestine, glycosylated forms of quercetin are not absorbed in the stomach and will be absorbed only in the small intestine, after deglycosylation, as quercetin aglycone. Quercetin biotransformation occurs by small intestinal and hepatic xenobiotic metabolism, which consists of three phases: phase I modification, phase II conjugation, and phase III elimination. Quercetin is rapidly eliminated via feces and urine. In addition to poor absorption, another factor limiting the bioavailability of quercetin is its hepatic biliary excretion, a significant proportion of absorbed quercetin being directed to biliary elimination and not to circulation [142][143].
Other factors that may affect quercetin bioavailability include the food matrix, nondigestible fiber, dietary fat, the presence of sugar moieties, and the botanical origin of quercetin. The results of a randomized crossover study, in which six women ingested the same amount of quercetin either in cereal bars or hard capsules, showed that the bioavailability of quercetin is higher when the quercetin aglycone is consumed as a whole food component [146][147]. In a study of rats, the influence of nondigestible oligosaccharides on the bioavailability of quercetin was examined. The co-administration of quercetin with short-chain fructooligosaccharides has been shown to improve the bioavailability of quercetin, as microbial degradation of the quercetin aglycone in the large intestine has been inhibited, thereby promoting the absorption of the quercetin glycoside [142][146]. Since quercetin aglycone is lipophilic, its co-ingestion along with fat has been able to increase the absorption of quercetin by incorporating it into micelles. This was observed in a study using pigs, but the improvement in the quercetin bioavailability in the case of fatty food ingestion was also demonstrated in another in vivo study in humans [142][146][147]. The bioavailability of quercetin may also be influenced by the presence or absence of the glucoside moiety, with studies in pigs showing the increased bioavailability of quercetin glycoside compared to quercetin aglycone, most likely due to the preferential absorption of quercetin glucoside, which is more water-soluble than quercetin aglycone [142]. In addition, the bioavailability of quercetin glycosides may be influenced by the type of sugar moiety [147]. Another factor on which quercetin bioavailability depends is its botanical origin. Thus, comparing the bioavailability of different forms of quercetin derivatives from onions, apples, and tea, it was observed that quercetin glycosides from onions had the highest bioavailability. The bioavailability of quercetin in humans may also be affected by health status, gut microbiota, genetic factors, and oxidative stress [146]. Various approaches have been used to improve the water solubility and bioavailability of quercetin, such as encapsulation in nanoparticles, emulsions and nanoemulsions, hydrogels, cyclodextrin complexation, size reduction (nanosuspension, nanocrystals, nanorods), co-crystallization, and amorphous solid dispersions [144][146][148].
6. Epigallocatechin-3-Gallate
EGCG is the most abundant catechin in tea, especially in green tea (Camellia sinensis L.). EGCG is a polyphenol with antioxidant and anti-inflammatory action. Besides tea, it is also found in smaller quantities in other foods, such as carob (Ceratonia siliqua L.) flour, apples (Malus domestica (Suckow) Borkh.), blackberries (Rubus plicatus L. Weihe and Nees), raspberries (Rubus idaeus L.), pistachios (Pistacia vera L.), prunes (Prunus domestica L.), peaches (Prunus persica (L.) Batsch), and avocados (Persea americana Mill.). From the tea plant, for production, the leaf bud and the two adjacent leaves are used together with their stem. Green tea is very rich in polyphenols, and among them, EGCG is the most-studied active ingredient with the highest antioxidant activity [149].
EGCG, and tea polyphenols in general, are capable of mediating the epigenetic induction of metalloproteinase inhibitors (TIMP), such as TIMP-3, whose levels have a key role in suppressing the gelatinolytic activity of MMP-2 and MMP-9, involved in the metastatic process. Therefore, EGCG is considered a modulator of metalloproteinase activity, with benefits at oncological levels [150].
EGCG also has the ability to inhibit acute promyelocytic leukemia (APL) by inhibiting cell proliferation and promoting apoptosis [151]. In cell culture and animal models of prostate, breast, skin, liver, bladder, lung, and digestive tract cancer, EGCG induces the inhibition of cell proliferation and apoptosis by affecting the MAPK/ERK pathways and growth factors IGF1, IGF, and IGFBP-3. By inhibiting PI3K/AKT/p-BAD, a cell survival pathway, EGCG controls apoptosis. Moreover, EGCG is able to inhibit angiogenesis, invasion, and VEGF [152]. EGCG is an important regulator of cancer-associated microRNAs and upregulates miR-16, miR-210, and miR-330 and decreases miR-21 and miR-98-5p expression in liver, prostate, and lung cancer [153].
As concerns the intestinal absorption of EGCG, this process seems to show low efficiency, as EGCG lacks specific receptors for its absorption and is carried by passive diffusion (e.g., paracellular diffusion, transcellular diffusion) across epithelial cells. Following absorption, EGCG undergoes a phenomenon of active outflow, mediated by components of the efflux transport system (e.g., P-glycoprotein, multidrug resistance-associated proteins, breast cancer resistance proteins) that actively efflux intracellular EGCG to the extracellular intestinal space [154][155]. At the level of the small intestine and liver, EGCG is metabolized by phase II enzymes, releasing glucuronidated, sulfated, and methylated conjugates. EGCG metabolites are excreted through both bile and urine. EGCG can be further reabsorbed from the intestine through the enterohepatic recirculation process [156][157][158].
To capitalize on the therapeutic potential of EGCG in humans, despite its reduced bioavailability, high intakes have been suggested (e.g., the consumption of 8 to 16 cups/day of green tea) [159]. Nevertheless, using high doses of catechins may be of concern in the context of their dose-dependent toxic effects. A recent report by the European Food Safety Agency indicated a risk of liver damage following the intake of EGCG in the form of dietary supplements, at doses of 800 mg/day or above [160][161]. In order to manage these issues and improve the bioavailability of EGCG, several approaches have been identified. One approach involves the co-administration of EGCG with other bioactives. For instance, a formulation with ascorbic acid and sucrose has been demonstrated to enhance EGCG bioavailability by increasing its bioaccessibility and intestinal uptake from green tea [162]. Likewise, it has been suggested that the ingestion of EGCG on an empty stomach may improve its systemic absorption [163]. Moreover, the structural modification of EGCG by methylation, acyclization, or glycoside modification seems to allow the management of its premature degradation and reduced absorption rate [156][164]. Finally, one promising approach to protect EGCG against unfavorable gastrointestinal conditions and improve its bioavailability includes the design of nanocarriers. Examples of carriers developed for the nanodelivery of green tea catechins comprise surfactant-based nanovesicles (liposomes, phytosomes, niosomes, bilosomes), polysaccharide nanostructures, protein nanoparticles, nanoemulsions, and nanostructured lipid carriers [154][165].
