Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kayla Socarras | -- | 3431 | 2022-09-29 18:16:14 | | | |
| 2 | Beatrix Zheng | Meta information modification | 3431 | 2022-09-30 03:38:12 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 3431 | 2022-09-30 03:40:17 | | | | |
| 4 | Beatrix Zheng | Meta information modification | 3431 | 2022-09-30 03:40:59 | | | | |
| 5 | Beatrix Zheng | Meta information modification | 3431 | 2022-09-30 05:37:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Socarras, K.M.; Haslund-Gourley, B.S.; Cramer, N.A.; Comunale, M.A.; Marconi, R.T.; Ehrlich, G.D. Borreliaceae Diagnostics. Encyclopedia. Available online: https://encyclopedia.pub/entry/28069 (accessed on 07 February 2026).
Socarras KM, Haslund-Gourley BS, Cramer NA, Comunale MA, Marconi RT, Ehrlich GD. Borreliaceae Diagnostics. Encyclopedia. Available at: https://encyclopedia.pub/entry/28069. Accessed February 07, 2026.
Socarras, Kayla M., Benjamin S. Haslund-Gourley, Nicholas A. Cramer, Mary Ann Comunale, Richard T. Marconi, Garth D. Ehrlich. "Borreliaceae Diagnostics" Encyclopedia, https://encyclopedia.pub/entry/28069 (accessed February 07, 2026).
Socarras, K.M., Haslund-Gourley, B.S., Cramer, N.A., Comunale, M.A., Marconi, R.T., & Ehrlich, G.D. (2022, September 29). Borreliaceae Diagnostics. In Encyclopedia. https://encyclopedia.pub/entry/28069
Socarras, Kayla M., et al. "Borreliaceae Diagnostics." Encyclopedia. Web. 29 September, 2022.
Copy Citation
The acceleration of climate change has been associated with an alarming increase in the prevalence and geographic range of tick-borne diseases (TBD), many of which have severe and long-lasting effects—particularly when treatment is delayed principally due to inadequate diagnostics and lack of physician suspicion. Moreover, there is a paucity of treatment options for many TBDs that are complicated by diagnostic limitations for correctly identifying the offending pathogens.
tick-borne diseases
Lyme disease
borrelia
pangenomics
diagnostics
borreliaceae
1. Introduction
Even in this era of modern medicine, including mass vaccination achievements and antibiotic treatment regimens, vector-borne diseases such as Lyme borreliosis, Malaria, Dengue fever, yellow fever, and bubonic plague still prevail. This group of ancient and persistent diseases is transmitted to humans through the bite of infected arthropod vectors including mosquitoes, lice, flies, and ticks. These vectors acquire their associated pathogens through blood feedings from multiple hosts throughout complex life cycles. This life-cycle complexity leads to difficulties in diagnoses and treatments, creating a persistent healthcare burden that has resulted in a push to identify biomarkers for the development of improved diagnostics, vaccines, and therapeutics [1].
Research on these fronts has been divided unevenly across different vector species. While a great deal of research has been performed on diseases transmitted by mosquitoes, tick-borne diseases (TBD) are less studied. As their name implies, TBDs are primarily transmitted through the bite of infected ticks. While these small ectoparasitic arachnids have long been documented to cause disease, some as early as 1550 B.C., their true clinical significance was not realized until 1893, when a publication by Smith and Killborne linked Rhipicephalus annaltus to the transmission of the protist parasite Babesia bigemina in cattle [2][3][4][5][6]. Despite this finding, research on ticks and their corresponding TBDs progressed slowly until the latter half of the 20th century.
2. Borreliosis, the Most Common Type of Tick-Borne Disease
The emergence of chronic cutaneous, neurologic, arthritic, and cardiac maladies have been documented worldwide for hundreds of years [7][8][9][10][11][12][13]. In the US, these outbreaks have occurred in waves for centuries. Some of the earliest mentions were documented in Long Island during the early 1600s [14]. These cases were called ‘Montauk’s knees’, ‘Southhampton knee’, or water on the knee due to the arthritic-like symptoms [15]. In the latter half of the twentieth century, another wave occurred at Lyme, Connecticut in the beginning of the 1970s. In this small community, several children presented with numerous non-specific and arthritic-like symptoms. These ailments were later formally recognized as Lyme borreliosis and is commonly referred to as Lyme disease [8][9][10][11][12][13]. Over the years, Lb has continually increased in prevalence within the US and Canada and is now the most common tick-borne disease, accounting for ~500,000 new cases each year in the US alone—due in large part to climate change, deforestation, habitat loss, and loss of predators of the primary mammalian species upon which the ticks feed [16].
In the years that followed the discovery of Lb, its etiological agent, Borreliella burgdorferi, and other Borreliella spirochetes were found to be pathogenic to humans [17][18][19][20][21][22]. Some of these spirochetes do not, however, have the same vectors or pathogenesis. In this instance, other Borreliaceae members can cause Relapsing fever (Rf) and may be transmitted through a tick or louse vector. While these distinctions were made primarily on pathology and geo-locale of origin, later comparative genomic research suggested splitting the genus into two distinct disease-causing genera [23]. The Lb causing spirochetes were then given the new designation of Borreliella, while all other Borreliaceae spirochetes which cause Rf retained its original name of Borrelia.
Lb is a multi-systemic infectious disease with a wide and seemingly unconnected variety of conditions (e.g., polyarthralgias; neurological diseases, including polydysthesias/parathesias, cardiomyopathy, multiple sclerosis, other demylenating diseases, and ataxia; and psychiatric conditions, such as pediatric bipolar disorder and PANS and PANDAS) [24][25][26][27][28][29][30][31][32]. The sole pathognomonic presentation of Lb is erythema migrans, commonly known as the bulls-eye rash. Unfortunately, this presentation may not occur or is not visible to all individuals, occurring in approximately 50% of Lb cases [33][34][35][36]. Lb is divided into distinct stages: localized and disseminated. The disease presentations vary wildly among individuals, as well as by the species of Borreliella. This is illustrated most clearly with common Borreliella spirochetes, B. burgdorferi, B.azfelii, and B. garinii, each of which is endemic to the US or Europe. B. burgdorferi, the most common cause of Lb in the US, is primarily associated with arthritis, while B. afzelii is associated with cutaneous infections and B. garinii with neurological disease in Europe [35].
Due to the spectrum of non-specific symptoms for Lb, diagnoses are often difficult. Currently, clinicians rely on imprecise serological diagnostics and proof of tick-bite before accepting a Lb differential. While the above approaches may be useful in some instances, these current diagnostics have severe limitations, including a highly unreliable negative predictive value. To understand why these diagnostics may fail, it is critical to understand the basic biology of these spirochetes.
Previously, researchers have noted that Borreliellal spirochetes share many features ranging from their obligate parasitic nature within a large network of reservoir hosts, rather organisms that sustain spirochetes and facilitate their reproduction, to dynamic morphology that facilitates their near-constant host invasion [17][37]. Their unique morphology is thought to be created by 11 anti-parallel inter-membrane flagella and a chitobiose peptidoglycan [38][39]. Interestingly, this morphology has been documented to change in response to varying external stimuli [40][41][42][43][44]. It is, however, unclear what the mechanisms underlying the Borreliaceae morphological shifts are.
