| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria C Jiménez-Martínez | + 2415 word(s) | 2415 | 2020-10-27 05:36:47 |
Video Upload Options
Ocular allergic diseases are frequently seen in ophthalmological clinical practice. Immunological damage is mediated by a local Th2 inflammatory microenvironment, accompanied by changes in circulating cell subsets, with more effector cells and fewer T regulatory cells (Tregs). This study aimed to evaluate the involvement of toll-like receptor 4 (TLR4) and a-melanocyte stimulating hormone (α-MSH) in the immune regulation associated with perennial allergic conjunctivitis (PAC). We performed an Ag-specific stimulation during 72 h of culturing with or without LPS or α-MSH in peripheral blood mononuclear cells (PBMC), analyzing the cell subsets and cytokines induced by the stimuli. We also determined α-MSH in tear samples from healthy donors (HD) or PAC patients. Our findings demonstrate an immunological dysregulation characterized by an increased frequency of CD4+TLR4+ in the PBMC of patients with PAC, compared to HD. Most of these CD4+TLR4+ cells were also CD25+, and when α-MSH was added to the culture, the percentage of CD4+CD25+FoxP3+ increased significantly, while the percentage of CD69+ cells and cytokines IL-4 and IL-6 were significantly decreased. In tears, we found an increased concentration of α-MSH in PAC patients, compared with HD. These findings indicate a novel mechanism involved in controlling ocular allergic diseases, in which α-MSH diminishes the concentration of IL-6 and IL-4, restoring the frequency of Tregs and down-regulating CD4 activation. Moreover, we demonstrate the involvement of CD4+TLR4+ cells, as an effector cell subset, in ocular allergy.
1. Introduction
The prevalence of allergic conjunctivitis (AC) varies from country to country, with rates between15% and 40% [1][2], and the most affected population is pediatric patients [3]. Clinical forms of AC include chronic inflammation of the ocular surface in atopic keratoconjunctivitis (AKC) and vernal keratoconjunctivitis (VKC). In contrast, in mild forms, the inflammation is mainly localized in the conjunctiva; it is persistent in perennial allergic conjunctivitis (PAC), and there are periods in which no damage is induced in seasonal allergic conjunctivitis (SAC) [1][4]. Immunological damage appears to be mediated by the activation of CD4+ T cell subsets [5] by environmental and ubiquitous allergens[6][7]. IL-4, IL-5, and IFN-γ are cytokines involved in ocular surface damage in chronic forms of allergy [8]. Interestingly, circulating CD4+T cells in patients with VKC produce IL-4, IL-5, and IFN-γ after Dermatophagoides pteronyssinus (Der p) stimulation [9]. By contrast, in the acute forms of AC, the cytokines released after Der p-stimulation are IL-5, IL-6, and-8, and their circulating CD4+T cells express CCR4 and CCR9, which are phenotypes of Th2 cells in transit (potential homing) to the conjunctiva [10]. Sensitization to Der p is clinically relevant in ocular allergy[7], and it is one of the most studied allergens in relation to the house dust mite (HDM) [11]. It has protease activity that is capable of disrupting the airway epithelial barrier, initiating the secretion of IL-25, IL-33, and TSLP by damaged cells. These soluble molecules promote Th2[10] differentiation and recruit eosinophils, basophils, and dendritic cells to the local airway[11][12]. In these models, damage to the epithelium increases the crossing of allergens across the epithelial barrier, favoring the activation of local CD4+T cells and the diminution of Treg activity. Remarkably, patients with PAC have diminished the frequency of circulating CD4+CD25+FoxP3+ (Tregs) cells[10]. The disruption of the epithelial barrier facilitates the recognition of pathogen patterns by innate receptors, such as toll-like receptors (TLR) [13]. TLR-4 has been well recognized in the development of asthma, with an increased expression on the stromal cells in the airways of a mouse model [14]. In line with this, TLR4 is increased in epithelial cells and co-expressed in conjunctiva-infiltrating CD4+T cells in patients with VKC [15]. After TLR4 recognizes lipopolysaccharide (LPS), it induces several intracellular signals leading to the secretion of inflammatory cytokines, such as TNF-α and IL-6 [13]. Notably, in a rat model of endotoxin-induced uveitis, alpha-MSH, a neuropeptide expressed the ocular level, suppressed LPS-induced inflammation[16], and in mouse-derived macrophages, alpha-MSH was able to suppress the activation of LPS-stimulated TLR4 [17]. These data are relevant because α-MSH has also been linked to the induction of Tregs in mice [18]. Nevertheless, neither the involvement of TLR4/α-MSH in the acute forms of ocular allergy nor their functional status in circulating CD4+T cells have been studied in patients with PAC yet. This work aims to evaluate the potential involvement of TLR4/α-MSH in CD4+T cells and their functional impact in the cells of patients with perennial allergic conjunctivitis.
