| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elba Mauriz | + 1120 word(s) | 1120 | 2020-11-02 09:04:52 | | | |
| 2 | Nicole Yin | Meta information modification | 1120 | 2020-11-03 04:58:36 | | |
Video Upload Options
Colorimetric analysis has become of great importance in recent years to improve the operationalization of plasmonic-based biosensors. The unique properties of nanomaterials have enabled the development of a variety of plasmonics applications on the basis of the colorimetric sensing provided by metal nanoparticles. In particular, the extinction of localized surface plasmon resonance (LSPR) in the visible range has permitted the exploitation of LSPR colorimetric-based biosensors as powerful tools for clinical diagnostics and drug monitoring. This review summarizes recent progress in the biochemical monitoring of clinical biomarkers by ultrasensitive plasmonic colorimetric strategies according to the distance- or the morphology/size-dependent sensing modes. Colorimetric analysis has become of great importance in recent years to improve the operationalization of plasmonic-based biosensors. The unique properties of nanomaterials have enabled the development of a variety of plasmonics applications on the basis of the colorimetric sensing provided by metal nanoparticles. In particular, the extinction of localized surface plasmon resonance (LSPR) in the visible range has permitted the exploitation of LSPR colorimetric-based biosensors as powerful tools for clinical diagnostics and drug monitoring. This review summarizes recent progress in the biochemical monitoring of clinical biomarkers by ultrasensitive plasmonic colorimetric strategies according to the distance- or the morphology/size-dependent sensing modes.
1. Introduction
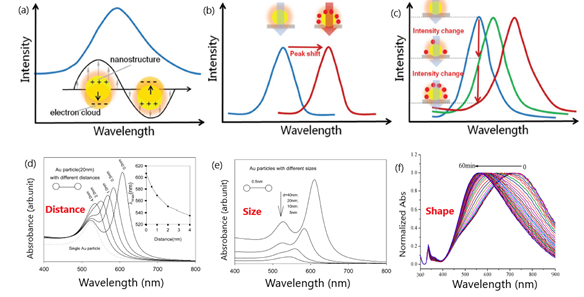
Colorimetric sensors take advantage of the localized surface plasmon resonance (LSPR) occurring in the surface of conductive nanoparticles under light stimulation as a result of the confinement of surface plasmons in two-dimensional thin metallic layers[1][2][3]. The LSPR phenomenon enables free electrons to oscillate collectively in the electric field of incident light, leading to the resonance condition[4] (Figure 1).
Figure 1. Schematic representation of: (a) peak in the extinction spectra of nanostructures as a result of the resonance between the electron clouds and the incident light; (b) peak shifts of the spectra due to the changes in refractive index (RI) adjacent to metallic surface induced by the adsorption of target molecules; (c) variation of the extinction intensity with the degree of the RI changes near localized surface plasmon resonance (LSPR) sensing substrate at a specific wavelength. Adapted with permission from Wang et al.[5] Copyright © 2017 Wiley; (d) change in extinction spectra for 20 nm diameter particles with interparticle distance (s). Inset is the peak shift vs. interparticle distance; (e) influence of Au nanoparticle diameter. Adapted with permission from Zhong et al.[6] Copyright © 2004 American Chemical Society; (f) SPR (Surface Plasmon Resonance) peak shift of triangular silver nanoprisms in the presence of glucose oxidase (GOD) and glucose. Adapted with permission from Yang et al. [7] Copyright © 2014 American Chemical Society.
2. Distance–Dependent Plasmonic Naked-Eye Colorimetric Assays
The sensing system relying on the inter particle distance is capable of measuring both LSPR spectral shifts and color variations due to the surface plasmon coupling induced by the aggregation and dispersion of nanoparticles[8][9]. In particular, the aggregation and dispersion of nanoparticles with diameters above 3.5 nm can cause shifts in the plasmon bands along with visible color transitions from red to blue and blue to red, respectively[10][11]. The decrease in the particle distance can be controlled by the presence of the target analyte, thereby setting the basis for colorimetric assays.