7. Lycopene
Lycopene is a non-provitamin A carotenoid, present particularly in tomatoes (Solanum lycopersicum L.), but also in apricots (Prunus armeniaca S. X. Sun L.), guava (Psidium guajava L.), papaya (Carica papaya L.), watermelon (Citrullus lanatus subsp. vulgaris (Schrad.) Fursa), and pink grapefruit (Citrus × paradisi Macfad.). The level of ripeness in these fruits influences their lycopene content. For example, the content of lycopene is 50 mg/kg in ripe tomatoes, but only 5 mg/kg in unripe yellow tomatoes [166]. In addition to having a powerful antioxidant action, lycopene can improve the fluidity of the circulating blood mass and reduce the inflammatory response [167].
Carotenoids, as well as their metabolites and oxidation products, improve communication at the level of the intercellular junction gate GJC (Gap Junction Communication), which is considered one of the mechanisms of cancer prevention. GJC is deficient in many forms of cancer and the restoration of this function leads to cell proliferation reduction [168]. Several studies have demonstrated that lycopene is capable of modulating the expression of genes involved in inflammation, apoptosis, and cancer progression and, in this manner, reducing prostate cancer risk [169][170].
Although the majority of the lycopene in unprocessed food is found in the all-trans isoform, human serum and tissues have been reported to contain mainly the cis isomers of this carotenoid. A potential mechanism to explain this phenomenon could be an intestinal absorption that is preferential for the forms of cis-lycopene [171]. Indeed, evidence from early in vitro and animal studies has suggested that the cis isomers of lycopene have greater bioavailability than the all-trans form, possibly due to cis isomers showing a shorter length, higher solubility in mixed micelles, and/or a lower tendency to aggregate into crystalline structures [172]. However, more recent studies in human subjects have reported that there are no significant differences between cis-lycopene and all-trans lycopene absorption, indicating heat-induced isomerization or enzymatic isomerization within body tissues as processes that could explain the enhanced cis isomeric profile in human serum and tissues [173].
As concerns the intestinal absorption of lycopene, this process occurs either by passive diffusion or via the scavenger receptor class B type 1 protein (SR-B1) transporter. Nevertheless, the intestinal absorption of lycopene seems to have limited efficiency [171]. In the study conducted by Moran et al. (2015), human subjects absorbed only about 24% of the ingested lycopene. Lycopene is mainly stored in the liver, but it can also accumulate within extrahepatic tissues (e.g., adipose tissue, adrenals, skin, kidneys, lungs, prostate, testes, ovaries, and breastmilk) [171][173]. During its initial metabolism, lycopene produces lycopenoids (e.g., APO-10′-lycopenoic acid). Some studies suggest that the health effects of lycopene are actually related to the biological activities of lycopenoids, but more research is required to clarify this aspect [174]. Lycopene is mainly excreted through the feces and in lower amounts through the urine [171].
In recent years, there has been a growing interest in identifying factors that could improve the bioavailability of lycopene from dietary sources such as tomatoes. The mechanical treatment of tomatoes (e.g., by mastication, grinding) appears to be important for lycopene bioaccessibility and hence for its plasmatic bioavailability [175]. Likewise, the heat processing of tomatoes may increase the bioavailability of lycopene in these foods by favoring its trans-to-cis isomerization. It has also been suggested by several studies that the addition of dietary fats (e.g., olive oil, avocado) to tomato dishes may increase the absorption and consequently the plasma levels of lycopene [176][177]. A recommendation has been issued to add a minimum of 10 g of fat in culinary preparations containing processed tomato products and 15 g of fat in fresh tomato recipes, respectively [171]. The encapsulation in nanoparticles of the lycopene extracted from food sources or biosynthesized also seems to help in solving its bioavailability issues and capitalizing on its nutraceutical potential [178].
Furthermore, there are several factors related to the characteristics of human subjects ingesting lycopene that have been reported to influence the bioavailability of this bioactive compound. Gender, adiposity, body mass index, and smoking habits appear to contribute to about 25% of the variation in serum lycopene concentrations [179]. In addition, there are studies to suggest that certain genetic variants linked to carotenoid metabolism may have an impact on lycopene bioavailability [179][180][181]. For example, the single nucleotide polymorphism rs6564851 in the β-carotene 15,15′-oxygenase-1 (BCO1) gene, which encodes for the BCO1 enzyme responsible for carotenoid cleavage in mammals, has been reported as being significantly associated with changes in lycopene circulating levels [179].
References
- Sharifi-Rad, J.; Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 1021.
- Hwang, K.W.; Son, D.; Jo, H.W.; Kim, C.H.; Seong, K.C.; Moon, J.K. Levels of curcuminoid and essential oil compositions in turmerics (Curcuma longa L.) grown in Korea. Appl. Biol. Chem. 2016, 59, 209–215.
- Dosoky, N.; Setzer, W. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196.
- Reuter, S.; Gupta, S.C.; Park, B.; Goel, A.; Aggarwal, B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011, 6, 93.
- Teiten, M.H.; Dicato, M.; Diederich, M. Curcumin as a regulator of epigenetic events. Mol. Nutr. Food Res. 2013, 57, 1619–1629.
- Yang, C.H.; Yue, J.; Sims, M.; Pfeffer, L.M. The Curcumin Analog EF24 Targets NF-κB and miRNA-21, and Has Potent Anticancer Activity In Vitro and In Vivo. PLoS ONE 2013, 8, e71130.
- Zhang, J.; Zhang, T.; Ti, X.; Shi, J.; Wu, C.; Ren, X.; Yin, H. Curcumin promotes apoptosis in A549/DDP multidrug-resistant human lung adenocarcinoma cells through a miRNA signaling pathway. Biochem. Biophys. Res. Commun. 2010, 399, 1–6.
