| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jason Zhu | -- | 2615 | 2022-10-08 05:38:02 |
Video Upload Options
Parvovirus is the common name applied to all the viruses in the Parvoviridae taxonomic family. Parvovirus can also be used specifically for members of one of the two Parvoviridae subfamilies: Parvovirinae, which infect vertebrate hosts, and Densovirinae, which infect invertebrate hosts, are more commonly referred to as densoviruses. In subfamily Parvovirinae there are eight genera, containing a total of 58 recognized species, while in subfamily Densovirinae there are 5 genera and a total of 21 species. Parvoviruses are linear, nonsegmented, single-stranded DNA viruses, with an average genome size of 5-6 kilo base pairs (kbp). They are classified as group II viruses in the Baltimore classification of viruses. Parvoviruses are among the smallest viruses (hence the name, from Latin parvus meaning small) and are 23–28 nm in diameter. Parvoviruses can infect and may cause disease in many animals, from arthropods such as insects and shrimp, to echinoderms such as starfish, and to mammals including humans. Because most of these viruses require actively dividing cells to replicate, the type of tissue infected varies with the age of the animal. The gastrointestinal tract and lymphatic system can be affected at any age, leading to vomiting, diarrhea, and immunosuppression, but cerebellar hypoplasia is only seen in cats that were infected with feline parvovirus (FPV) in the womb or at less than two weeks of age, and disease of the myocardium is seen in puppies infected with canine parvovirus 2 (CPV2) between the ages of three and eight weeks.
1. History
Perhaps due to their extremely small size, the first parvoviruses were not discovered until the late 1950s.[1] Parvovirus B19, the first known parvovirus to cause disease in humans, was discovered in London by Australian virologist Yvonne Cossart in 1974. Cossart and her group were focused on hepatitis B and were processing blood samples when they discovered a number of "false positives" later identified as parvovirus B19. The virus is named for the patient code of one of the blood-bank samples involved in the discovery.[2]
2. Structure
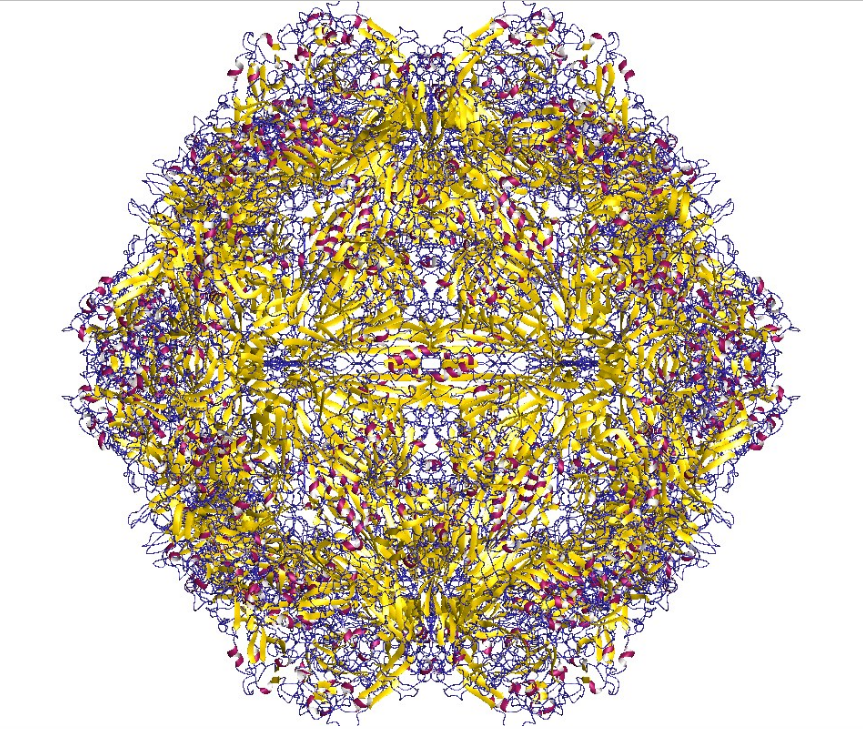
The viral capsid of a parvovirus is made up of 60 copies of two or more size variants of a single protein sequence, called VP1, VP2 etc., which form a resilient structure with T=1 icosahedral symmetry. These virions are typically resistant to dilute acids, bases, solvents, and temperatures up to 50°C (122°F). Parvoviruses do not have envelopes, thus are considered "naked" viruses. In addition, the shape of the virion is roughly spherical, with surface protrusions and canyons.[3]
Inside the capsid is a linear, single-stranded DNA genome in the size range 4–6 kbp, so the small genome of parvovirus can encode only a few proteins. At the 5’ and 3’ ends of this genome are short complementary sequences of roughly 120 to 550 nucleotides that form secondary structures as hairpins for example inverted terminal repeats (ITRs, which are two identical secondary structures at the termini) or unique sequences at the termini (two unique and different secondary structures are at each end of the DNA) and are essential for viral genome replication mechanism called rolling-hairpin replication.[3]
3. Genome Size
These viruses have small genomes, encoding just two genes, and must rely on the synthetic machinery of their host cell for their own preferential replication. This means that many parvoviruses require host cells to enter S-phase before viral DNA replication can initiate, but they do not encode any gene products that can drive this transition. Parvoviruses overcome this problem in various ways: viruses in many genera simply wait within the cell for it to enter S-phase under its own cell cycle control, which means that they can only infect actively-dividing cell populations.