LSPR colorimetric sensors based on the aggregation of nanoparticles may provide high sensitivities and functional designs. Thus, the stability of the assemblies from plasmonic nanoparticles can be modulated by either covalent or non-covalent interactions (i.e., electrostatic interactions, hydrophobic forces, hydrogen bonding and specific biological bonding) and are strongly dependent on external conditions such as temperature, pH and buffer solution[12][13]. However, practical applications for naked-eye detection must face poor resolution, especially for lower target concentrations, because the color readouts by visual inspections only make use of monocolor changes[14]. On the other hand, the auto-aggregation of nanoparticles caused by non-target-mediated factors such as pH, temperature, solvent and molecules charges may lead to false positive/negative results[14]. In spite of these potential limitations, many colorimetric applications have reported ultrasensitive detection levels of biologically relevant targets for molecular diagnostic purposes and drug discovery by merely using the unaided eye[15][16][17][18].
3. Colorimetric Sensors Based on Etching and Growth of Noble Metal Nanoparticles
The term ‘non aggregation’ is usually applied to colorimetric sensors that depend on the morphology and size of noble metal nanoparticles for detecting target analytes. In comparison to classical nanoparticle-based aggregation methods, ‘non aggregation’ colorimetric sensors are reported to improve the sensing readout by making use of specific catalytic conversion processes (chemical or biological) based on monocolor or multicolor variations. Another important merit is the possibility of avoiding false/positive results due to the stability of the color substrate and the ability to prevent both auto-aggregation and the interference of foulants in complex biological matrices[8]. The possibility of developing colorimetric sensors based on test strips are further advantages over other plasmonic sensing schemes[10][19][20]. These features become essential for clinical analysis, thereby setting the fabrication of ‘non aggregation’ platforms in a prominent position for the development of POCT applications.
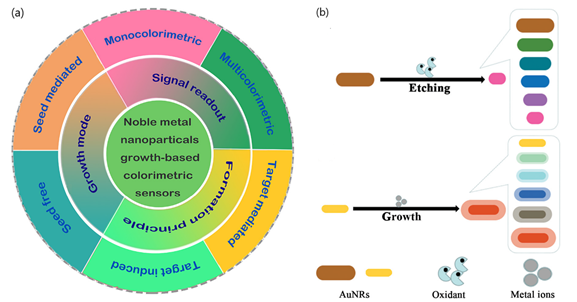
According to the sensing mechanism, ‘non aggregation’ colorimetric sensors can be classified in relation to the growth and etching of metal nanoparticles. On the one hand, the nanoparticles’ growth implies the production of new nanostructures that yield changes in the extinction spectra of the LSPR band and the color of the solution[10][8]. The growth of newly generated nanoparticles is induced by metal ions in the absence or presence of seeds and can be determined by different signal readouts (monocolorimetric, multicolorimetric), generation mechanisms (seed-free, seed-mediated) and sensing principles (target-induced/mediated) (Figure 2)[8]. The utilization of different precursors, reducing or capping agents for the generation of nanoparticles can also contribute to modifying LSPR properties, yielding different visual color readouts.
Figure 2. (a) Schematic representation of seed-free and seed-mediated based colorimetric sensors which depend on different signal generation mechanisms. Target-induced and target-mediated colorimetric protocols which rely on different sensing principles. Monocolorimetric and multicolorimetric sensors based on different signal readouts. Adapted with permission from Wang et al.[8] Copyright © 2019 Elsevier; (b) two routes for the evolution of the shape of AuNRs used in AuNR multicolorimetric assays: AuNR-etching based process and AuNRs growth-based process. Adapted with permission from Rao et al. [20]Copyright © 2019 The Royal Society of Chemistry.
In contrast, etching processes are associated with the change in color and the shift of the LSPR band extinction spectra resulting from the tuning of the size, shape, and composition of nanoparticles. By controlling the morphology/size and kinetics of nanoparticles in the presence of the etchant, usually through oxidation, the recognition events induced by the target can generate a variation of LSPR and detected by naked-eye[19]. Additionally, etching-based colorimetric sensors can benefit from enzyme-mediated strategies in which the products of a reaction catalyzed by an enzyme can induce the etching of metal nanoparticles, thus offering further applications in plasmonic DNA assays and enzyme linked immunosorbent assays (ELISAs).
References
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species.Chem. Rev.2008,108, 462–493.
- Couture, M.; Zhao, S.S.; Masson, J.-F. Modern surface plasmon resonance for bioanalytics and biophysics.Phys. Chem. Chem. Phys.2013,15, 11190.
- Masson, J.-F. Portable and field-deployed surface plasmon resonance and plasmonic sensors.Analyst2020,145, 3776–3800
- Ming Li; Scott K. Cushing; Nianqiang Wu; Plasmon-enhanced optical sensors: a review. The Analyst 2015, 140, 386-406, 10.1039/c4an01079e.