- Hassan, F.U.; Rehman, M.S.U.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an alternative epigenetic modulator: Mechanism of action and potential effects. Front. Genet. 2019, 10, 514.
- Yu, J.; Peng, Y.; Wu, L.C.; Xie, Z.; Deng, Y.; Hughes, T.; He, S.; Mo, X.K.; Chiu, M.; Wang, Q.E.; et al. Curcumin Down-Regulates DNA Methyltransferase 1 and Plays an Anti-Leukemic Role in Acute Myeloid Leukemia. PLoS ONE 2013, 8, e55934.
- Boyanapalli, S.S.S.; Kong, A.N.T. “Curcumin, the King of Spices”: Epigenetic Regulatory Mechanisms in the Prevention of Cancer, Neurological, and Inflammatory Diseases. Curr. Pharmacol. Rep. 2015, 1, 129–139.
- Nagoor, N.H.; Aggarwal, B. Cancer-linked targets modulated by curcumin. Int. J. Biochem. Mol. Biol. 2012, 3, 328–351.
- Kasi, P.D.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.F.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H.; Nabavi, S.M. Molecular targets of curcumin for cancer therapy: An updated review. Tumour Biol. 2016, 37, 13017–13028.
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376.
- Farghadani, R.; Naidu, R. Curcumin: Modulator of Key Molecular Signaling Pathways in Hormone-Independent Breast Cancer. Cancers 2021, 13, 3427.
- Zhou, H.; Beevers, C.S.; Huang, S. Targets of curcumin. Curr. Drug Targets 2011, 12, 332.
- Qadir, M.; Naqvi, S.; Muhammad, S. Curcumin: A Polyphenol with Molecular Targets for Cancer Control. Asian Pac. J. Cancer Prev. 2016, 17, 2735–2739.
- Vadukoot, A.K.; Mottemmal, S.; Vekaria, P.H. Curcumin as a Potential Therapeutic Agent in Certain Cancer Types. Cureus 2022, 14, e22825.
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214.
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791.
- Dai, C.; Zhang, X.; Zhang, K. New Discovery of Curcumin Combination Therapy and Action Mechanism. Evid. Based Complement. Altern. Med. 2020, 2020, 4793058.
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.H.; Kumar, A.P.; Sethi, G. The Multifaceted Role of Curcumin in Cancer Prevention and Treatment. Molecules 2015, 20, 2728–2769.
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21.
- Ruan, D.; Zhu, Y.W.; Fouad, A.M.; Yan, S.J.; Chen, W.; Zhang, Y.N.; Xia, W.G.; Wang, S.; Jiang, S.Q.; Yang, L.; et al. Dietary curcumin enhances intestinal antioxidant capacity in ducklings via altering gene expression of antioxidant and key detoxification enzymes. Poult. Sci. 2019, 98, 3705–3714.
- Shaikh, S.; Shaikh, J.; Naba, Y.S.; Doke, K.; Ahmed, K.; Yusufi, M. Curcumin: Reclaiming the lost ground against cancer resistance. Cancer Drug Resist. 2021, 4, 298–320.
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; McGillivray, D.J. Recent advances to improve curcumin oral bioavailability. Trends Food Sci. Technol. 2021, 110, 253–266.
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397.
- Rahimi, H.R.; Nedaeinia, R.; Shamloo, A.S.; Nikdoust, S.; Oskuee, R.K. Novel delivery system for natural products: Nano-curcumin formulations. Avicenna J. Phytomed. 2016, 6, 383.
- Porat, D.; Dahan, A. Active intestinal drug absorption and the solubility-permeability interplay. Int. J. Pharm. 2018, 537, 84–93.
- Kharat, M.; McClements, D.J. Recent advances in colloidal delivery systems for nutraceuticals: A case study—Delivery by Design of curcumin. J. Colloid Interface Sci. 2019, 557, 506–518.
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15.
- Cuomo, F.; Cofelice, M.; Venditti, F.; Ceglie, A.; Miguel, M.; Lindman, B.; Lopez, F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids Surf. B. Biointerfaces 2018, 168, 29–34.
- Lamuela-Raventós, R.M.; Romero-Pérez, A.I.; Waterhouse, A.L.; de la Torre-Boronat, M.C. Direct HPLC Analysis of cis- and trans-Resveratrol and Piceid Isomers in Spanish Red Vitis vinifera Wines. J. Agric. Food Chem. 1995, 43, 281–283.
- Akinwumi, B.C.; Bordun, K.A.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792.
- Anisimova, N.Y.U.; Kiselevsky, M.V.; Sosnov, A.V.; Sadovnikov, S.V.; Stankov, I.N.; Gakh, A.A. Trans-, cis-, and dihydro-resveratrol: A comparative study. Chem. Cent. J. 2011, 5, 88.
- Ducimetiere, P.; Cambien, F.; Richard, J.L.; Rakotovao, R.; Claude, J.R. Coronary heart disease in middle-aged Frenchmen. Comparisons between Paris Prospective Study, Seven Countries Study, and Pooling Project. Lancet 1980, 1, 1346–1350.
- Ferrières, J. The French paradox: Lessons for other countries. Heart 2004, 90, 107.
- Miura, T.; Muraoka, S.; Ikeda, N.; Watanabe, M.; Fujimoto, Y. Antioxidative and prooxidative action of stilbene derivatives. Pharmacol. Toxicol. 2000, 86, 203–208.
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (NOX4). J. Physiol. Pharmacol. 2009, 60, 111–116.
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84.
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049.
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234.
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8.
- Issinger, O.G.; Guerra, B. Phytochemicals in cancer and their effect on the PI3K/AKT-mediated cellular signalling. Biomed. Pharmacother. 2021, 139, 111650.
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466.
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005, 280, 17187–17195.
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Caloric restriction, resveratrol and melatonin: Role of SIRT1 and implications for aging and related-diseases. Mech. Ageing Dev. 2015, 146–148, 28–41.
- Mierziak, J.; Kostyn, K.; Boba, A.; Czemplik, M.; Kulma, A.; Wojtasik, W. Influence of the Bioactive Diet Components on the Gene Expression Regulation. Nutrients 2021, 13, 3673.