In addition to altering their morphology as a stress response, Borreliaceace spirochetes can manipulate their host’s immune and inflammatory response to their advantage. This is most clearly seen within I. scapularis ticks where Borreliella spp. reside within the tick midgut. These spirochetes are bound to the tick receptor for OspA (TROSPA) until the initiation of a blood meal [45]. Through feeding, the Borreliella dissociate from TROSPA, then switch their outer membrane surface protein (Osp) composition. The act of feeding induces tick salivary proteins to cover presenting Borreliella OspA and translocate to the tick salivary gland before peritrophic membrane formation [46][47]. Once in the salivary gland, the spirochete can then be transmitted into the new host dermis. During transmission, the Borreliella OspA in the outer membrane decreases and OspC rises [46]. For humans, Borreliella/Borrelia can be transmitted at varying rates depending on the tick species, tick feeding status, microbial strain, and microbial load, e.g., an I. scapularis tick can transmit B. burgdorferi within 24–48 h of initiating blood feeding [48][49][50].
Once Borrelieceae spirochetes have successfully entered the human body, they can persist within the dermis before disseminating. There are two proposed dissemination methods for Borreliella spirochetes: the hematogenous and non-hematogenous routes including the lymphatics or tissue [51]. In both dissemination routes, the spirochetes mitigate the host immune response to prevent recognition by the innate immune system and ultimately delay and distort the development of a T-cell-dependent B-cell response [52][53][54][55][56]. In addition, Borreliaceae spirochetes, can also evade the host immune system through various other means [57]. Both Borrelieceae can utilize the antigenic variation system, vls, present within the genome to evade the complement cascade. Additionally, Borreliella can achieve complement evasion by binding to Factor H, a negative regulator of host complement, to outer membrane proteins CspA, CspZ, and OspE [58][59][60]. Borreliella can also inhibit the classical complement pathway by binding C1r to outer membrane protein BBK32 [61][62]. Through antigenic switching of Borreliella outer membrane proteins, including hypervariable OspC and BBA70, the overall outer membrane composition and pathogenesis of the spirochete can be altered in situ [63][64]. Through these virulence mechanisms, it is believed that if Borrelieceae spirochetes are not successfully cleared by the immune system, they may colonize host tissues to form a persistent infection.
While Lyme borreliosis has become highly prevalent, the impact of elapsing fever (Rf) still remains a significant health concern. The RF-causing Borrelia genus can be transmitted either by ticks or lice around the world [57][65][66][67]. Within the United States, this infectious disease has remained endemic solely within western mountainous regions [67]. Regardless of geo-locale of origin, all variants of Rf have the same symptomology. The illness does have nonspecific symptoms like fatigue, headache, nausea, and muscle/joint aches [68][69]. Important diagnostically, however, is that uniquely induces periodic fever spikes associated with Borrelial septicemias. The fever dissipates during periods of time when there are decreased levels of Borrelia present within the blood but return on a cyclical basis over the course of weeks. Due to the elevated numbers of Borrelia within the blood, Rf is commonly diagnosed through microscopic examination of blood smears (Figure 1).
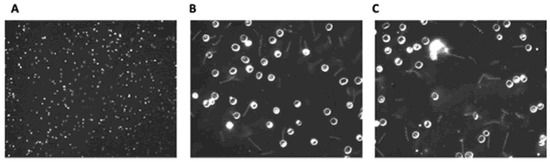
Figure 1. Dark field microscopic images of wet mount Borrelial spirochaetes (Borrelia hermsii strain DAH). Blood from C3H-Hej mice infected with B. hermsii was diluted 4-fold with phosphate buffered saline. (A): 100×, (B,C): 400×.
3. Borreliaceae Diagnostics
In the US, the cost of preventing and treating Lb has been estimated to range from $712 million to $1.3 billion per year, but this is likely a gross underestimate as many Lb patients go undiagnosed for years while seeking care for their ‘nonspecific’ symptoms [70]. Often, patients may pay out of pocket for additional diagnostic tests and treatments. In contrast, acute diagnostics for other common bacteria such as Streptococcus, Treponema pallidum, and Staphylococcus infections are accurate and lead to effective treatment before the bacteria can progress to later stages of infection [71][72][73][74][75]. Meanwhile, the economic impact of Lb infections continues to rise in large part due to an inadequate diagnosis. Thus, it is critical to develop and implement better diagnostics, prognostics, and therapeutics for borrelioses [76][77].
Many factors contribute to acute Lb diagnoses being missed. For example, persons with darker skin pigmentation will often not display a visible EM rash, others lack access to medical care or only have non-specific symptoms of acute Lb, while still others have a non-traditional EM rash that is not recognized during the acute phase of Lb [34][78][79]. If a patient suspected of acute Lb presents to healthcare providers, clinicians will assess risk factors for contracting Lyme borreliosis, including symptom presentation timing, geographic location, recent travel history, pet ownership, and history of other TBDs or rashes [80]. Laboratory-based tests are then utilized by clinicians to confirm a suspected acute case of Lyme borreliosis. Currently, there are several indirect and direct approaches to assist in diagnosing an individual with Lb. The Center for Disease Control and Prevention (CDC) recommends a two-tiered serological (ELISA and Western blot) system to confirm a suspected Lyme borreliosis case [81]. The two-tiered approach relies on a patient’s adaptive immune response towards transiently expressed surface proteins of Borreliella. Producing an IgG antibody response with strong avidity towards specific antigen targets takes between 2–3 weeks following infection [81]. Furthermore, most Lb western blots utilize B. burgdorferi sensu stricto strain B31 (Bb B31) as the source of the proteins utilized in their assay [82]. The B31 subtype was isolated over 30 years ago and the Bb B31 antigens do not represent other Borreliella antigens produced from other closely related Lb causing spirochetes [76][83]. Thus, patients who seroconvert during acute Lb infection could produce antibodies targeting antigens that are not included on the standard western blot. In addition, these serology-based diagnostic approaches cannot serve as prognostics to track treatment outcomes. This forces physicians to primarily rely on a patient’s symptoms to guide clinical outcomes or antibiotic treatment efficacy studies [84].
Serologic Lb diagnostics are further complicated by variation in the human adaptive immune response. If patients are diagnosed with Lb based on an EM rash and antibiotic treatment is promptly initiated, they might not seroconvert [85][86]. This fact further complicates the surveillance and confirmation of Lb. The lack of seroconversion could be due to Bb’s profound immunomodulatory and immunosuppressive effects which depend on the combination of host and pathogen genetics [55][87]. Accurate diagnosis of Lb is further complicated when a patient is co-infected with other Borreliellal spp. or additional tick-borne disease pathogens that are also commonly transferred from the tick’s mid-gut [76][88][89][90][91][92]. Taken together, the average sensitivity of the Lb two-tiered test for the acute Lb is less than 50% [82]. This poor sensitivity produces high rates of false-negatives and delays treatment which contributes to the development of chronic/late-stage Lb. During late-stage Lb, such as Lyme carditis or Lyme arthritis, a two-tiered test can confirm the diagnosis of the patient with high sensitivity. Unfortunately, patients in the later stages of Lb face permanent tissue damage and require longer antibiotic treatments [93][94].