In contrast, the so-called adeno-associated viruses (AAVs) from genus Dependoparvovirus must wait until the cell is co-infected by a helper DNA virus, commonly an adenovirus or herpes virus, which does encode gene products that can drive the cell into S-phase, allowing AAV infection to initiate and out-compete the helper virus.[4] A third strategy is used by human bocavirus 1 (HBoV1) from genus Bocaparvovirus, which appears to invoke a specific DNA-damage response in its host cell that ultimately supports viral DNA amplification and progeny virus production. [5]
4. Examples of Parvoviruses
Parvoviruses that infect vertebrate hosts make up the subfamily Parvovirinae, while those that infect invertabrates (currently only known to infect insects, crustacea, and echinoderms) make up the subfamily Densovirinae. Prior to 2014, the name parvovirus was also applied to a genus within subfamily Parvovirinae, but this genus name has been amended to Protoparvovirus to avoid confusion between taxonomic levels.[6][7]
Many mammalian species sustain infection by multiple parvoviruses. Parvoviruses tend to be specific about the species of animal they will infect, but this is a somewhat flexible characteristic. Thus, all isolates of canine parvovirus affect dogs, wolves, and foxes, but only some of them will infect cats.
Humans can be infected by viruses from five of the eight genera in the subfamily Parvovirinae: i) Bocaparvovirus (e.g. human bocavirus 1), ii) Dependoparvovirus (e.g. adeno-associated virus 2), iii) Erythroparvovirus (e.g. parvovirus B19), iv) Protoparvovirus (e.g. bufavirus 1a), and v) Tetraparvovirus (e.g. human parv4 G1). As of 2018, no known human viruses were in the remaining three recognized genera: vi) Amdoparvovirus (e.g. Aleutian mink disease virus), vii) Aveparvovirus (e.g. chicken parvovirus), and viii) Copiparvovirus (e.g. bovine parvovirus 2).[3]
4.1. Dependoparvoviruses
Dependoparvoviruses, previously known as dependoviruses, require helper viruses (e.g. adenoviruses or herpesviruses) to replicate.[8] They are excellent vectors for experimental or clinical gene transduction and are now being used in patients for the therapeutic treatment of certain genetic diseases. The biggest advantage for such applications is that they are not known to cause any diseases.
4.2. Autonomous parvoviruses
Unlike dependoparvoviruse, autonomous parvoviruses do not require a helper virus. The autonomous parvovirus genome is a linear ssDNA with terminal palindromes, and several viruses have different sorts of palindromes at each end which are known to package the negative sense DNA strand for the most part. Most Autonomous Parvoviruses are known to encode only two NS structural proteins. Similar to RNA viruses, autonomous parvoviruses are highly effective when cells are in the S phase.
Autonomous Parvovirus Replication occurs through hairpins being formed by the palindrome at the 3′ end of the viral genome. This acts as a primer for the synthesis of complementary strands. Elongating strands then become covalently linked to hairpins at the 5' end of the template to create a linear duplex model that has covalent cross-links at the ends by DNA hairpins. Right end of the hairpin is nicked on the new strand by NS1. The genomic ssDNA is generated and packaged when the capsid is available. The RF structures create ssDNA but they are not linear duplex monomers. These Rf structures are potentially NS1/DNA complexes that are related to the structure of capsid.
