
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dhanesh G. Mohan | -- | 2425 | 2022-10-11 10:44:38 | | | |
| 2 | Amina Yu | + 9 word(s) | 2434 | 2022-10-12 03:44:55 | | |
Video Upload Options
The coronavirus disease 2019 (COVID-19) rapidly spread to over 180 countries and abruptly disrupted production rates and supply chains worldwide. Since then, 3D printing, also recognized as additive manufacturing (AM) and known to be a novel technique that uses layer-by-layer deposition of material to produce intricate 3D geometry, has been engaged in reducing the distress caused by the outbreak. During the early stages of this pandemic, shortages of personal protective equipment (PPE), including facemasks, shields, respirators, and other medical gear, were significantly answered by remotely 3D printing them. Amidst the growing testing requirements, 3D printing emerged as a potential and fast solution as a manufacturing process to meet production needs due to its flexibility, reliability, and rapid response capabilities.
1. Additive Manufacturing (AM)/3D Printing in Medical Applications during COVID-19
1.1. AM/3D-Printed Personal Protective Equipment (PPE)
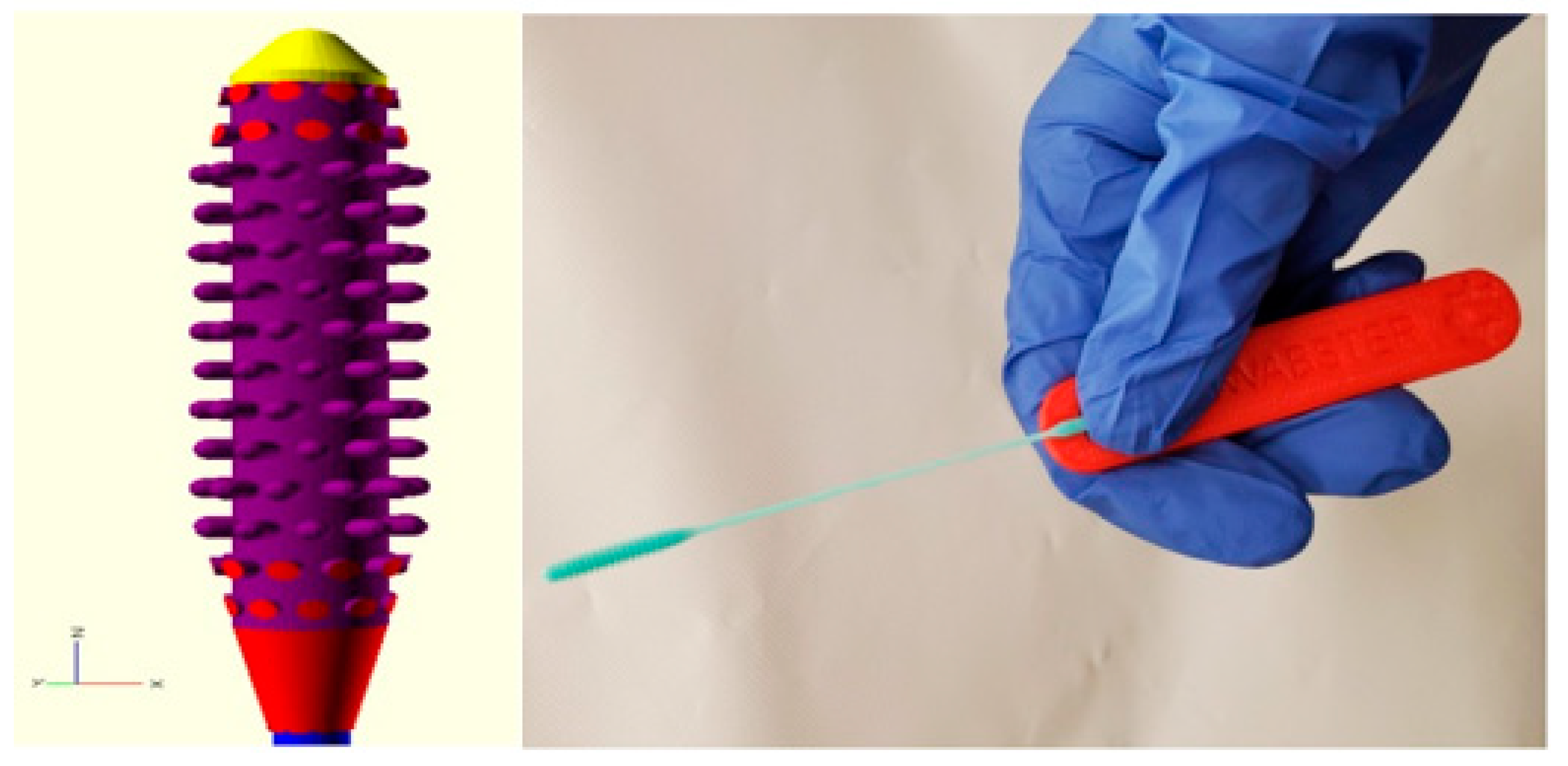