- Komorowska, J.; Wątroba, M.; Szukiewicz, D. Review of beneficial effects of resveratrol in neurodegenerative diseases such as Alzheimer’s disease. Adv. Med. Sci. 2020, 65, 415–423.
- Ahmed, T.; Javed, S.; Javed, S.; Tariq, A.; Šamec, D.; Tejada, S.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Resveratrol and Alzheimer’s Disease: Mechanistic Insights. Mol. Neurobiol. 2017, 54, 2622–2635.
- Park, D.; Jeong, H.; Lee, M.N.; Koh, A.; Kwon, O.; Yang, Y.R.; Noh, J.; Suh, P.G.; Park, H.; Ryu, S.H. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 2016, 6, 21772.
- Taniguchi, T.; Iizumi, Y.; Watanabe, M.; Masuda, M.; Morita, M.; Aono, Y.; Toriyama, S.; Oishi, M.; Goi, W.; Sakai, T. Resveratrol directly targets DDX5 resulting in suppression of the mTORC1 pathway in prostate cancer. Cell Death Dis. 2016, 7, e2211.
- Neves, A.R.; Martins, S.; Segundo, M.A.; Reis, S. Nanoscale Delivery of Resveratrol towards Enhancement of Supplements and Nutraceuticals. Nutrients 2016, 8, 131.
- Vesely, O.; Baldovska, S.; Kolesarova, A. Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids. Nutrients 2021, 13, 3095.
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53, S7–S15.
- Quarta, A.; Gaballo, A.; Pradhan, B.; Patra, S.; Jena, M.; Ragusa, A. Beneficial Oxidative Stress-Related trans-Resveratrol Effects in the Treatment and Prevention of Breast Cancer. Appl. Sci. 2021, 11, 11041.
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170.
- De Vries, K.; Strydom, M.; Steenkamp, V. Bioavailability of resveratrol: Possibilities for enhancement. J. Herb. Med. 2018, 11, 71–77.
- Brotons-Canto, A.; Gonzalez-Navarro, C.J.; Gurrea, J.; González-Ferrero, C.; Irache, J.M. Zein nanoparticles improve the oral bioavailability of resveratrol in humans. J. Drug Deliv. Sci. Technol. 2020, 57, 101704.
- de Vries, K.; Strydom, M.; Steenkamp, V. A Brief Updated Review of Advances to Enhance Resveratrol’s Bioavailability. Molecules 2021, 26, 4367.
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-González, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-based formulations for resveratrol delivery: Effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surf. B. Biointerfaces 2019, 180, 127–140.
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates from Cruciferous Vegetables and Their Potential Role in Chronic Disease: Investigating the Preclinical and Clinical Evidence. Front. Pharmacol. 2021, 12, 2964.
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24.
- Iahtisham-Ul-Haq; Khan, S.; Awan, K.A.; Iqbal, M.J. Sulforaphane as a potential remedy against cancer: Comprehensive mechanistic review. J. Food Biochem. 2022, 46, e13886.
- Fahey, J.W.; Holtzclaw, W.D.; Wehage, S.L.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Sulforaphane Bioavailability from Glucoraphanin-Rich Broccoli: Control by Active Endogenous Myrosinase. PLoS ONE 2015, 10, e0140963.
- Shekarri, Q.; Dekker, M. A physiological-based model for simulating the bioavailability and kinetics of sulforaphane from broccoli products. Foods 2021, 10, 2761.
- Aronica, L.; Ordovas, J.M.; Volkov, A.; Lamb, J.J.; Stone, P.M.; Minich, D.; Leary, M.; Class, M.; Metti, D.; Larson, I.A.; et al. Genetic Biomarkers of Metabolic Detoxification for Personalized Lifestyle Medicine. Nutrients 2022, 14, 768.
- Hauder, J.; Winkler, S.; Bub, A.; Rüfer, C.E.; Pignitter, M.; Somoza, V. LC-MS/MS quantification of sulforaphane and indole-3-carbinol metabolites in human plasma and urine after dietary intake of selenium-fortified broccoli. J. Agric. Food Chem. 2011, 59, 8047–8057.
- Sun, J.; Charron, C.S.; Novotny, J.A.; Peng, B.; Yu, L.; Chen, P. Profiling glucosinolate metabolites in human urine and plasma after broccoli consumption using non-targeted and targeted metabolomic analyses. Food Chem. 2020, 309, 125660.
- Fu, L.-J.; Ding, Y.-B.; Wu, L.-X.; Wen, C.-J.; Qu, Q.; Zhang, X.; Zhou, H.-H. The Effects of Lycopene on the Methylation of the GSTP1 Promoter and Global Methylation in Prostatic Cancer Cell Lines PC3 and LNCaP. Int. J. Endocrinol. 2014, 2014, 1–9.
- Bradlow, H.; Zeligs, M. Diindolylmethane (DIM) spontaneously forms from indole-3-carbinol (I3C) during cell culture experiments. In Vivo 2010, 24, 387–391.
- Williams, D.E. Indoles Derived from Glucobrassicin: Cancer Chemoprevention by Indole-3-Carbinol and 3,3′-Diindolylmethane. Front. Nutr. 2021, 8, 4334.
- Anderton, M.J.; Manson, M.M.; Verschoyle, R.; Gescher, A.; Steward, W.P.; Williams, M.L.; Mager, D.E. Physiological modeling of formulated and crystalline 3,3’-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab. Dispos. 2004, 32, 632–638.
- Anderton, M.J.; Manson, M.M.; Verschoyle, R.D.; Gescher, A.; Lamb, J.H.; Farmer, P.B.; Steward, W.P.; Williams, M.L. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin. Cancer Res. 2004, 10, 5233–5241.
- Acharya, A.; Das, I.; Singh, S.; Saha, T. Chemopreventive properties of indole-3-carbinol, diindolylmethane and other constituents of cardamom against carcinogenesis. Recent Pat. Food. Nutr. Agric. 2010, 2, 166–177.
- Nian, H.; Delage, B.; Ho, E.; Dashwood, R.H. Modulation of Histone Deacetylase Activity by Dietary Isothiocyanates and Allyl Sulfides: Studies with Sulforaphane and Garlic Organosulfur Compounds. Environ. Mol. Mutagen. 2009, 50, 213.