Autonomous Parvoviruses have a very unique scheme of transcription. Several are known to utilize promoters at map regions 4 and 38 to create variable transcripts along with polyadenylation signals at the right end of the genome. Small introns exist between map positions 444 and 46, and large introns exist between map positions 10 and 39. All messengers in MVM have small introns spliced while alternative splice donors and acceptor sites can also be used. During genome replication, read-through of the internal poly S site occurs and the large transcript is translated into a capsid protein.
Protein synthesis is dependent on transcription. The earliest transcripts during the course of infection were the NS protein transcripts and they now are able to regulate gene expression. NS1 and NS2 are phosphorylated after translation, and NS1 is known to dimerize before nuclear localization. NS2 has been found to have profound impact on virus growth in a host-dependent manner.[9]
5. Disease Information on Parvoviridae
The remainder of this article discusses the disease-causing Parvoviridae.
5.1. Diseases caused by members of the Parvoviridae family
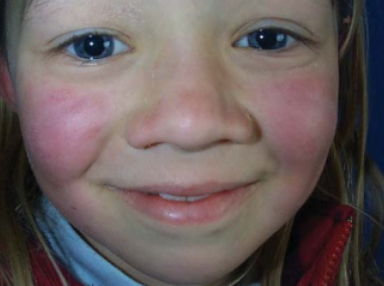
Parvovirus B19 which causes fifth disease in humans, is a member of species Primate erythroparvovirus 1 in the genus Erythroparvovirus. It infects red blood cell precursors and was the first parvovirus shown to cause human disease.[10] Some infections do not result in visible infection, while some manifest with visible effects, such as fifth disease (erythema infectiosum), which can give children a ‘slapped-cheek’ appearance.
Canine parvovirus is a member of species Carnivore protoparvovirus 1 in the genus Protoparvovirus. It causes a particularly deadly disease among young puppies, about 80% fatal, causing gastrointestinal tract damage and dehydration, as well as a cardiac syndrome in very young animals. It is spread by contact with an infected dog's feces. Symptoms include lethargy, severe diarrhea, fever, vomiting, loss of appetite, and dehydration.[11]
However, mouse parvovirus 1, a member of species Rodent protoparvovirus 1, causes no symptoms but can contaminate immunology experiments in biological research laboratories.
Porcine parvovirus, a member of species Ungulate Protoparvovirus 1, causes a reproductive disease in swine known as SMEDI, which stands for stillbirth, mummification, embryonic death, and infertility.
Like canine parvovirus, feline panleukopenia, now more commonly known as feline parvovirus, is also a member of species Carnivore protoparvovirus 1 in the genus Protoparvovirus. This virus is common in kittens and causes fever, low white blood cell count, diarrhea, and death. Infection of the cat fetus and kittens less than two weeks old causes cerebellar hypoplasia.
Mink enteritis virus, also a member of species Carnivore protoparvovirus 1, is similar in effect to feline parvovirus, except that it does not cause cerebellar hypoplasia. A different parvovirus causes aleutian disease in mink and other mustelids, characterized by lymphadenopathy, splenomegaly, glomerulonephritis, anemia, and death.
5.2. Replication as disease vector
To enter host cells, parvoviruses typically bind to a sialic acid-bearing cell surface receptor and penetration into the cytoplasm is mediated by a phospholipase A2 activity carried on the amino-terminal peptide of the capsid VP1 polypeptide.[3] Once in the cytoplasm, the intact virus is translocated into the nucleus prior to uncoating. Transcription only initiates when the host cell enters S-phase under its own cell cycle control, when the cell's replication machinery converts the incoming single strand into a duplex transcription template, allowing synthesis of mRNAs encoding the nonstructural proteins, NS1 and NS2. The mRNAs are transported out of the nucleus into the cytoplasm, where the host ribosomes translate them into viral proteins. Viral DNA replication proceeds through a series of monomeric and concatemeric duplex intermediates by a unidirectional strand-displacement mechanism that is mediated by components of the host replication fork, aided and orchestrated by the viral NS1 polypeptide. NS1 also transactivates an internal transcriptional promoter that directs synthesis of the structural VP polypeptides. Once assembled capsids are available, replication shifts from synthesizing duplex DNA to displacement of progeny single strands, which are typically negative-sense and are packaged in a 3'-to-5' direction into formed particles within the nucleus. Mature virions may be released from infected cells prior to cell lysis, which promotes rapid transmission of the virus, but if this fails, then the virus is released at cell lysis.[3]
Unlike most other DNA viruses, parvoviruses are unable to activate DNA synthesis in host cells. Thus, for viral replication to take place, the infected cells must typically be nonquiescent (i.e. must be actively mitotic). Their inability to force host cells into S-phase means that parvoviruses are nontumorigenic. Indeed, they are commonly oncolytic, showing a strong tendency to replicate preferentially in cells with transformed phenotypes.[3]
6. Use of HeLa Cells in Parvovirus Testing
Testing for how feline parvovirus and canine parvovirus infect cells and what pathways are taken, scientists used cat cells, mouse cells, cat and mouse hybrid cells, mink cells, dog cells, human cells, and HeLa cells.[12] Both feline parvovirus and canine parvovirus enter their hosts, follow specific pathways, and infect at certain parts of cells before infecting major organs. Parvoviruses are specific viruses that are characterized in part by which receptors they attack.[13] Testing found that parvovirus infects carnivorous animals through the oropharyngeal pathway. Parvovirus infects the oropharyngeal cells by binding to transferrin receptors, a glycoprotein, on the plasma membrane.[14][15] The parvovirus plasmid is stored in a small non-enveloped capsid.[14][16] Once oropharyngeal cells become infected the virus spreads to dividing lymph cells and continues to work to the bone marrow and spread to target organs through blood.