Newer serologically-based diagnostics present recombinantly expressed surface proteins from multiple pathogens and strains of Borreliella [89][95]. These approaches increase the chance of detecting antibodies produced towards B. burgdorferi strains other than B31 or identifying co-infections. These methods, however, still have their limitations, as Bb is immunosuppressive and, depending on the infecting strain and host genetics, a significant percentage of infected persons will fail to appropriately produce antibodies.
Clinicians seeking diagnosis for suspected Lb patients may venture beyond CDC guidelines. Traditional pathologic assays such as dark-field microscopy and primary culture from blood or skin biopsies have poor sensitivity and are not employed as a reliable diagnostic for Lb, however they have a very high positive predictive value [96]. Attempting to culture or detect Borreliella spirochetes from human tissue biopsies using PCR methods (standard or quantitative) is invasive and insensitive [76][97][98][99][100][101]. These direct methods are limited by the low spirochete load in tissues and the bloodstream, unlike many other human bacterial pathologies [76][89][90][102][103][104]. Thus, patients and clinicians require alternative methods of acute Lb diagnosis.
In contrast to the limitations of above-mentioned PCR-based methods for detecting Borreliella DNA within humans, Next-Generation Sequencing (NGS) approaches can be highly specific. As Borreliella spirochetes are rarely present in the blood after initial disease onset, the challenge for NGS is to obtain enough of a sample to confidently detect genes associated with acute Lb bacteria [105][106]. Previously, many Borreliella NGS approaches targeted highly conserved genes throughout the genera such as the ribosomal 16S in ticks or human samples [107]. However, this approach was limited to identifying Lb within ticks rather than humans due to low titers of Borreliella in the bloodstream.
In a new NGS approach, the limitations of the sample sources may be circumvented by using patient urine [108]. While most NGS-based assays are limited by the low counts of Borreliella genomic material present in human samples such as blood, this approach aims to ensure a higher Borreliella DNA yield with claims that Lb bacteria infect the kidneys. While accuracy was stated to be ‘superior’ to the standard two-tiered testing approach, the sensitivity of the test has yet to be reported in the literature. This is slightly different than previous diagnostic iterations which used the same biosample but targeted solely OspA, a protein which would not be expressed in high quantities on the outersurface of Borreliaceae within a mammalian host [109].
Other efforts have been made to increase the sensitivity of Lb bloodborne detection. Traditional PCR-based diagnostics for Lyme borreliosis have been improved by isothermal amplification of DNA, followed by PCR amplification of Borreliellal DNA, which is then detected by electrospray ionization mass spectrometry (PCR/ESI-MSI). In PCR/ESI-MSI, it was possible to detect the presence of B. burgdorferi in 13 of 21 blood samples from patients with an acute Lb cases confirmed with positive serology and a history of at least one EM rash [104][105]. The assay required 1.25 mL of EDTA-treated whole blood and could detect 0.6 or greater copies of Borreliella genomes in whole blood.
In a follow-up study, the PCR/ESI-MSI method attempted to survey the presence of B. burgdorferi sensu stricto within four patients during their antibiotic treatment for Lyme borreliosis [110]. In this research, the investigators increased the blood volume from 1.25 mL to 20 mL, with the aim of increasing the diagnostic sensitivity. B. burgdorferi genes were detected in 2 of the 4 patients acutely infected with the aforementioned spirochete. In addition, they did not determine if the increased blood volume increased sensitivity. The genomic amplification approach relied on detecting and targeting conserved genes present within the Borreliella genome such as rpoC, FlaB, and OspC [111]. While such targets can indicate the presence of this spirochetal genus and may provide species-level resolution, there are some complications [110]. One such complication is that use of consistently expressed proteins like OspC may be insufficient due to the protein’s high diversity.
Another NGS diagnostic approach utilizes unbiased metagenomic cell-free DNA sequencing of human plasma. This cell-free DNA (cfDNA) approach was used to detect B. burgdorferi DNA from 64% (18 of 28) human plasma samples during acute Lb [112]. The cfDNA sequencing method’s sensitivity was further improved by combining the results of the modified two-tiered serology testing to identify 86% of acute Lyme borreliosis cases. Additionally, a recent NGS detection study identified core genes within the Borreliella pangenome to increase the sensitivity of DNA-based Borreliaceae diagnostics [113]. Taken together, genomic Borreliella detection methods have significantly improved over the last decade. However, more work is required to deliver a robust and sensitive diagnosis for patients and clinicians. One possibility is to use innate immune proteins that recognize specific PAMPs, such as Apolipoprotein H linked to paramagnetic beads, to ‘sweep’ a much larger volume of blood [114][115][116][117][118].
Next-generation sequencing has been used to detect host responses to acute Lb rather than attempting to directly detect the Borreliellal genome. Sequencing human T-cell receptors (TCRs) is a novel approach to Lyme disease diagnostics and began clinical trials in 2021 [119]. T-cells respond to Lb infection earlier than B-cells can produce antibodies, and thus the expansion of Borreliellal-specific T-cell receptor sequences in a patient’s circulating lymphocytes has the potential to confirm acute cases of Lyme disease earlier than traditional serologic methods [54]. This TCR immuno-sequencing assay differentiated acute Lb patients from healthy controls with a sensitivity of 54%, while the standard two-tiered serological testing approach had a sensitivity of only 30%. Clearly, human T-cell responses significantly vary between patients and this approach will not detect all acute Lyme disease cases. However, TCR immuno-sequencing assay’s increased sensitivity is a move in the right direction and has the potential to be combined with other diagnostic approaches to further increase sensitivity.
In addition to genomic detection, researchers have explored xenodiagnosis, metabolomics, and biomarker profiling [120][121][122]. Xenodiagnostics use an uninfected, natural vector for the isolation of the targeted pathogen from the infected host [123]. In the case of Borreliellal spirochetes, ticks facilitate the reacquisition of Borreliella from a variety of hosts during a 24-h feeding cycle [124][125]. This feature was noted in the past with Lyme-infected monkeys and mice but, was not substantiated in humans until 2014 [126][127][128]. Over the last 8 years, a clinical trial of tick-based recapture of Borreliellal pathogens from infected human hosts has been underway [123]. No results from this research have been released at this time. This approach could be further bolstered by applying NGS to characterize the pathogens recaptured after the tick feeds on the patient suspected of contracting Lyme borreliosis to increase diagnostic sensitivity. It is important to note, that while it could prove useful, much more work would be necessary to make it feasible as a diagnostic.
Metabolomic analyses of Lyme borreliosis patients has also made great strides in recent years. These studies have identified altered abundances of circulating metabolites produced by host tissues during Lyme borreliosis. A recent assay was able to discriminate between acute Lyme borreliosis and uninfected controls using their metabolic profiles [129][130]. Diagnostics relying on specific metabolic profiles are limited by the time and cost associated to prepare samples for analysis but offer yet another promising avenue for future Lyme borreliosis diagnostics.
Proteomic studies of serum collected from humans afflicted with Lb by Zhou et al. identified host acute phase protein abundance alterations during acute Lyme borreliosis [122]. The abundance of proteins—APOA4, C9, CRP, CST6, PGLYRP2, and S100A9—were validated using a second sample set of acute Lyme borreliosis samples and discriminated between healthy controls and acute Lb patients with a 78% sensitivity. Developing a multiplexed ELISA to identify acute-phase proteins associated with Lyme borreliosis could yield a high-throughput diagnostic, yet the issue of cross-reactivity with other infection markers must first be addressed. “Mimic diseases” such as rheumatoid arthritis or fibromyalgia often have similar acute phase protein alterations. Thus, the Lb proteomic study should be validated for Lb-specificity by testing against a panel of sera from patients with other mimic diseases [129].