- Beaver, L.M.; Yu, T.W.; Sokolowski, E.I.; Williams, D.E.; Dashwood, R.H.; Ho, E. 3,3′-Diindolylmethane, but not indole-3-carbinol, inhibits histone deacetylase activity in prostate cancer cells. Toxicol. Appl. Pharmacol. 2012, 263, 345–351.
- Lubecka-Pietruszewska, K.; Kaufman-Szymczyk, A.; Stefanska, B.; Cebula-Obrzut, B.; Smolewski, P.; Fabianowska-Majewska, K. Sulforaphane Alone and in Combination with Clofarabine Epigenetically Regulates the Expression of DNA Methylation-Silenced Tumour Suppressor Genes in Human Breast Cancer Cells. J. Nutrigenet. Nutr. 2015, 8, 91–101.
- Hsu, A.; Wong, C.P.; Yu, Z.; Williams, D.E.; Dashwood, R.H.; Ho, E. Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clin. Epigenetics 2011, 3, 3.
- Phuah, N.H.; Nagoor, N.H. Regulation of microRNAs by natural agents: New strategies in cancer therapies. Biomed. Res. Int. 2014, 2014, 804510.
- El-Daly, S.M.; Gamal-Eldeen, A.M.; Gouhar, S.A.; Abo-elfadl, M.T.; El-Saeed, G. Modulatory Effect of Indoles on the Expression of miRNAs Regulating G1/S Cell Cycle Phase in Breast Cancer Cells. Appl. Biochem. Biotechnol. 2020, 192, 1208–1223.
- Jump, S.M.; Kung, J.; Staub, R.; Kinseth, M.A.; Cram, E.J.; Yudina, L.N.; Preobrazhenskaya, M.N.; Bjeldanes, L.F.; Firestone, G.L. N-Alkoxy derivatization of indole-3-carbinol increases the efficacy of the G1 cell cycle arrest and of I3C-specific regulation of cell cycle gene transcription and activity in human breast cancer cells. Biochem. Pharmacol. 2008, 75, 713–724.
- Szaefer, H.; Licznerska, B.; Krajka-Kuniak, V.; Bartoszek, A.; Baer-Dubowska, W. Modulation of CYP1A1, CYP1A2 and CYP1B1 expression by cabbage juices and indoles in human breast cell lines. Nutr. Cancer 2012, 64, 879–888.
- Reed, G.A.; Arneson, D.W.; Putnam, W.C.; Smith, H.J.; Gray, J.C.; Sullivan, D.K.; Mayo, M.S.; Crowell, J.A.; Hurwitz, A. Single-dose and multiple-dose administration of indole-3-carbinol to women: Pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2477–2481.
- Arslan, A.A.; Koenig, K.L.; Lenner, P.; Afanasyeva, Y.; Shore, R.E.; Chen, Y.; Lundin, E.; Toniolo, P.; Hallmans, G.; Zeleniuch-Jacquotte, A. Circulating Estrogen Metabolites and Risk of Breast Cancer in Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1290.
- Zeleniuch-Jacquotte, A.; Shore, R.E.; Afanasyeva, Y.; Lukanova, A.; Sieri, S.; Koenig, K.L.; Idahl, A.; Krogh, V.; Liu, M.; Ohlson, N.; et al. Postmenopausal circulating levels of 2- and 16α-hydroxyestrone and risk of endometrial cancer. Br. J. Cancer 2011, 105, 1458–1464.
- McCann, S.E.; Wactawski-Wende, J.; Kufel, K.; Olson, J.; Ovando, B.; Kadlubar, S.N.; Davis, W.; Carter, L.; Muti, P.; Shields, P.G.; et al. Changes in 2-hydroxyestrone and 16alpha-hydroxyestrone metabolism with flaxseed consumption: Modification by COMT and CYP1B1 genotype. Cancer Epidemiol. Biomark. Prev. 2007, 16, 256–262.
- Bradlow, H.L.; Davis, D.L.; Lin, G.; Sepkovic, D.; Tiwari, R. Effects of pesticides on the ratio of 16 alpha/2-hydroxyestrone: A biologic marker of breast cancer risk. Environ. Health Perspect. 1995, 103, 147.
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689.
- Chen, C.Y.; Yu, Z.Y.; Chuang, Y.S.; Huang, R.M.; Wang, T.C.V. Sulforaphane attenuates EGFR signaling in NSCLC cells. J. Biomed. Sci. 2015, 22, 1–9.
- Ganai, S.A. Histone deacetylase inhibitor sulforaphane: The phytochemical with vibrant activity against prostate cancer. Biomed. Pharmacother. 2016, 81, 250–257.
- Abbas, A.; Hall, J.A.; Patterson, W.L.; Ho, E.; Hsu, A.; Al-Mulla, F.; Georgel, P.T. Sulforaphane modulates telomerase activity via epigenetic regulation in prostate cancer cell lines. Biochem. Cell Biol. 2016, 94, 71–81.
- Meeran, S.M.; Patel, S.N.; Tollefsbol, T.O. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS ONE 2010, 5, e11457.
- Kim, E.J.; Park, H.; Kim, J.; Park, J.H.Y. 3,3’-diindolylmethane suppresses 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and tumor promotion in mouse skin via the downregulation of inflammatory mediators. Mol. Carcinog. 2010, 49, 672–683.
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.N.T. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: Anti-Oxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharmacol. Rep. 2015, 1, 179.
- Royston, K.J.; Tollefsbol, T.O. The Epigenetic Impact of Cruciferous Vegetables on Cancer Prevention. Curr. Pharmacol. Rep. 2015, 1, 46–51.
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxid. Med. Cell. Longev. 2019, 2019, 2716870.
- Thomson, C.A.; Ho, E.; Strom, M.B. Chemopreventive properties of 3,30-diindolylmethane in breast cancer: Evidence from experimental and human studies. Nutr. Rev. 2016, 74, 432–443.
- Kotsopoulos, J.; Zhang, S.; Akbari, M.; Salmena, L.; Llacuachaqui, M.; Zeligs, M.; Sun, P.; Narod, S.A. BRCA1 mRNA levels following a 4–6-week intervention with oral 3,3′-diindolylmethane. Br. J. Cancer 2014, 111, 1269.