2. Results
2.1. Increased Frequency of CD4+TLR4+T Cells in Peripheral Blood Mononuclear Cells in Patients with
Perennial Allergic Conjunctivitis
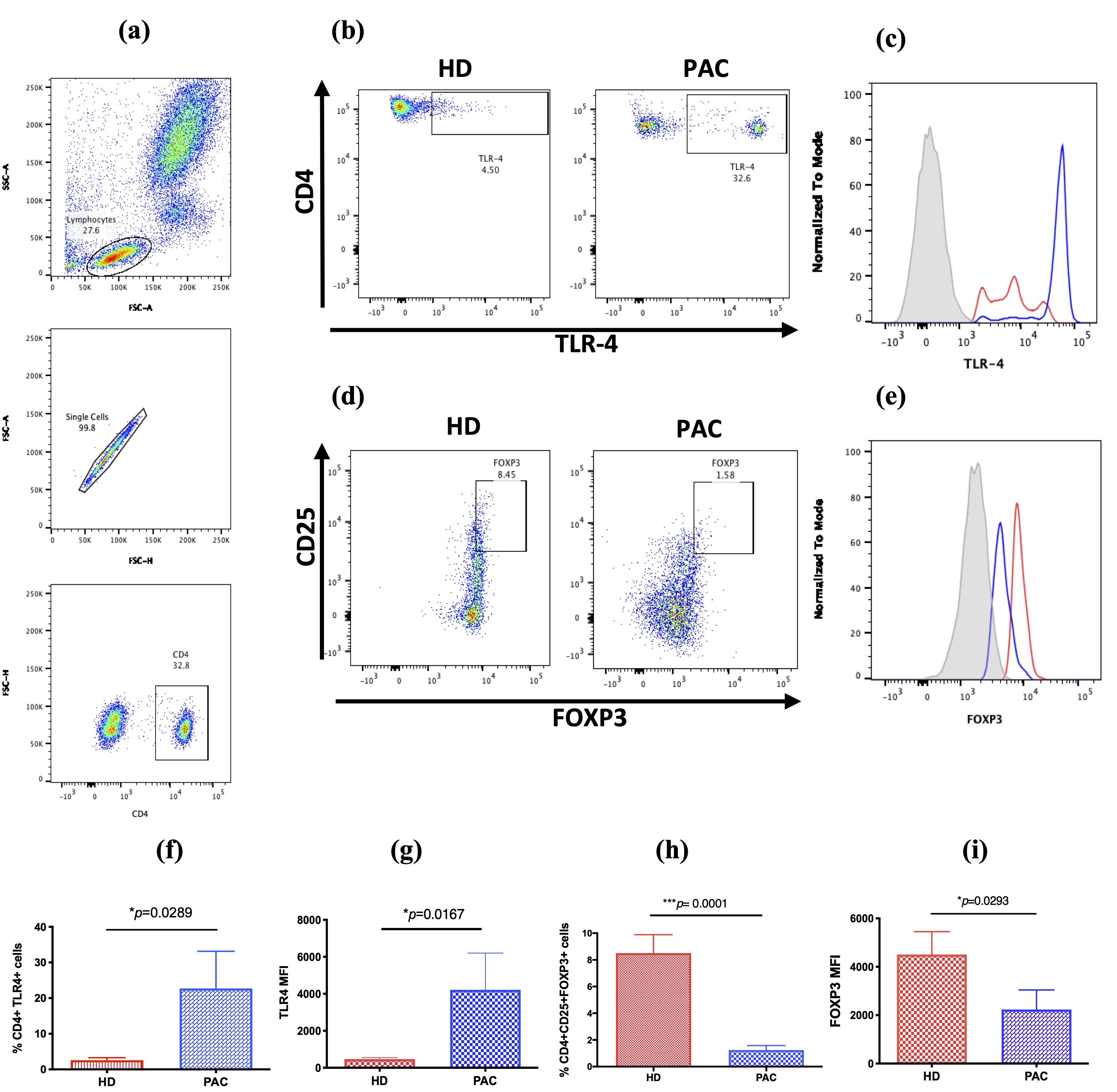
Figure 1. TLR4 expression in CD4+T cells and CD4+CD25+FOXP3+T reg cell subset. (a) Both subsets of cells were analyzed from the lymphocyte population through the selection of singlets and gathering of CD4+T cells; (b) representative dot plots, showing the frequency of CD4+TLR4+T cells in peripheral blood from patients diagnosed with PAC, compared to healthy donors (HD); (c) the TLR-4 expression in CD4 T cells is higher in PAC patients (blue line) than in HD (red line); (d) representative dot plots showing the frequency of CD4+CD25+FoxP3+ in PAC patients, compared with that in HD; (e)the FOXP3 expression in CD4 T cells is lower in PAC patients (blue line) than in HD (red line); (f) percentage of CD4+TLR4+ cells in the peripheral blood of HD and PAC patients; (g) the TLR4 expression in the CD4+T cells of HD and PAC patients; (h) the percentage of CD4+CD25+FOXP3+ cells in the peripheral blood mononuclear (PBMC) in HD, compared to that in PAC patients; (i) the FOXP3 expression in the CD4+CD25+ cells in the peripheral blood of HD and PAC patients. MFI, mean fluorescence intensity. *p≤0.005; **p≤0.01; ***p≤0.001.