Lastly, a glycoproteomic approach using MALDI-FT-ICR mass spectroscopy has been demonstrated to detect changes in the IgG N-glycan profile during acute Lyme disease with a sensitivity of 75% and specificity of 100%. Moreover, this assay can differentiate between acute Lb cases and patients who have received successful doxycycline treatment [131].
In summary, Borreliella diagnostics are improving, but have a long way to go as each has strengths, as well as limitations [82]. In this era of increasing TBDs, the best path forward may be to combine multiple diagnostics to complement the strengths of each method to construct a testing protocol that is highly sensitive and specific. In doing so, better measures can be taken to initiate early treatments and prevent chronic disease progression.
References
- Gubler, D.J. Arthropods in Disease Transmission. In Hunter’s Tropical Medicine and Emerging Infectious Disease; Saunders: Philadelphia, PA, USA, 2013.
- Anderson, J.F.; Magnarelli, L.A. Biology of Ticks. Infect. Dis. Clin. North Am. 2008, 22, 195–215.
- Anderson, J.F. The Natural History of Ticks. Med. Clin. North Am. 2002, 86, 205–218.
- Obenchain, F.D.; Galun, R. Physiology of Ticks: Current Themes in Tropical Science; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9781483162348.
- Smith, T.; Kilborne, F.L. Investigations into the Nature, Causation, and Prevention of Texas Or Southern Cattle Fever; U.S. Government Printing Office: Washington, DC, USA, 1893.
- Beard, C.B.; Eisen, L.; Eisen, R.J. The Rise of Ticks and Tickborne Diseases in the United States-Introduction. J. Med. Entomol. 2021, 58, 1487–1489.
- Steere, A.C.; Malawista, S.E.; Snydman, D.R.; Shope, R.E.; Andiman, W.A.; Ross, M.R.; Steele, F.M. Lyme arthritis: An epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 1977, 20, 7–17.
- Afzelius, A. Verhandlungen Der Dermatologischen Gesellschaft Zu Stockholm. Arch. Dermatol. Syph. 1910, 101, 404.
- Hellerstrom, S. Erythema Chronicum Migrans Afzelius with Meningitis. Acta Derm. Venereol. 1951, 31, 227–234.
- Garin, C.H.; Bujadoux, C.H. Paralysie Par Les Tiques. J. Med. Lyon 1922, 71, 7.
- Benach, J.; Garcia Monco, J. The Woldwide Saga of Lyme Borreliosis. Borrelia. Molecular Biology, Host Interaction and Pathogenesis; Caister Academic Press: Norwich, UK, 2010; pp. 7–26.
- Steere, A.C.; Malawista, S.E.; Hardin, J.A.; Ruddy, S.; Askenase, W.; Andiman, W.A. Erythema Chronicum Migrans and Lyme Arthritis. The Enlarging Clinical Spectrum. Ann. Intern. Med. 1977, 86, 685–698.
- Reik, L.; Steere, A.C.; Bartenhagen, N.H.; Shope, R.E.; Malawista, S.E. Neurologic Abnormalities of Lyme Disease. Medicine 1979, 58, 281–294.
- Walter, K.S.; Carpi, G.; Caccone, A.; Diuk-Wasser, M.A. Genomic Insights into the Ancient Spread of Lyme Disease across North America. Nat Ecol Evol 2017, 1, 1569–1576.
- Earnhart, C.G.; Marconi, R.T. Chapter 52—Lyme Disease. In Vaccines for Biodefense and Emerging and Neglected Diseases; Barrett, A.D.T., Stanberry, L.R., Eds.; Academic Press: London, UK, 2009; pp. 1031–1060. ISBN 9780123694089.
- Data and Surveillance Lyme Disease|CDC. Available online: https://www.cdc.gov/lyme/datasurveillance/index.html (accessed on 25 April 2019).
- Burgdorfer, W.; Barbour, A.G.; Hayes, S.F.; Benach, J.L.; Grunwaldt, E.; Davis, J.P. Lyme Disease—A Tick-Borne Spirochetosis? Science 1982, 216, 1317–1319.
- Dworkin, M.S.; Schwan, T.G.; Anderson, D.E., Jr.; Borchardt, S.M. Tick-Borne Relapsing Fever. Infect. Dis. Clin. North Am. 2008, 22, 449–468.
- Pritt, B.S.; Mead, P.S.; Johnson, D.K.H.; Neitzel, D.F.; Respicio-Kingry, L.B.; Davis, J.P.; Schiffman, E.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; et al. Identification of a Novel Pathogenic Borrelia Species Causing Lyme Borreliosis with Unusually High Spirochaetaemia: A Descriptive Study. Lancet Infect. Dis. 2016, 16, 556–564.
- Crowder, C.D.; Matthews, H.E.; Schutzer, S.; Rounds, M.A.; Luft, B.J.; Nolte, O.; Campbell, S.R.; Phillipson, C.A.; Li, F.; Sampath, R.; et al. Genotypic Variation and Mixtures of Lyme Borrelia in Ixodes Ticks from North America and Europe. PLoS ONE 2010, 5, e10650.
- Margos, G.; Vollmer, S.A.; Ogden, N.H.; Fish, D. Population Genetics, Taxonomy, Phylogeny and Evolution of Borrelia Burgdorferi Sensu Lato. Infect. Genet. Evol. 2011, 11, 1545–1563.
- Ras, N.M.; Lascola, B.; Postic, D.; Cutler, S.J.; Rodhain, F.; Baranton, G.; Raoult, D. Phylogenesis of Relapsing Fever Borrelia spp. Int. J. Syst. Bacteriol. 1996, 46, 859–865.
- Adeolu, M.; Gupta, R.S. A Phylogenomic and Molecular Marker Based Proposal for the Division of the Genus Borrelia into Two Genera: The Emended Genus Borrelia Containing Only the Members of the Relapsing Fever Borrelia, and the Genus Borreliella Gen. Nov. Containing the Members of the Lyme Disease Borrelia (Borrelia Burgdorferi Sensu Lato Complex). Antonie Van Leeuwenhoek 2014, 105, 1049–1072.
- Müllegger, R.R.; Glatz, M. Skin Manifestations of Lyme Borreliosis. Am. J. Clin. Dermatol. 2008, 9, 355–368.
- Verma, V.; Roman, M.; Shah, D.; Zaretskaya, M.; Yassin, M.H. A Case of Chronic Progressive Lyme Encephalitis as a Manifestation of Late Lyme Neuroborreliosis. Infect. Dis. Rep. 2014, 6, 5496.
- Brodziński, S.; Nasierowski, T. Psychosis in Borrelia Burgdorferi Infection—Part I: Epidemiology, Pathogenesis, Diagnosis and Treatment of Neuroborreliosis. Psychiatr. Pol. 2019, 53, 629–640.
- Arav-Boger, R.; Crawford, T.; Steere, A.C.; Halsey, N.A. Cerebellar Ataxia as the Presenting Manifestation of Lyme Disease. Pediatr. Infect. Dis. J. 2002, 21, 353–356.