- Fujioka, N.; Fritz, V.; Upadhyaya, P.; Kassie, F.; Hecht, S.S. Research on cruciferous vegetables, indole-3-carbinol, and cancer prevention: A tribute to Lee, W. Wattenberg. Mol. Nutr. Food Res. 2016, 60, 1228–1238.
- Wang, Q.; Bao, Y. Nanodelivery of natural isothiocyanates as a cancer therapeutic. Free Radic. Biol. Med. 2021, 167, 125–140.
- Luo, Y.; Wang, T.T.Y.; Teng, Z.; Chen, P.; Sun, J.; Wang, Q. Encapsulation of indole-3-carbinol and 3,3′-diindolylmethane in zein/carboxymethyl chitosan nanoparticles with controlled release property and improved stability. Food Chem. 2013, 139, 224–230.
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196.
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152.
- Yan, T.; Li, H.Y.; Wu, J.S.; Niu, Q.; Duan, W.H.; Han, Q.Z.; Ji, W.M.; Zhang, T.; Lv, W. Astaxanthin inhibits gemcitabine-resistant human pancreatic cancer progression through EMT inhibition and gemcitabine resensitization. Oncol. Lett. 2017, 14, 5400–5408.
- Yang, Y.; Fuentes, F.; Shu, L.; Wang, C.; Pung, D.; Li, W.; Zhang, C.; Guo, Y.; Kong, A.N. Epigenetic CpG Methylation of the Promoter and Reactivation of the Expression of GSTP1 by Astaxanthin in Human Prostate LNCaP Cells. AAPS J. 2017, 19, 421–430.
- Yang, Y.; Yang, I.; Cao, M.; Su, Z.; Wu, R.; Guo, Y.; Fang, M.; Kong, A.N. Fucoxanthin Elicits Epigenetic Modifications, Nrf2 Activation and Blocking Transformation in Mouse Skin JB6 P+ Cells. AAPS J. 2018, 20, 32.
- Ni, X.; Yu, H.; Wang, S.; Zhang, C.; Shen, S. Astaxanthin Inhibits PC-3 Xenograft Prostate Tumor Growth in Nude Mice. Mar. Drugs 2017, 15, 66.
- Karimian, A.; Hadi Bahadori, M.; Moghaddam, A.H.; Mir Mohammadrezaei, F.; Mohammadrezaei, F.M. Effect of Astaxanthin on cell viability in T-47D and MDA-MB-231 Breast Cancer Cell Lines. Multidiscip. Cancer Investig. 2017, 124, 151832.
- McCall, B.; McPartland, C.K.; Moore, R.; Frank-Kamenetskii, A.; Booth, B.W. Effects of Astaxanthin on the Proliferation and Migration of Breast Cancer Cells In Vitro. Antioxidants 2018, 7, 135.
- Zhang, Z.; Sun, D.; Cheng, K.W.; Chen, F. Inhibition of autophagy modulates astaxanthin and total fatty acid biosynthesis in Chlorella zofingiensis under nitrogen starvation. Bioresour. Technol. 2018, 247, 610–615.
- Kim, H.Y.; Kim, Y.M.; Hong, S. Astaxanthin suppresses the metastasis of colon cancer by inhibiting the MYC-mediated downregulation of microRNA-29a-3p and microRNA-200a. Sci. Rep. 2019, 9, 9457.
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA. Cancer J. Clin. 2011, 61, 69–90.
- Liao, K.S.; Wei, C.L.; Chen, J.C.; Zheng, H.Y.; Chen, W.C.; Wu, C.H.; Wang, T.J.; Peng, Y.S.; Chang, P.Y.; Lin, Y.W. Astaxanthin enhances pemetrexed-induced cytotoxicity by downregulation of thymidylate synthase expression in human lung cancer cells. Regul. Toxicol. Pharmacol. 2016, 81, 353–361.
- Chen, J.C.; Wu, C.H.; Peng, Y.S.; Zheng, H.Y.; Lin, Y.C.; Ma, P.F.; Yen, T.C.; Chen, T.Y.; Lin, Y.W. Astaxanthin enhances erlotinib-induced cytotoxicity by p38 MAPK mediated xeroderma pigmentosum complementation group C (XPC) down-regulation in human lung cancer cells. Toxicol. Res. 2018, 7, 1247.
- Tomasini, P.; Barlesi, F.; Mascaux, C.; Greillier, L. Pemetrexed for advanced stage nonsquamous non-small cell lung cancer: Latest evidence about its extended use and outcomes. Ther. Adv. Med. Oncol. 2016, 8, 198–208.
- Yang, L.; Qiao, X.; Gu, J.; Li, X.; Cao, Y.; Xu, J.; Xue, C. Influence of molecular structure of astaxanthin esters on their stability and bioavailability. Food Chem. 2021, 343, 128497.
- Kim, S.; Lee, H.; Lim, J.W.; Kim, H. Astaxanthin induces NADPH oxidase activation and receptor-interacting protein kinase 1-mediated necroptosis in gastric cancer AGS cells. Mol. Med. Rep. 2021, 24, 1–12.
- Honda, M.; Kageyama, H.; Hibino, T.; Osawa, Y.; Hirasawa, K.; Kuroda, I. Evaluation and improvement of storage stability of astaxanthin 3 isomers in oils and fats. Food Chem. 2021, 352, 129371.
- Martínez-Álvarez, Ó.; Calvo, M.M.; Gómez-Estaca, J. Recent Advances in Astaxanthin Micro/Nanoencapsulation to Improve Its Stability and Functionality as a Food Ingredient. Mar. Drugs 2020, 18, 406.
- Madhavi, D.; Kagan, D.; Seshadri, S. A Study on the Bioavailability of a Proprietary, Sustained-release Formulation of Astaxanthin. Integr. Med. A Clin. J. 2018, 17, 38.
- Odeberg, J.M.; Lignell, Å.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304.
- Edelman, R.; Engelberg, S.; Fahoum, L.; Meyron-Holtz, E.G.; Livney, Y.D. Potato protein- based carriers for enhancing bioavailability of astaxanthin. Food Hydrocoll. 2019, 96, 72–80.