Testing of HeLa cells and human cells to exposure of both feline parvovirus and canine parvovirus resulted in infections of the cells at human transferrin receptors.[12] When antibodies and parvovirus samples were added at the same time to human cells and HeLa cells, no infection was found to take place; both human cells and HeLa cells have transferrin receptors, but no evidence of humans contracting parvovirus was found.
Certain chromosomes in cells show more susceptibility to parvovirus than others. Testing of feline parvovirus on cat cells and cat mouse hybrid cells found cultures with cells having the highest concentrations of the C2 chromosome were the most highly infected cells.[12] Slight mutations of binding sites were found to slow down or completely stop the infection of the given parvovirus, whereas cells that are naturally missing the receptors or are mutants lacking them cannot be mutated.[15] Both feline parvovirus and canine parvovirus express plasticity during cellular infection.[16][17] Although transferrin receptors may be limited on cell surfaces, the parvovirus will find available transferrin receptors and will use different pathways to gain entry to the cell. Unlike plasma membrane infection plasticity, all strains of parvovirus show related routes to the cell nucleus.
7. Canine and Feline
Canine parvovirus is a mutant strain of feline parvovirus.[12][13][14] A very specific mutation is necessary for the virus to change species of infection. The mutation affects capsid proteins of feline parvovirus, giving it the ability to infect dogs.[15] Both forms of the virus are very similar, so once the mutation has occurred, canine parvovirus is still able to infect cats. The canine parvovirus has the tradeoff of gaining the ability to infect canine cells, while becoming less effective at infecting feline cells. Both feline parvovirus and canine parvovirus bind to and infect the transferrin receptors, but both have different sequences in the cells and animals. Infection by both feline parvovirus and canine parvovirus are relatively quick, but because of constant mutation of canine parvovirus, canine parvovirus has a slower infection time than feline parvovirus.[16] Studies of other strains of mutated canine parvovirus have revealed that changes in the viral capsid by just one protein can be fatal to the virus. Deleterious mutations have been noted to lead to inability to bind to transferrin receptors, bind to nonreceptive parts of the cell membrane, and identification of the virus by the host’s antibody cells.[17]
Dogs, cats, and swine can be vaccinated against parvovirus. In pet dogs and cats, this is a normal vaccine commonly administered when they are young. The dog vaccine that protects against parvovirus is DHPP, and the cat vaccine is FPV.
8. Management and Therapy
Currently, no vaccine exists to prevent infection by all parvoviruses, but recently, the virus's capsid proteins, which are noninfectious molecules, have been suggested acting as antigens for improving of vaccines. For pig vaccine, inactivated live, monovalent combined, most contain old PPV-1 strains to protect already positive sows. Vaccinate after 6 months, once or twice before mating, and repeat yearly.
Antivirals and human immunoglobulin-sourced treatments are usually for relief of symptoms. Using immunoglobulins is a logical solution for treatment as neutralizing antibodies because a majority of adults have been in danger from the parvoviruses, especially B19 virus.[18]
References
- Kilham, L; Olivier, LJ (1959). "A latent virus of rats isolated in tissue culture". Virology 7 (4): 428–437. doi:10.1016/0042-6822(59)90071-6. PMID 13669314. https://dx.doi.org/10.1016%2F0042-6822%2859%2990071-6
- "Human parvovirus B19". Clin Microbiol Rev 15 (3): 485–505. 2002. doi:10.1128/cmr.15.3.485-505.2002. PMID 12097253. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=118081
- "ICTV 10th Report (2018)". https://talk.ictvonline.org/ictv-reports/ictv_online_report/ssdna-viruses/w/parvoviridae.