- Rauer, S.; Kastenbauer, S.; Fingerle, V.; Hunfeld, K.-P.; Huppertz, H.-I.; Dersch, R. Lyme Neuroborreliosis. Dtsch. Arztebl. Int. 2018, 115, 751–756.
- Cardenas-de la Garza, J.A.; De la Cruz-Valadez, E.; Ocampo-Candiani, J.; Welsh, O. Clinical Spectrum of Lyme Disease. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 201–208.
- Cassarino, D.S.; Quezado, M.M.; Ghatak, N.R.; Duray, P.H. Lyme-Associated Parkinsonism: A Neuropathologic Case Study and Review of the Literature. Arch. Pathol. Lab. Med. 2003, 127, 1204–1206.
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme Borreliosis. Lancet 2012, 379, 461–473.
- Blanc, F.; Philippi, N.; Cretin, B.; Kleitz, C.; Berly, L.; Jung, B.; Kremer, S.; Namer, I.J.; Sellal, F.; Jaulhac, B.; et al. Lyme Neuroborreliosis and Dementia. J. Alzheimers. Dis. 2014, 41, 1087–1093.
- Steere, A.C.; Malawista, S.E.; Bartenhagen, N.H.; Spieler, P.N.; Newman, J.H.; Rahn, D.W.; Hutchinson, G.J.; Green, J.; Snydman, D.R.; Taylor, E. The Clinical Spectrum and Treatment of Lyme Disease. Yale J. Biol. Med. 1984, 57, 453–461.
- Fix, A.D.; Peña, C.A.; Strickland, G.T. Racial Differences in Reported Lyme Disease Incidence. Am. J. Epidemiol. 2000, 152, 756–759.
- Coburn, J.; Garcia, B.; Hu, L.T.; Jewett, M.W.; Kraiczy, P.; Norris, S.J.; Skare, J. Lyme Disease Pathogenesis. Curr. Issues Mol. Biol. 2020, 42, 473–518.
- Torbahn, G.; Hofmann, H.; Rücker, G.; Bischoff, K.; Freitag, M.H.; Dersch, R.; Fingerle, V.; Motschall, E.; Meerpohl, J.J.; Schmucker, C. Efficacy and Safety of Antibiotic Therapy in Early Cutaneous Lyme Borreliosis: A Network Meta-Analysis. JAMA Dermatol. 2018, 154, 1292–1303.
- Tilly, K.; Rosa, P.A.; Stewart, P.E. Biology of Infection with Borrelia Burgdorferi. Infect. Dis. Clin. North Am. 2008, 22, 217–234.
- DeHart, T.G.; Kushelman, M.R.; Hildreth, S.B.; Helm, R.F.; Jutras, B.L. The Unusual Cell Wall of the Lyme Disease Spirochaete Borrelia Burgdorferi Is Shaped by a Tick Sugar. Nat. Microbiol. 2021, 6, 1583–1592.
- Motaleb, M.A.; Corum, L.; Bono, J.L.; Elias, A.F.; Rosa, P.; Samuels, D.S.; Charon, N.W. Borrelia Burgdorferi Periplasmic Flagella Have Both Skeletal and Motility Functions. Proc. Natl. Acad. Sci. USA 2000, 97, 10899–10904.
- Sapi, E.; Bastian, S.L.; Mpoy, C.M.; Scott, S.; Rattelle, A.; Pabbati, N.; Poruri, A.; Burugu, D.; Theophilus, P.A.S.; Pham, T.V.; et al. Characterization of Biofilm Formation by Borrelia Burgdorferi in Vitro. PLoS ONE 2012, 7, e48277.
- Sapi, E.; Balasubramanian, K.; Poruri, A.; Maghsoudlou, J.S.; Socarras, K.M.; Timmaraju, A.V.; Filush, K.R.; Gupta, K.; Shaikh, S.; Theophilus, P.A.S.; et al. Evidence of In Vivo Existence of Borrelia Biofilm in Borrelial Lymphocytomas. Eur. J. Microbiol. Immunol. 2016, 6, 9–24.
- Timmaraju, V.A.; Theophilus, P.A.S.; Balasubramanian, K.; Shakih, S.; Luecke, D.F.; Sapi, E. Biofilm Formation by Borrelia Burgdorferi Sensu Lato. FEMS Microbiol. Lett. 2015, 362, fnv120.
- Shaikh, S.; Timmaraju, V.A.; Torres, J.P.; Socarras, K.M.; Theophilus, P.A.S.; Sapi, E. Influence of Tick and Mammalian Physiological Temperatures on Borrelia Burgdorferi Biofilms. Microbiology 2016, 162, 1984–1995.
- Rudenko, N.; Golovchenko, M.; Kybicova, K.; Vancova, M. Metamorphoses of Lyme Disease Spirochetes: Phenomenon of Borrelia Persisters. Parasit. Vectors 2019, 12, 237.
- Pal, U.; Li, X.; Wang, T.; Montgomery, R.R.; Ramamoorthi, N.; Desilva, A.M.; Bao, F.; Yang, X.; Pypaert, M.; Pradhan, D.; et al. TROSPA, an Ixodes Scapularis Receptor for Borrelia Burgdorferi. Cell 2004, 119, 457–468.
- Kurokawa, C.; Lynn, G.E.; Pedra, J.H.F.; Pal, U.; Narasimhan, S.; Fikrig, E. Interactions between Borrelia Burgdorferi and Ticks. Nat. Rev. Microbiol. 2020, 18, 587–600.
- Hovius, J.W.R.; van Dam, A.P.; Fikrig, E. Tick–host–pathogen Interactions in Lyme Borreliosis. Trends Parasitol. 2007, 23, 434–438.
- CDC Transmission. Available online: https://www.cdc.gov/lyme/transmission/index.html (accessed on 17 March 2022).
- Eisen, L. Pathogen Transmission in Relation to Duration of Attachment by Ixodes Scapularis Ticks. Ticks Tick Borne Dis. 2018, 9, 535–542.
- Cook, M.J. Lyme Borreliosis: A Review of Data on Transmission Time after Tick Attachment. Int. J. Gen. Med. 2015, 8, 1–8.
- Hyde, J.A. Borrelia Burgdorferi Keeps Moving and Carries on: A Review of Borrelial Dissemination and Invasion. Front. Immunol. 2017, 8, 114.
- Tracy, K.E.; Baumgarth, N. Borrelia Burgdorferi Manipulates Innate and Adaptive Immunity to Establish Persistence in Rodent Reservoir Hosts. Front. Immunol. 2017, 8, 116.
- Oosting, M.; Buffen, K.; van der Meer, J.W.M.; Netea, M.G.; Joosten, L.A.B. Innate Immunity Networks during Infection with Borrelia Burgdorferi. Crit. Rev. Microbiol. 2016, 42, 233–244.
- Bockenstedt, L.K.; Wooten, R.M.; Baumgarth, N. Immune response to Borrelia: Lessons from Lyme disease spirochetes. Curr. Issues Mol. Biol. 2021, 42, 145–190.
- Elsner, R.A.; Barthold, S.W.; Baumgarth, N. Delays and Diversions Mark the Development of B Cell Responses to Borrelia Burgdorferi Infection. J. Immunol. 2012, 188, 5612–5622.