- Yang, J.; Hua, S.; Huang, Z.; Gu, Z.; Cheng, L.; Hong, Y. Comparison of bioaccessibility of astaxanthin encapsulated in starch-based double emulsion with different structures. Carbohydr. Polym. 2021, 272, 118475.
- Gomez-Estaca, J.; Comunian, T.A.; Montero, P.; Ferro-Furtado, R.; Favaro-Trindade, C.S. Encapsulation of an astaxanthin-containing lipid extract from shrimp waste by complex coacervation using a novel gelatin–cashew gum complex. Food Hydrocoll. 2016, 61, 155–162.
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021, 256, 117426.
- Banc, R.; Loghin, F.; Miere, D.; Ranga, F.; Socaciu, C. Phenolic composition and antioxidant activity of red, rosé and white wines originating from Romanian grape cultivars. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 716–734.
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S.
- Toma, C.-C.; Simu, G.M.; Hanganu, D.; Olah, N.; Vata, F.M.G.; Hammami, C.; Hammami, M. Chemical composition of the Tunisian Nigella sativa. Note I. Profile on essential oil. Farmacia 2010, 58, 458–464.
- Zhang, Q.; Zhao, X.H.; Wang, Z.J. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol. In Vitro 2009, 23, 797–807.
- Tan, J.; Wang, B.; Zhu, L. Regulation of survivin and Bcl-2 in HepG2 cell apoptosis induced by quercetin. Chem. Biodivers. 2009, 6, 1101–1110.
- Gupta, K.; Panda, D. Perturbation of microtubule polymerization by quercetin through tubulin binding: A novel mechanism of its antiproliferative activity. Biochemistry 2002, 41, 13029–13038.
- Moon, S.K.; Cho, G.O.; Jung, S.Y.; Gal, S.W.; Kwon, T.K.; Lee, Y.C.; Madamanchi, N.R.; Kim, C.H. Quercetin exerts multiple inhibitory effects on vascular smooth muscle cells: Role of ERK1/2, cell-cycle regulation, and matrix metalloproteinase-9. Biochem. Biophys. Res. Commun. 2003, 301, 1069–1078.
- Tanigawa, S.; Fujii, M.; Hou, D.X. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biosci. Biotechnol. Biochem. 2008, 72, 797–804.
- Xi, L.; Zhang, Y.; Kong, S.; Liang, W. miR-34 inhibits growth and promotes apoptosis of osteosarcoma in nude mice through targetly regulating TGIF2 expression. Biosci. Rep. 2018, 38, 20180078.
- Yang, Y.; Wang, T.; Chen, D.; Ma, Q.; Zheng, Y.; Liao, S.; Wang, Y.; Zhang, J. Quercetin preferentially induces apoptosis in KRAS-mutant colorectal cancer cells via JNK signaling pathways. Cell Biol. Int. 2019, 43, 117–124.
- Ghafouri-Fard, S.; Shabestari, F.A.; Vaezi, S.; Abak, A.; Shoorei, H.; Karimi, A.; Taheri, M.; Basiri, A. Emerging impact of quercetin in the treatment of prostate cancer. Biomed. Pharmacother. 2021, 138, 111548.
- Vijayababu, M.R.; Arunkumar, A.; Kanagaraj, P.; Arunakaran, J. Effects of quercetin on insulin-like growth factors (IGFs) and their binding protein-3 (IGFBP-3) secretion and induction of apoptosis in human prostate cancer cells. J. Carcinog. 2006, 5, 10.
- Dong, Y.; Yang, J.; Yang, L.; Li, P. Quercetin Inhibits the Proliferation and Metastasis of Human Non-Small Cell Lung Cancer Cell Line: The Key Role of Src-Mediated Fibroblast Growth Factor-Inducible 14 (Fn14)/ Nuclear Factor kappa B (NF-κB) pathway. Med. Sci. Monit. 2020, 26, e920537-1–e920537-11.
- Luo, C.L.; Liu, Y.Q.; Wang, P.; Song, C.H.; Wang, K.J.; Dai, L.P.; Zhang, J.Y.; Ye, H. The effect of quercetin nanoparticle on cervical cancer progression by inducing apoptosis, autophagy and anti-proliferation via JAK2 suppression. Biomed. Pharmacother. 2016, 82, 595–605.
- Alvarez, M.C.; Maso, V.; Torello, C.O.; Ferro, K.P.; Saad, S.T.O. The polyphenol quercetin induces cell death in leukemia by targeting epigenetic regulators of pro-apoptotic genes. Clin. Epigenetics 2018, 10, 139.
- Kim, D.H.; Khan, H.; Ullah, H.; Hassan, S.T.S.; Šmejkal, K.; Efferth, T.; Mahomoodally, M.F.; Xu, S.; Habtemariam, S.; Filosa, R.; et al. MicroRNA targeting by quercetin in cancer treatment and chemoprotection. Pharmacol. Res. 2019, 147, 104346.
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210.
- Guo, Y.; Mah, E.; Bruno, R.S. Quercetin bioavailability is associated with inadequate plasma vitamin C status and greater plasma endotoxin in adults. Nutrition 2014, 30, 1279–1286.
- Liu, K.; Zha, X.Q.; Shen, W.; Li, Q.M.; Pan, L.H.; Luo, J.P. The hydrogel of whey protein isolate coated by lotus root amylopectin enhance the stability and bioavailability of quercetin. Carbohydr. Polym. 2020, 236, 116009.
- Lin, J.; Teo, L.M.; Leong, L.P.; Zhou, W. In vitro bioaccessibility and bioavailability of quercetin from the quercetin-fortified bread products with reduced glycemic potential. Food Chem. 2019, 286, 629–635.
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200.
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23.
- Manzoor, M.F.; Hussain, A.; Sameen, A.; Sahar, A.; Khan, S.; Siddique, R.; Aadil, R.M.; Xu, B. Novel extraction, rapid assessment and bioavailability improvement of quercetin: A review. Ultrason. Sonochem. 2021, 78, 1350–4177.
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821.
- Deb, G.; Thakur, V.S.; Limaye, A.M.; Gupta, S. Epigenetic induction of tissue inhibitor of matrix metalloproteinase-3 by green tea polyphenols in breast cancer cells. Mol. Carcinog. 2015, 54, 485–499.