1.2. AM/3D-Printed Nasopharyngeal Swabs
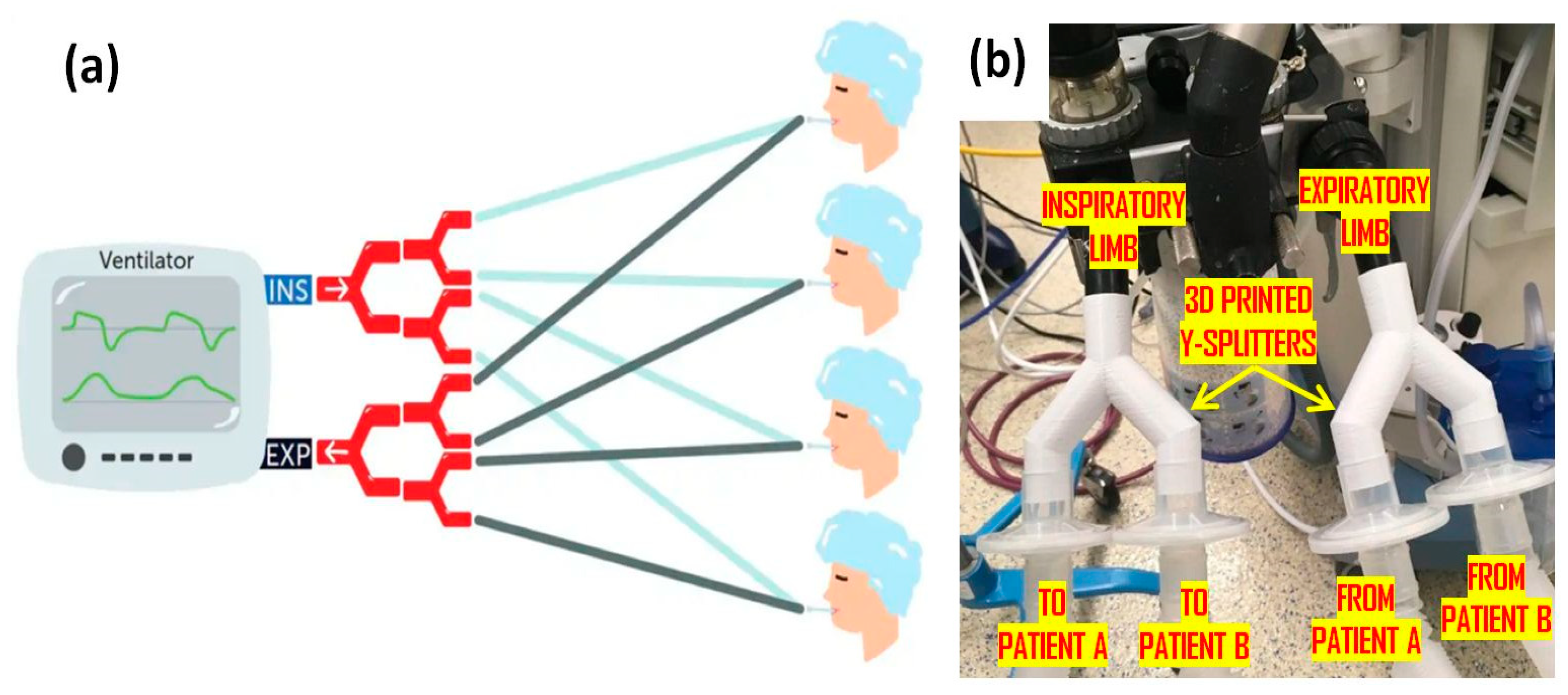
1.3. AM/3D-Printed Ventilators and Valves
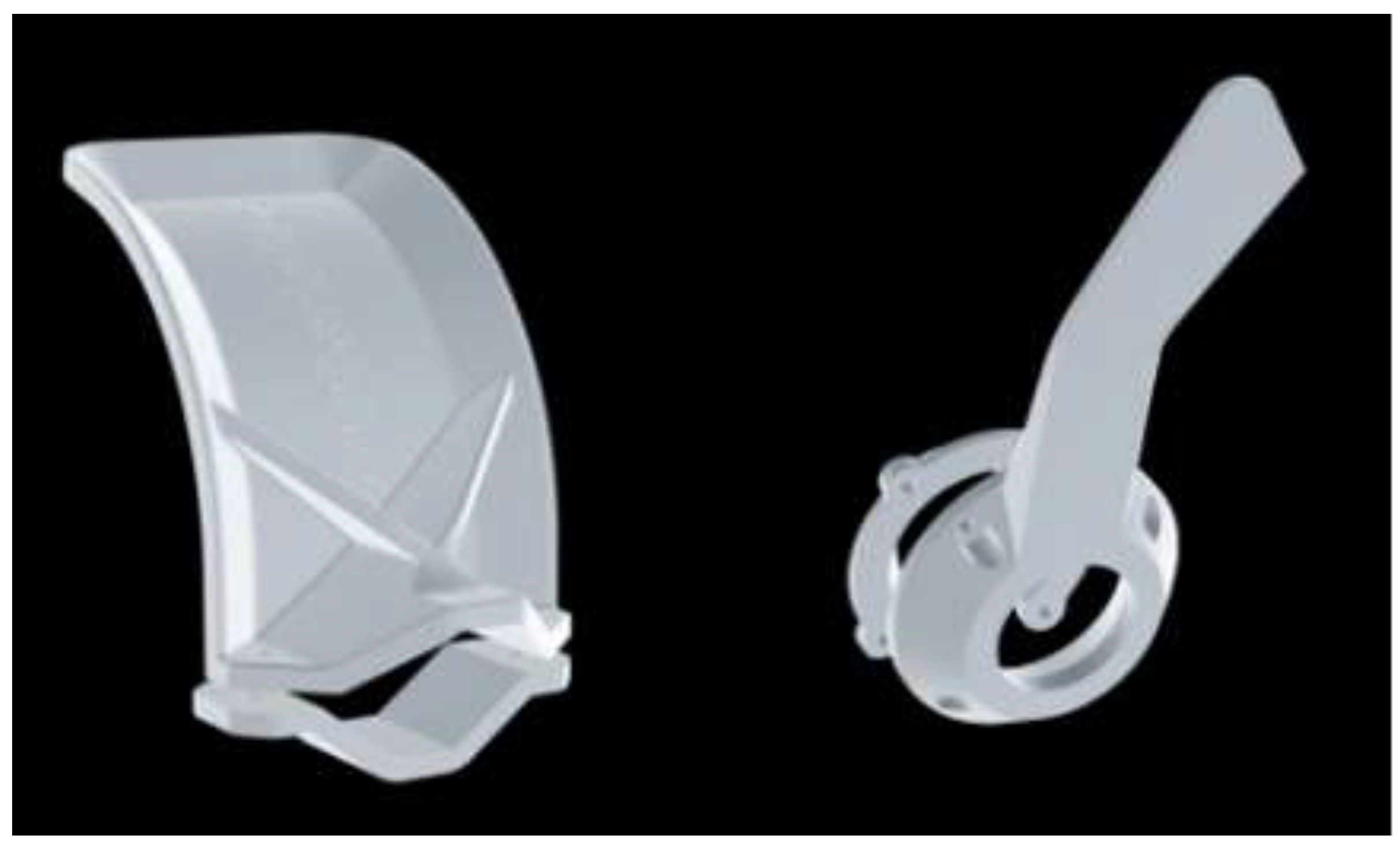
2. AM/3D Printing in Non-Medical Applications of COVID-19
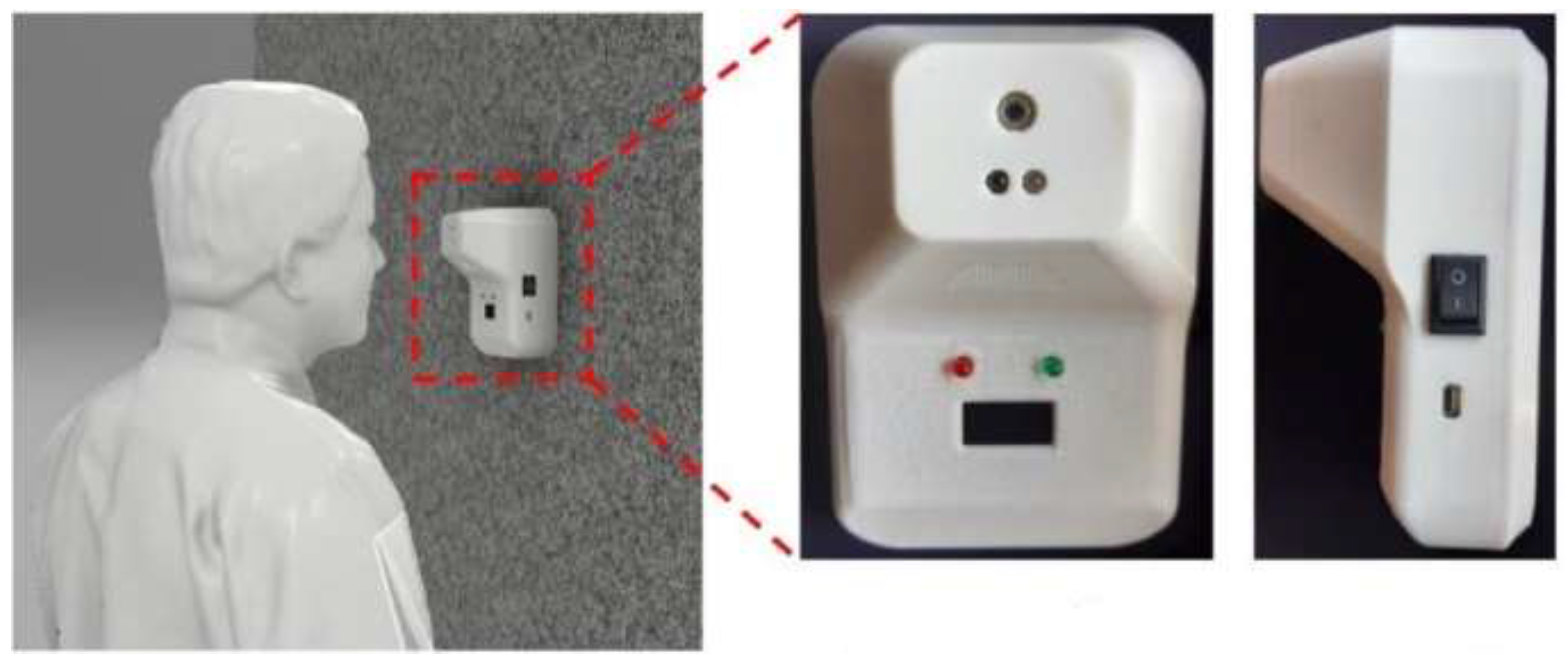
3. AM/3D Concrete Printing and COVID-19 Pandemic
References
- Vordos, N.; Gkika, D.A.; Maliaris, G.; Tilkeridis, K.E.; Antoniou, A.; Bandekas, D.V.; Mitropoulos, A.C. How 3D printing and social media tackles the PPE shortage during COVID-19 pandemic. Saf. Sci. 2020, 130, 104870.
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Devices and Radiological Health (CDRH); Office of Product Evaluation and Quality (OPEQ). Enforcement Policy for Telethermographic Systems During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency. In Guidance for Industry and Food and Drug Administration Staff; Food and Drug Administration: Rockville, MD, USA, 2020; Volume 2.
- Ford Partners with 3M, GE Healthcare to Make Respirators, Ventilators and Face Shields Amid Coronavirus Outbreak. Available online: https://www.detroitnews.com/story/business/autos/ford/2020/03/24/ford-partners-3-m-ge-healthcare-respirators-ventilators-face-shields/2905986001/ (accessed on 8 February 2021).