- Elsner, R.A.; Hastey, C.J.; Baumgarth, N. CD4+ T Cells Promote Antibody Production but Not Sustained Affinity Maturation during Borrelia Burgdorferi Infection. Infect. Immun. 2015, 83, 48–56.
- Röttgerding, F.; Kraiczy, P. Immune Evasion Strategies of Relapsing Fever Spirochetes. Front. Immunol. 2020, 11, 1560.
- Hallström, T.; Siegel, C.; Mörgelin, M.; Kraiczy, P.; Skerka, C.; Zipfel, P.F. CspA from Borrelia Burgdorferi Inhibits the Terminal Complement Pathway. MBio 2013, 4, e00481-13.
- Hart, T.; Nguyen, N.T.T.; Nowak, N.A.; Zhang, F.; Linhardt, R.J.; Diuk-Wasser, M.; Ram, S.; Kraiczy, P.; Lin, Y.-P. Polymorphic Factor H-Binding Activity of CspA Protects Lyme Borreliae from the Host Complement in Feeding Ticks to Facilitate Tick-to-Host Transmission. PLoS Pathog. 2018, 14, e1007106.
- Lin, Y.-P.; Frye, A.M.; Nowak, T.A.; Kraiczy, P. New Insights Into CRASP-Mediated Complement Evasion in the Lyme Disease Enzootic Cycle. Front. Cell. Infect. Microbiol. 2020, 10, 1.
- Garcia, B.L.; Zhi, H.; Wager, B.; Höök, M.; Skare, J.T. Borrelia Burgdorferi BBK32 Inhibits the Classical Pathway by Blocking Activation of the C1 Complement Complex. PLoS Pathog. 2016, 12, e1005404.
- Kraiczy, P. Hide and Seek: How Lyme Disease Spirochetes Overcome Complement Attack. Front. Immunol. 2016, 7, 385.
- Locke, J.W. Complement Evasion in Borrelia Spirochetes: Mechanisms and Opportunities for Intervention. Antibiotics 2019, 8, 80.
- Koenigs, A.; Hammerschmidt, C.; Jutras, B.L.; Pogoryelov, D.; Barthel, D.; Skerka, C.; Kugelstadt, D.; Wallich, R.; Stevenson, B.; Zipfel, P.F.; et al. BBA70 of Borrelia Burgdorferi Is a Novel Plasminogen-Binding Protein. J. Biol. Chem. 2013, 288, 25229–25243.
- Talagrand-Reboul, E.; Boyer, P.H.; Bergström, S.; Vial, L.; Boulanger, N. Relapsing Fevers: Neglected Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2018, 8, 98.
- Cutler, S.J. Relapsing Fever—A Forgotten Disease Revealed. J. Appl. Microbiol. 2010, 108, 1115–1122.
- Forrester, J.D.; Kjemtrup, A.M.; Fritz, C.L.; Marsden-Haug, N.; Nichols, J.B.; Tengelsen, L.A.; Sowadsky, R.; DeBess, E.; Cieslak, P.R.; Weiss, J.; et al. Tickborne Relapsing Fever—United States, 1990–2011. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 58–60.
- CDC Symptoms. Available online: https://www.cdc.gov/relapsing-fever/symptoms/index.html (accessed on 10 February 2022).
- Lopez, J.; Hovius, J.W.; Bergström, S. Pathogenesis of Relapsing Fever. In Lyme Disease and Relapsing Fever Spirochetes: Genomics, Molecular Biology, Host Interactions and Disease Pathogenesis 2021; Caister Academic Press: Wymondham, UK, 2021.
- Adrion, E.R.; Aucott, J.; Lemke, K.W.; Weiner, J.P. Health Care Costs, Utilization and Patterns of Care Following Lyme Disease. PLoS ONE 2015, 10, e0116767.
- Pfoh, E.; Wessels, M.R.; Goldmann, D.; Lee, G.M. Burden and Economic Cost of Group A Streptococcal Pharyngitis. Pediatrics 2008, 121, 229–234.
- Luo, Y.; Xie, Y.; Xiao, Y. Laboratory Diagnostic Tools for Syphilis: Current Status and Future Prospects. Front. Cell. Infect. Microbiol. 2020, 10, 574806.
- Park, I.U.; Fakile, Y.F.; Chow, J.M.; Gustafson, K.J.; Jost, H.; Schapiro, J.M.; Novak-Weekley, S.; Tran, A.; Nomura, J.H.; Chen, V.; et al. Performance of Treponemal Tests for the Diagnosis of Syphilis. Clin. Infect. Dis. 2019, 68, 913–918.
- Worrall, G.; Hutchinson, J.; Sherman, G.; Griffiths, J. Diagnosing Streptococcal Sore Throat in Adults: Randomized Controlled Trial of in-Office Aids. Can. Fam. Phys. 2007, 53, 666–671.
- Tansarli, G.S.; LeBlanc, L.; Auld, D.B.; Chapin, K.C. Diagnostic Accuracy of Presurgical Staphylococcus Aureus PCR Assay Compared with Culture and Post-PCR Implementation Surgical Site Infection Rates. J. Mol. Diagn. 2020, 22, 1063–1069.
- Aguero-Rosenfeld, M.E.; Wang, G.; Schwartz, I.; Wormser, G.P. Diagnosis of Lyme Borreliosis. Clin. Microbiol. Rev. 2005, 18, 484–509.
- Schmidt, B.L. PCR in Laboratory Diagnosis of Human Borrelia Burgdorferi Infections. Clin. Microbiol. Rev. 1997, 10, 185–201.
- Aucott, J.; Morrison, C.; Munoz, B.; Rowe, P.C.; Schwarzwalder, A.; West, S.K. Diagnostic Challenges of Early Lyme Disease: Lessons from a Community Case Series. BMC Infect. Dis. 2009, 9, 79.
- Barrett, J. It’s about Lyme: Why Congress Must Enact Medical Insurance Coverage Laws for Lyme Disease Patients Now. Seattle U. L. Rev. SUpra 2022, 45, 50.
- St Pierre, S.E.; Gould, O.N.; Lloyd, V. Knowledge and Knowledge Needs about Lyme Disease among Occupational and Recreational Users of the Outdoors. Int. J. Environ. Res. Public Health 2020, 17, 355.
- Moore, A.; Nelson, C.; Molins, C.; Mead, P.; Schriefer, M. Current Guidelines, Common Clinical Pitfalls, and Future Directions for Laboratory Diagnosis of Lyme Disease, United States. Emerg. Infect. Dis. 2016, 22, 1167–1177.
- Waddell, L.A.; Greig, J.; Mascarenhas, M.; Harding, S.; Lindsay, R.; Ogden, N. The Accuracy of Diagnostic Tests for Lyme Disease in Humans, A Systematic Review and Meta-Analysis of North American Research. PLoS ONE 2016, 11, e0168613.
- Radolf, J.D.; Scott Samuels, D. Borrelia: Molecular Biology, Host Interaction and Pathogenesis; Horizon Scientific Press: Norwich, UK, 2010; ISBN 9781904455585.
- Marques, A.R. Laboratory Diagnosis of Lyme Disease: Advances and Challenges. Infect. Dis. Clin. North Am. 2015, 29, 295–307.
- Zagorac, G.B.; Kezele, T.G. Ceftriaxone and Doxycycline Induced Seroconversion in Previously Seronegative Patient with Clinically Suspected Disseminated Lyme Disease: Case Report. Infect Chemother. 2021, 53, 582–588.