- Carter, John; Saunders, Venetia (2007). Virology: Principles and Applications. Wiley. pp. 137–146. ISBN 978-0-470-02386-0.
- Deng, X; Yan, Z; Cheng, F; Engelhardt, JF; Qiu, J (2016). "Replication of an Autonomous Human Parvovirus in Non-dividing Human Airway Epithelium Is Facilitated through the DNA Damage and Repair Pathways". PLoS Pathogens 12 (1): e1005399. doi:10.1371/journal.ppat.1005399. PMID 26765330. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4713420
- Cotmore, S. F; Agbandje-Mckenna, M; Chiorini, J. A; Mukha, D. V; Pintel, D. J; Qiu, J; Soderlund-Venermo, M; Tattersall, P et al. (2014). "The family Parvoviridae". Arch. Virol. 159 (5): 1239–47. doi:10.1007/s00705-013-1914-1. PMID 24212889. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4013247
- "2013.001a-aaaV.A.v4.Parvoviridae.pdf". http://talk.ictvonline.org/files/ictv_official_taxonomy_updates_since_the_8th_report/m/vertebrate-official/4844.aspx. Retrieved 20 February 2016.
- "ICTV 10th Report (2018)Dependoparvovirus". https://talk.ictvonline.org/ictv-reports/ictv_online_report/ssdna-viruses/w/parvoviridae/1043/genus-dependoparvovirus.
- Fields Viology, 6th ed.
- "ICTV 10th Report (2018)Erythroparvovirus". https://talk.ictvonline.org/ictv-reports/ictv_online_report/ssdna-viruses/w/parvoviridae/1044/genus-erythroparvovirus.
- "ICTV 10th Report (2018)Protoparvovirus". https://talk.ictvonline.org/ictv-reports/ictv_online_report/ssdna-viruses/w/parvoviridae/1045/genus-protoparvovirus.
- Parker, J. S; Murphy, W. J; Wang, D; O'Brien, S. J; Parrish, C. R (2001). "Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells". Journal of Virology 75 (8): 3896–3902. doi:10.1128/JVI.75.8.3896-3902.2001. PMID 11264378. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=114880
- Ross, S. R; Schofield, J. J; Farr, C. J; Bucan, M (2002). "Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus". Proceedings of the National Academy of Sciences of the United States of America 99 (19): 12386–90. doi:10.1073/pnas.192360099. PMID 12218182. Bibcode: 2002PNAS...9912386R. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=129454
- Hueffer, K; Parker, J. S; Weichert, W. S; Geisel, R. E; Sgro, J. Y; Parrish, C. R (2003). "The natural host range shift and subsequent evolution of canine parvovirus resulted from virus-specific binding to the canine transferrin receptor". Journal of Virology 77 (3): 1718–26. doi:10.1128/jvi.77.3.1718-1726.2003. PMID 12525605. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=140992
- Goodman, L. B; Lyi, S. M; Johnson, N. C; Cifuente, J. O; Hafenstein, S. L; Parrish, C. R (2010). "Binding site on the transferrin receptor for the parvovirus capsid and effects of altered affinity on cell uptake and infection". Journal of Virology 84 (10): 4969–78. doi:10.1128/jvi.02623-09. PMID 20200243. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2863798
- Harbison, C. E; Lyi, S. M; Weichert, W. S; Parrish, C. R (2009). "Early steps in cell infection by parvoviruses: host-specific differences in cell receptor binding but similar endosomal trafficking". Journal of Virology 83 (20): 10504–14. doi:10.1128/jvi.00295-09. PMID 19656887. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2753109
- Nelson, C. D; Minkkinen, E; Bergkvist, M; Hoelzer, K; Fisher, M; Bothner, B; Parrish, C. R (2008). "Detecting small changes and additional peptides in the canine parvovirus capsid structure". Journal of Virology 82 (21): 10397–407. doi:10.1128/jvi.00972-08. PMID 18701590. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2573191
- White, David O.; Fenner, Frank (1994). "17 Parvoviridae: Parvovirus B19". Medical Virology (4th ed.). Academic Press. pp. 288–291. ISBN 978-0-12-746642-2. https://books.google.com/books?id=_EMHrP_jqC0C&pg=PA288.