- People Are 3D Printing Personal Protective Equipment to Help Hospitals With Shortage: NPR. Available online: https://www.npr.org/local/305/2020/04/01/825217523/people-are-3-d-printing-personal-protective-equipment-to-help-hospitals-with-shortage (accessed on 8 February 2021).
- Decker, S.J.; Goldstein, T.A.; Ford, J.M.; Teng, M.N.; Pugliese, R.S.; Berry, G.J.; Pettengill, M.; Silbert, S.; Hazelton, T.R.; Wilson, J.W.; et al. 3D Printed Alternative to the Standard Synthetic Flocked Nasopharyngeal Swabs Used for COVID-19 testing. Clin. Infect. Dis. 2020, 73, e3027–e3032.
- Starosolski, Z.; Admane, P.; Dunn, J.; Kaziny, B.; Huisman, T.A.G.M.; Annapragada, A. Design of 3D-printed nasopharyngeal swabs for children is enabled by radiologic imaging. Am. J. Neuroradiol. 2020, 41, 2345–2347.
- Bennett, I.; Bulterys, P.L.; Chang, M.; DeSimone, J.M.; Fralick, J.; Herring, M.; Kabaria, H.; Kong, C.S.; Larson, B.; Lu, O.; et al. The rapid deployment of a 3D printed ‘Latticed’ nasopharyngeal swab for COVID-19 testing made using digital light synthesis. medRxiv 2020.
- Williams, E.; Bond, K.; Isles, N.; Chong, B.; Johnson, D.; Druce, J.; Hoang, T.; Ballard, S.A.; Hall, V.; Muhi, S.; et al. Pandemic printing: A novel 3D-printed swab for detecting SARS-CoV-2. Med. J. Aust. 2020, 213, 276–279.
- Gallup, N.; Pringle, A.M.; Oberloier, S.; Tanikella, N.G.; Pearce, J.M. Parametric nasopharyngeal swab for sampling COVID-19 and other respiratory viruses: Open source design, SLA 3-D printing and UV curing system. HardwareX 2020, 8, e00135.
- Oland, G.; Garner, O.; de St Maurice, A. Prospective clinical validation of 3D printed nasopharyngeal swabs for diagnosis of COVID-19. Diagn. Microbiol. Infect. Dis. 2021, 99, 115257.
- 8 Ways 3D Printing is Helping to Fight Coronavirus—ASME. Available online: https://www.asme.org/topics-resources/content/8-ways-3d-printing-is-helping-to-fight-coronavirus (accessed on 8 February 2021).
- 3D Printed COVID-19 Test Swabs|Formlabs. Available online: https://formlabs.com/asia/covid-19-response/covid-test-swabs/ (accessed on 8 February 2021).
- Alyouha, S.; Almazeedi, S.; Alghounaim, M.; Al-Mutawa, Y.; Alsabah, S. Polyester tipped 3-dimensionally printed swab that costs less than US$0.05 and can easily and rapidly be mass produced. BMJ Innov. 2020, 6, 262–264.
- Lyengar, K.; Bahl, S.; Vaishya, R.; Vaish, A. Challenges and solutions in meeting up the urgent requirement of ventilators for COVID-19 patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 499–501.
- Advincula, R.C.; Dizon, J.R.C.; Chen, Q.; Niu, I.; Chung, J.; Kilpatrick, L.; Newman, R. Additive manufacturing for COVID-19: Devices, materials, prospects, and challenges. MRS Commun. 2020, 10, 413–427.
- Rowan, N.J.; Laffey, J.G. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic—Case study from the Republic of Ireland. Sci. Total Environ. 2020, 725, 138532.
- Tino, R.; Moore, R.; Antoline, S.; Ravi, P.; Wake, N.; Ionita, C.N.; Morris, J.M.; Decker, S.J.; Sheikh, A.; Rybicki, F.J.; et al. COVID-19 and the role of 3D printing in medicine. 3D Print. Med. 2020, 6, 11.
- Neyman, G.; Irvin, C.B. A Single Ventilator for Multiple Simulated Patients to Meet Disaster Surge. Acad. Emerg. Med. 2006, 13, 1246–1249.
- ventsplitter.org—Free 3D Printable Ventilator Circuit Splitter. Available online: http://ventsplitter.org/ (accessed on 8 February 2021).
- Coronavirus: 3D Printers Save Hospital with Valves—BBC News. Available online: https://www.bbc.com/news/technology-51911070 (accessed on 8 February 2021).