- Coyle, P.K. Advances and Pitfalls in the Diagnosis of Lyme Disease. FEMS Immunol. Med. Microbiol. 1997, 19, 103–109.
- Anderson, C.; Brissette, C.A. The Brilliance of Borrelia: Mechanisms of Host Immune Evasion by Lyme Disease-Causing Spirochetes. Pathogens 2021, 10, 281.
- Wang, G.; Aguero-Rosenfeld, M.E.; Wormser, G.P. Detection of Borrelia Burgdorferi; Caister Academic Press: Norfolk, UK, 2010.
- Dumler, J. Molecular Diagnosis of Lyme Disease: Review and Meta-Analysis. Mol. Diagn. 2001, 6, 1–11.
- Singh, S.K.; Girschick, H.J. Lyme Borreliosis: From Infection to Autoimmunity. Clin. Microbiol. Infect. 2004, 10, 598–614.
- Norris, S.J.; Coburn, J.; Leong, J.M.; Hu, L.T.; Hook, M. Pathobiology of Lyme Disease Borrelia. In Borrelia: Molecular Biology, Host Interaction And Pathogenesis; Horizon Scientific Press: Norwich, UK, 2010; pp. 293–325.
- Barbour, A.G.; Guo, B.P. Pathogenesis of Relapsing Fever. Borrelia Mol. Biol. Host Interact. Pathogenesis 2010, 333–357.
- Dattwyler, R.J.; Wormser, G.P.; Rush, T.J.; Finkel, M.F.; Schoen, R.T.; Grunwaldt, E.; Franklin, M.; Hilton, E.; Bryant, G.L.; Agger, W.A.; et al. A Comparison of Two Treatment Regimens of Ceftriaxone in Late Lyme Disease. Wien. Klin. Wochenschr. 2005, 117, 393–397.
- Donta, S.T. What We Know and Don’t Know About Lyme Disease. Front Public Health 2021, 9, 819541.
- Tokarz, R.; Mishra, N.; Tagliafierro, T.; Sameroff, S.; Caciula, A.; Chauhan, L.; Patel, J.; Sullivan, E.; Gucwa, A.; Fallon, B.; et al. A Multiplex Serologic Platform for Diagnosis of Tick-Borne Diseases. Sci. Rep. 2018, 8, 3158.
- Perronne, C. Lyme and Associated Tick-Borne Diseases: Global Challenges in the Context of a Public Health Threat. Front. Cell. Infect. Microbiol. 2014, 4, 74.
- Maraspin, V.; Ogrinc, K.; Ružić-Sabljić, E.; Lotrič-Furlan, S.; Strle, F. Isolation of Borrelia Burgdorferi Sensu Lato from Blood of Adult Patients with Borrelial Lymphocytoma, Lyme Neuroborreliosis, Lyme Arthritis and Acrodermatitis Chronica Atrophicans. Infection 2011, 39, 35–40.
- Schmidli, J.; Hunziker, T.; Moesli, P.; Schaad, U.B. Cultivation of Borrelia Burgdorferi from Joint Fluid Three Months after Treatment of Facial Palsy due to Lyme Borreliosis. J. Infect. Dis. 1988, 158, 905–906.
- Stanek, G.; Klein, J.; Bittner, R.; Glogar, D. Isolation of Borrelia Burgdorferi from the Myocardium of a Patient with Long-Standing Cardiomyopathy. N. Engl. J. Med. 1990, 322, 249–252.
- Preac-Mursic, V.; Pfister, H.W.; Spiegel, H.; Burk, R.; Wilske, B.; Reinhardt, S.; Böhmer, R. First Isolation of Borrelia Burgdorferi from an Iris Biopsy. J. Clin. Neuroophthalmol. 1993, 13, 155–161, discussion 162.
- Chao, L.-L.; Chen, Y.-J.; Shih, C.-M. First Isolation and Molecular Identification of Borrelia Burgdorferi Sensu Stricto and Borrelia Afzelii from Skin Biopsies of Patients in Taiwan. Int. J. Infect. Dis. 2011, 15, e182–e187.
- Liveris, D.; Schwartz, I.; McKenna, D.; Nowakowski, J.; Nadelman, R.; DeMarco, J.; Iyer, R.; Bittker, S.; Cooper, D.; Holmgren, D.; et al. Comparison of Five Diagnostic Modalities for Direct Detection of Borrelia Burgdorferi in Patients with Early Lyme Disease. Diagn. Microbiol. Infect. Dis. 2012, 73, 243–245.
- Liveris, D.; Wang, G.; Girao, G.; Byrne, D.W.; Nowakowski, J.; McKenna, D.; Nadelman, R.; Wormser, G.P.; Schwartz, I. Quantitative Detection of Borrelia Burgdorferi in 2-Millimeter Skin Samples of Erythema Migrans Lesions: Correlation of Results with Clinical and Laboratory Findings. J. Clin. Microbiol. 2002, 40, 1249–1253.
- Eshoo, M.W.; Crowder, C.C.; Rebman, A.W.; Rounds, M.A.; Matthews, H.E.; Picuri, J.M.; Soloski, M.J.; Ecker, D.J.; Schutzer, S.E.; Aucott, J.N. Direct Molecular Detection and Genotyping of Borrelia Burgdorferi from Whole Blood of Patients with Early Lyme Disease. PLoS ONE 2012, 7, e36825.
- Mosel, M.R.; Aucott, J.; Schutzer, S.E.; Marques, A.; Arnaboldi, P.M.; Dattwyler, R.; Eshoo, M.W. Lyme Disease Diagnostics. In Lyme Disease and Relapsing Fever Spirochetes: Genomics, Molecular Biology, Host Interactions and Disease Pathogenesis 2021; Caister Academic Press: Wymondham, UK, 2021.
- Branda, J.A.; Steere, A.C. Laboratory Diagnosis of Lyme Borreliosis. Clin. Microbiol. Rev. 2021, 34, e00018-19.
- Lambert, J.S.; Cook, M.J.; Healy, J.E.; Murtagh, R.; Avramovic, G.; Lee, S.H. Metagenomic 16S rRNA Gene Sequencing Survey of Borrelia Species in Irish Samples of Ixodes Ricinus Ticks. PLoS ONE 2019, 14, e0209881.
- Schettig, R.; Tan-Lim, R.; Warren, D.; Poteet, Z.; Sears, R.; Hummel, M.; Coffin, C.; Quart, K.; Aussems, C.; Matthias, R., Jr.; et al. PathoDNA, an Advanced Diagnostic for Lyme Disease & Co-Infections Utilizing next Generation DNA Sequencing with Greater Sensitivity and Selectivity than ELISA/western Blot. Adv. Infect. Dis. 2021, 11, 405–429.
- Rauter, C.; Mueller, M.; Diterich, I.; Zeller, S.; Hassler, D.; Meergans, T.; Hartung, T. Critical Evaluation of Urine-Based PCR Assay for Diagnosis of Lyme Borreliosis. Clin. Diagn. Lab. Immunol. 2005, 12, 910–917.