- Borutinskaitė, V.; Virkšaitė, A.; Gudelytė, G.; Navakauskienė, R. Green tea polyphenol EGCG causes anti-cancerous epigenetic modulations in acute promyelocytic leukemia cells. Leuk. Lymphoma 2018, 59, 469–478.
- Henning, S.M.; Wang, P.; Carpenter, C.L.; Heber, D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics 2013, 5, 729.
- Cadieux, Z.; Lewis, H.; Esquela-Kerscher, A. Role of Nutrition, the Epigenome, and MicroRNAs in Cancer Pathogenesis. In MicroRNAs in Diseases and Disorders: Emerging Therapeutic Targets; Royal Society of Chemistry: London, UK, 2019; pp. 1–35. ISBN 9781782621454.
- Rashidinejad, A.; Boostani, S.; Babazadeh, A.; Rehman, A.; Rezaei, A.; Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Opportunities and challenges for the nanodelivery of green tea catechins in functional foods. Food Res. Int. 2021, 142, 110186.
- Hong, J.; Lambert, J.D.; Lee, S.H.; Sinko, P.J.; Yang, C.S. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (-)-epigallocatechin-3-gallate and its methyl metabolites. Biochem. Biophys. Res. Commun. 2003, 310, 222–227.
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Khan, M.I.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022, 46, e14189.
- Shpigelman, A.; Israeli, G.; Livney, Y.D. Thermally-induced protein–polyphenol co-assemblies: Beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG. Food Hydrocoll. 2010, 24, 735–743.
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients 2015, 7, 5443–5468.
- Chow, H.H.S.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin. Cancer Res. 2005, 11, 4627–4633.
- Murakami, A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch. Biochem. Biophys. 2014, 557, 3–10.
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of green tea catechins|EFSA. EFSA J. 2018, 16, e05239.
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95.
- Naumovski, N.; Blades, B.L.; Roach, P.D. Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants 2015, 4, 373–393.
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732.
- Wang, L.; Huang, X.; Jing, H.; Ma, C.; Wang, H. Bilosomes as effective delivery systems to improve the gastrointestinal stability and bioavailability of epigallocatechin gallate (EGCG). Food Res. Int. 2021, 149, 110631.
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110.
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and Its Antioxidant Role in the Prevention of Cardiovascular Diseases-A Critical Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879.
- Aust, O.; Ale-Agha, N.; Zhang, L.; Wollersen, H.; Sies, H.; Stahl, W. Lycopene oxidation product enhances gap junctional communication. Food Chem. Toxicol. 2003, 41, 1399–1407.
- Ilic, D.; Misso, M. Lycopene for the prevention and treatment of benign prostatic hyperplasia and prostate cancer: A systematic review. Maturitas 2012, 72, 269–276.
- Kolberg, M.; Pedersen, S.; Bastani, N.E.; Carlsen, H.; Blomhoff, R.; Paur, I. Tomato paste alters NF-κB and cancer-related mRNA expression in prostate cancer cells, xenografts, and xenograft microenvironment. Nutr. Cancer 2015, 67, 305–315.
- Arballo, J.; Amengual, J.; Erdman, J.W. Lycopene: A Critical Review of Digestion, Absorption, Metabolism, and Excretion. Antioxidants 2021, 10, 342.
- Boileau, T.W.M.; Boileau, A.C.; Erdman, J.W. Bioavailability of all-trans and cis-isomers of lycopene. Exp. Biol. Med. 2002, 227, 914–919.
- Moran, N.E.; Cichon, M.J.; Riedl, K.M.; Grainger, E.M.; Schwartz, S.J.; Novotny, J.A.; Erdman, J.W.; Clinton, S.K. Compartmental and noncompartmental modeling of 13C-lycopene absorption, isomerization, and distribution kinetics in healthy adults. Am. J. Clin. Nutr. 2015, 102, 1436–1449.
- Wang, X.D. Lycopene metabolism and its biological significance. Am. J. Clin. Nutr. 2012, 96, 1214S–1222S.
- Vitucci, D.; Amoresano, A.; Nunziato, M.; Muoio, S.; Alfieri, A.; Oriani, G.; Scalfi, L.; Frusciante, L.; Rigano, M.M.; Pucci, P.; et al. Nutritional Controlled Preparation and Administration of Different Tomato Purées Indicate Increase of β-Carotene and Lycopene Isoforms, and of Antioxidant Potential in Human Blood Bioavailability: A Pilot Study. Nutrients 2021, 13, 1336.
- Unlu, N.Z.; Bohn, T.; Clinton, S.K.; Schwartz, S.J. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J. Nutr. 2005, 135, 431–436.
- Lee, A.; Thurnham, D.I.; Chopra, M. Consumption of tomato products with olive oil but not sunflower oil increases the antioxidant activity of plasma. Free Radic. Biol. Med. 2000, 29, 1051–1055.
- Amorim, A.D.G.N.; Vasconcelos, A.G.; Souza, J.; Oliveira, A.; Gullón, B.; de Souza de Almeida Leite, J.R.; Pintado, M. Bio-Availability, Anticancer Potential, and Chemical Data of Lycopene: An Overview and Technological Prospecting. Antioxidants 2022, 11, 360.
- Crowe-White, K.M.; Voruganti, V.S.; Talevi, V.; Dudenbostel, T.; Nagabooshanam, V.A.; Locher, J.L.; Ellis, A.C. Variation of Serum Lycopene in Response to 100% Watermelon Juice: An Exploratory Analysis of Genetic Variants in a Randomized Controlled Crossover Study. Curr. Dev. Nutr. 2020, 4, nzaa102.
- Zubair, N.; Kooperberg, C.; Liu, J.; Di, C.; Peters, U.; Neuhouser, M.L. Genetic variation predicts serum lycopene concentrations in a multiethnic population of postmenopausal women. J. Nutr. 2015, 145, 187–192.
- Ferrucci, L.; Perry, J.R.B.; Matteini, A.; Perola, M.; Tanaka, T.; Silander, K.; Rice, N.; Melzer, D.; Murray, A.; Cluett, C.; et al. Common variation in the β-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: A genome-wide association study. Am. J. Hum. Genet. 2008, 84, 123–133.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
12 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