- Meet The Italian Engineers 3D-Printing Respirator Parts For Free To Help Keep Coronavirus Patients Alive. Available online: https://www.forbes.com/sites/amyfeldman/2020/03/19/talking-with-the-italian-engineers-who-3d-printed-respirator-parts-for-hospitals-with-coronavirus-patients-for-free/?sh=277a072778f1 (accessed on 8 February 2021).
- Viera-Artiles, J.; Valdiande, J.J. 3D-printable headlight face shield adapter. Personal protective equipment in the COVID-19 era. Am. J. Otolaryngol.—Head Neck Med. Surg. 2020, 41, 102576.
- 3D Printing of Medical Devices, Accessories, Components, and Parts during the COVID-19 Pandemic|FDA. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/3d-printing-medical-devices-accessories-components-and-parts-during-covid-19-pandemic (accessed on 20 September 2022).
- WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 7 February 2021).
- Hsiao, W.K.; Lorber, B.; Paudel, A. Can 3D printing of oral drugs help fight the current COVID-19 pandemic (and similar crisis in the future)? Expert Opin. Drug Deliv. 2020, 17, 899–902.
- Martin-Noguerol, T.; Paulano-Godino, F.; Menias, C.O.; Luna, A. Lessons learned from COVID-19 and 3D printing. Am. J. Emerg. Med. 2020, 46, 659–660.
- Manekiya, M.; Donelli, M. Monitoring the COVID-19 diffusion by combining wearable biosensors and smartphones. Prog. Electromagn. Res. M 2021, 100, 13–21.
- Additive manufacturing and coronavirus 3D Printing Media Network—The Pulse of the AM Industry. Available online: https://www.3dprintingmedia.network/category/industry-2/additive-manufacturing-and-coronavirus/ (accessed on 7 February 2021).
- François, P.M.; Bonnet, X.; Kosior, J.; Adam, J.; Khonsari, R.H. 3D-printed contact-free devices designed and dispatched against the COVID-19 pandemic: The 3D COVID initiative. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 381–385.
- Hands-Free Door Opener: Technical Information—Materialise. Available online: https://www.materialise.com/en/hands-free-door-opener/technical-information (accessed on 8 February 2021).
- Abuzairi, T.; Sumantri, N.I.; Irfan, A.; Mohamad, R.M. Infrared thermometer on the wall (iThermowall): An open source and 3-D print infrared thermometer for fever screening. HardwareX 2021, 9, e00168.
- Sanjayan, J.G.; Nematollahi, B. 3D Concrete Printing for Construction Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019.
- Siddika, A.; al Mamun, M.A.; Ferdous, W.; Saha, A.K.; Alyousef, R. 3D-printed concrete: Applications, performance, and challenges. J. Sustain. Cem. Mater. 2020, 9, 127–164.
- Alhumayani, H.; Gomaa, M.; Soebarto, V.; Jabi, W. Environmental assessment of large-scale 3D printing in construction: A comparative study between cob and concrete. J. Clean. Prod. 2020, 270, 122463.
- Nair, A.; Aditya, S.D.; Adarsh, R.N.; Nandan, M.; Dharek, M.S.; Sreedhara, B.M.; Prashant, S.C.; Sreekeshava, K.S. Additive Manufacturing of Concrete: Challenges and opportunities. IOP Conf. Ser. Mater. Sci. Eng. 2020, 814, 012022.
- Chennai: 350 Sqft House Can Be Built in Week at Low Cost. Available online: https://www.deccanchronicle.com/nation/current-affairs/251018/chennai-350-sqft-house-can-be-built-in-week-at-low-cost.html (accessed on 9 February 2021).
- This Startup Will Build Your House in Three Weeks at Half the Cost—Times of India. Available online: https://timesofindia.indiatimes.com/companies/this-startup-will-build-your-house-in-three-weeks-at-half-the-cost/articleshow/79614197.cms (accessed on 9 February 2021).
- WinSun Deploys 3D Printed Isolation Wards for Coronavirus Medical Staff » 3D Printing Media Network—The Pulse of the AM Industry. Available online: https://www.3dprintingmedia.network/winsun-3d-printed-isolation-wards-coronavirus-medical-workers/ (accessed on 9 February 2021).