- Mosel, M.R.; Carolan, H.E.; Rebman, A.W.; Castro, S.; Massire, C.; Ecker, D.J.; Soloski, M.J.; Aucott, J.N.; Eshoo, M.W. Molecular Testing of Serial Blood Specimens from Patients with Early Lyme Disease during Treatment Reveals Changing Coinfection with Mixtures of Borrelia Burgdorferi Genotypes. Antimicrob. Agents Chemother. 2019, 63, e00237-19.
- Eshoo, M.W.; Schutzer, S.E.; Crowder, C.D.; Carolan, H.E.; Ecker, D.J. Achieving Molecular Diagnostics for Lyme Disease. Expert Rev. Mol. Diagn. 2013, 13, 875–883.
- Branda, J.A.; Lemieux, J.E.; Blair, L.; Ahmed, A.A.; Hong, D.K.; Bercovici, S.; Blauwkamp, T.A.; Hollemon, D.; Ho, C.; Strle, K.; et al. Detection of Borrelia Burgdorferi Cell-Free DNA in Human Plasma Samples for Improved Diagnosis of Early Lyme Borreliosis. Clin. Infect. Dis. 2021, 73, e2355–e2361.
- Lee, S.H.; Healy, J.E.; Lambert, J.S. Single Core Genome Sequencing for Detection of Both Borrelia Burgdorferi Sensu Lato and Relapsing Fever Borrelia Species. Int. J. Environ. Res. Public Health 2019, 16, 1779.
- Stefas, I.; Dubois, G.; Tigrett, S.; Lucarz, E.; Veas, F. Apolipoprotein H, an acute phase protein, a performing tool for ultra-sensitive detection and isolation of microorganisms from different origins. In Acute Phase Proteins as Early Non-Specific Biomarkers of Human and Veterinary Diseases; Veas, F., Ed.; InTechOpen: London, UK, 2011; pp. 21–42.
- Lacout, A.; Mone, Y.; Franck, M.; Marcy, P.-Y.; Mas, M.; Veas, F.; Perronne, C. Blood Cell Disruption to Significantly Improve the Borrelia PCR Detection Sensitivity in Borreliosis in Humans. Med. Hypotheses 2018, 116, 1–3.
- Adlhoch, C.; Kaiser, M.; Hoehne, M.; Mas Marques, A.; Stefas, I.; Veas, F.; Ellerbrok, H. Highly Sensitive Detection of the Group A Rotavirus Using Apolipoprotein H-Coated ELISA Plates Compared to Quantitative Real-Time PCR. Virol. J. 2011, 8, 63.
- Stefas, I.; Rucheton, M.; D’Angeac, A.D.; Morel-Baccard, C.; Seigneurin, J.M.; Zarski, J.P.; Martin, M.; Cerutti, M.; Bossy, J.P.; Missé, D.; et al. Hepatitis B Virus Dane Particles Bind to Human Plasma Apolipoprotein H. Hepatology 2001, 33, 207–217.
- Stefas, E.; Rucheton, M.; Graafland, H.; Moynier, M.; Sompeyrac, C.; Bahraoui, E.M.; Veas, F. Human Plasmatic Apolipoprotein H Binds Human Immunodeficiency Virus Type 1 and Type 2 Proteins. AIDS Res. Hum. Retroviruses 1997, 13, 97–104.
- Greissl, J.; Pesesky, M.; Dalai, S.C.; Rebman, A.W.; Soloski, M.J.; Horn, E.J.; Dines, J.N.; Gittelman, R.M.; Snyder, T.M.; Emerson, R.O.; et al. Immunosequencing of the T-Cell Receptor Repertoire Reveals Signatures Specific for Diagnosis and Characterization of Early Lyme Disease. bioRxiv 2021.
- Hefter, Y.; D’Arco, C.; Shute, T.; Dattwyler, R.; Arnaboldi, P.; Nolan, S. 1760. Interferon Gamma Release Assay for Diagnosis of Lyme Disease. Open Forum Infect Dis 2018, 5, S61.
- Shan, J.; Jia, Y.; Teulières, L.; Patel, F.; Clokie, M.R.J. Targeting Multicopy Prophage Genes for the Increased Detection of Borrelia Burgdorferi Sensu Lato (s.l.), the Causative Agents of Lyme Disease, in Blood. Front. Microbiol. 2021, 12, 651217.
- Zhou, Y.; Qin, S.; Sun, M.; Tang, L.; Yan, X.; Kim, T.-K.; Caballero, J.; Glusman, G.; Brunkow, M.E.; Soloski, M.J.; et al. Measurement of Organ-Specific and Acute-Phase Blood Protein Levels in Early Lyme Disease. J. Proteome Res. 2020, 19, 346–359.
- Marques, A.; Telford, S.R., 3rd; Turk, S.-P.; Chung, E.; Williams, C.; Dardick, K.; Krause, P.J.; Brandeburg, C.; Crowder, C.D.; Carolan, H.E.; et al. Xenodiagnosis to Detect Borrelia Burgdorferi Infection: A First-in-Human Study. Clin. Infect. Dis. 2014, 58, 937–945.
- Murfin, K.E.; Kleinbard, R.; Aydin, M.; Salazar, S.A.; Fikrig, E. Borrelia Burgdorferi Chemotaxis toward Tick Protein Salp12 Contributes to Acquisition. Ticks Tick-Borne Dis. 2019, 10, 1124–1134.
- Shih, C.-M.; Chao, L.-L.; Yu, C.-P. Chemotactic Migration of the Lyme Disease Spirochete (Borrelia Burgdorferi) to Salivary Gland Extracts of Vector Ticks. Am. J. Trop. Med. Hyg. 2002, 66, 616–621.
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia Burgdorferi in Rhesus Macaques Following Antibiotic Treatment of Disseminated Infection. PLoS ONE 2012, 7, e29914.
- Embers, M.E.; Hasenkampf, N.R.; Jacobs, M.B.; Tardo, A.C.; Doyle-Meyers, L.A.; Philipp, M.T.; Hodzic, E. Variable Manifestations, Diverse Seroreactivity and Post-Treatment Persistence in Non-Human Primates Exposed to Borrelia Burgdorferi by Tick Feeding. PLoS ONE 2017, 12, e0189071.
- Bockenstedt, L.K.; Mao, J.; Hodzic, E.; Barthold, S.W.; Fish, D. Detection of Attenuated, Noninfectious Spirochetes inBorrelia burgdorferi–Infected Mice after Antibiotic Treatment. J. Infect. Dis. 2002, 186, 1430–1437.
- Molins, C.R.; Ashton, L.V.; Wormser, G.P.; Hess, A.M.; Delorey, M.J.; Mahapatra, S.; Schriefer, M.E.; Belisle, J.T. Development of a Metabolic Biosignature for Detection of Early Lyme Disease. Clin. Infect. Dis. 2015, 60, 1767–1775.
- Fitzgerald, B.L.; Molins, C.R.; Islam, M.N.; Graham, B.; Hove, P.R.; Wormser, G.P.; Hu, L.; Ashton, L.V.; Belisle, J.T. Host Metabolic Response in Early Lyme Disease. J. Proteome Res. 2020, 19, 610–623.
- Haslund-Gourley, B.S.; Grauzam, S.; Mehta, A.S.; Wigdahl, B.; Comunale, M.A. Acute lyme disease IgG N-linked glycans contrast the canonical inflammatory signature. Front. Immunol. 2022, 13, 949118.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
5 times
(View History)
Update Date:
30 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