- Yangyang Wang; Jianhua Zhou; Jinghong Li; Construction of Plasmonic Nano-Biosensor-Based Devices for Point-of-Care Testing. Small Methods 2017, 1, 1700197, 10.1002/smtd.201700197.
- Ziyi Zhong; ‡ Sergiy Patskovskyy; † Pierre Bouvrette; † And John H. T. Luong; § Aharon Gedanken; The Surface Chemistry of Au Colloids and Their Interactions with Functional Amino Acids. The Journal of Physical Chemistry B 2004, 108, 4046-4052, 10.1021/jp037056a.
- Xinjian Yang; Yuebo Yu; Zhiqiang Gao; A Highly Sensitive Plasmonic DNA Assay Based on Triangular Silver Nanoprism Etching. ACS Nano 2014, 8, 4902-4907, 10.1021/nn5008786.
- Hongqiang Wang; Honghong Rao; Minyue Luo; Xin Xue; Zhonghua Xue; Xiaoquan Lu; Noble metal nanoparticles growth-based colorimetric strategies: From monocolorimetric to multicolorimetric sensors. Coordination Chemistry Reviews 2019, 398, 113003, 10.1016/j.ccr.2019.06.020.
- Yunlei Xianyu; Yangzhouyun Xie; Nuoxin Wang; Zhuo Wang; Xingyu Jiang; A Dispersion-Dominated Chromogenic Strategy for Colorimetric Sensing of Glutathione at the Nanomolar Level Using Gold Nanoparticles. Small 2015, 11, 5510-5514, 10.1002/smll.201500903.
- Longhua Tang; Jinghong Li; Plasmon-Based Colorimetric Nanosensors for Ultrasensitive Molecular Diagnostics. ACS Sensors 2017, 2, 857-875, 10.1021/acssensors.7b00282.
- Chifang Peng; Xiaohui Duan; Gabriel Wafala Khamba; Zhengjun Xie; Highly sensitive “signal on” plasmonic ELISA for small molecules by the naked eye. Analytical Methods 2014, 6, 9616-9621, 10.1039/c4ay01993h.
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures toFunctional Materials.Chem. Rev.2016,116, 11220–11289.
- Vogel, N.; Retsch, M.; Fustin, C.-A.; del Campo, A.; Jonas, U. Advances in Colloidal Assembly: The Designof Structure and Hierarchy in Two and Three Dimensions.Chem. Rev.2015,115, 6265–6311
- Sujit Kumar Ghosh; § Tarasankar Pal; Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chemical Reviews 2007, 107, 4797-4862, 10.1021/cr0680282.
- Krishnendu Saha; Sarit S. Agasti; Chaekyu Kim; Xiaoning Li; Vincent M. Rotello; Gold Nanoparticles in Chemical and Biological Sensing. Chemical Reviews 2012, 112, 2739-2779, 10.1021/cr2001178.
- Kumar, V.; Kumar, P.; Pournara, A.; Vellingiri, K.; Kim, K.-H. Nanomaterials for the sensing of narcotics:Challenges and opportunities.Trac Trends Anal. Chem.2018,106, 84–115.
- Garzón, V.; Pinacho, D.G.; Bustos, R.-H.; Garzón, G.; Bustamante, S. Optical Biosensors for Therapeutic DrugMonitoring.Biosensors2019,9, 132.
- Taddeo, A.; Prim, D.; Bojescu, E.-D.; Segura, J.-M.; Pfeifer, M.E. Point-of-Care Therapeutic Drug Monitoringfor Precision Dosing of Immunosuppressive Drugs.J. Appl. Lab. Med.2020,5, 738–761.
- Zhiyang Zhang; Han Wang; Zhaopeng Chen; Xiaoyan Wang; Jaebum Choo; Lingxin Chen; Plasmonic colorimetric sensors based on etching and growth of noble metal nanoparticles: Strategies and applications. Biosensors and Bioelectronics 2018, 114, 52-65, 10.1016/j.bios.2018.05.015.
- Honghong Rao; Xin Xue; Hongqiang Wang; Zhonghua Xue; Gold nanorod etching-based multicolorimetric sensors: strategies and applications. J. Mater. Chem. C 2019, 7, 4610-4621, 10.1039/c9tc00757a.