2.2. CD4+TLR4+T Cells Are Induced after Der p Stimulation
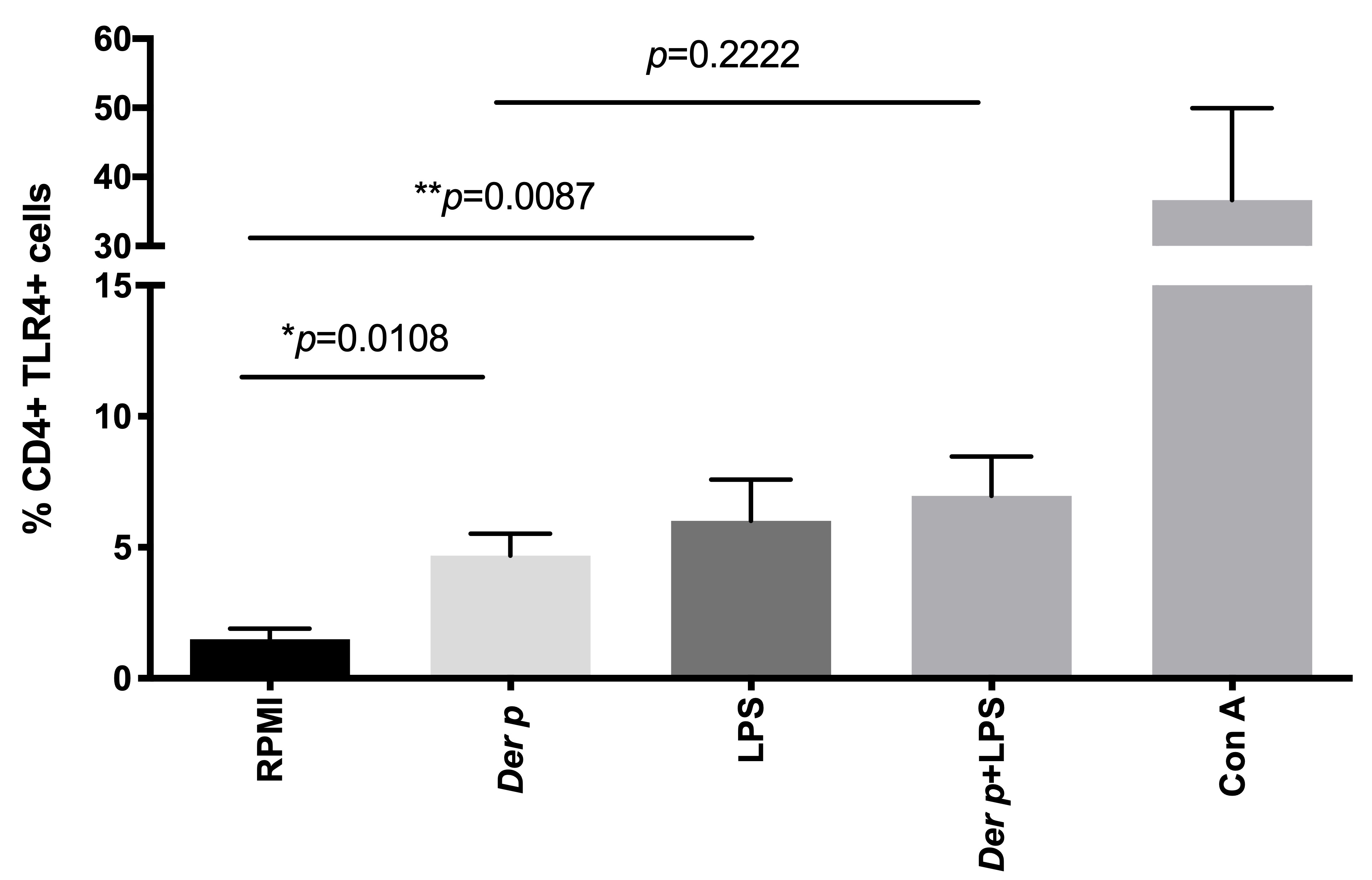
Figure 2. TLR4 expression in CD4+T cells. After 72 h of culturing with Der p and LPS, we observed an increased percentage of CD4+TLR4+ cells. *p≤0.005; **p≤0.01; ***p≤0.001.
2.3. α-MSH Induces Treg Cell Differentiation In Vitro from PBMC after Der p Stimulation
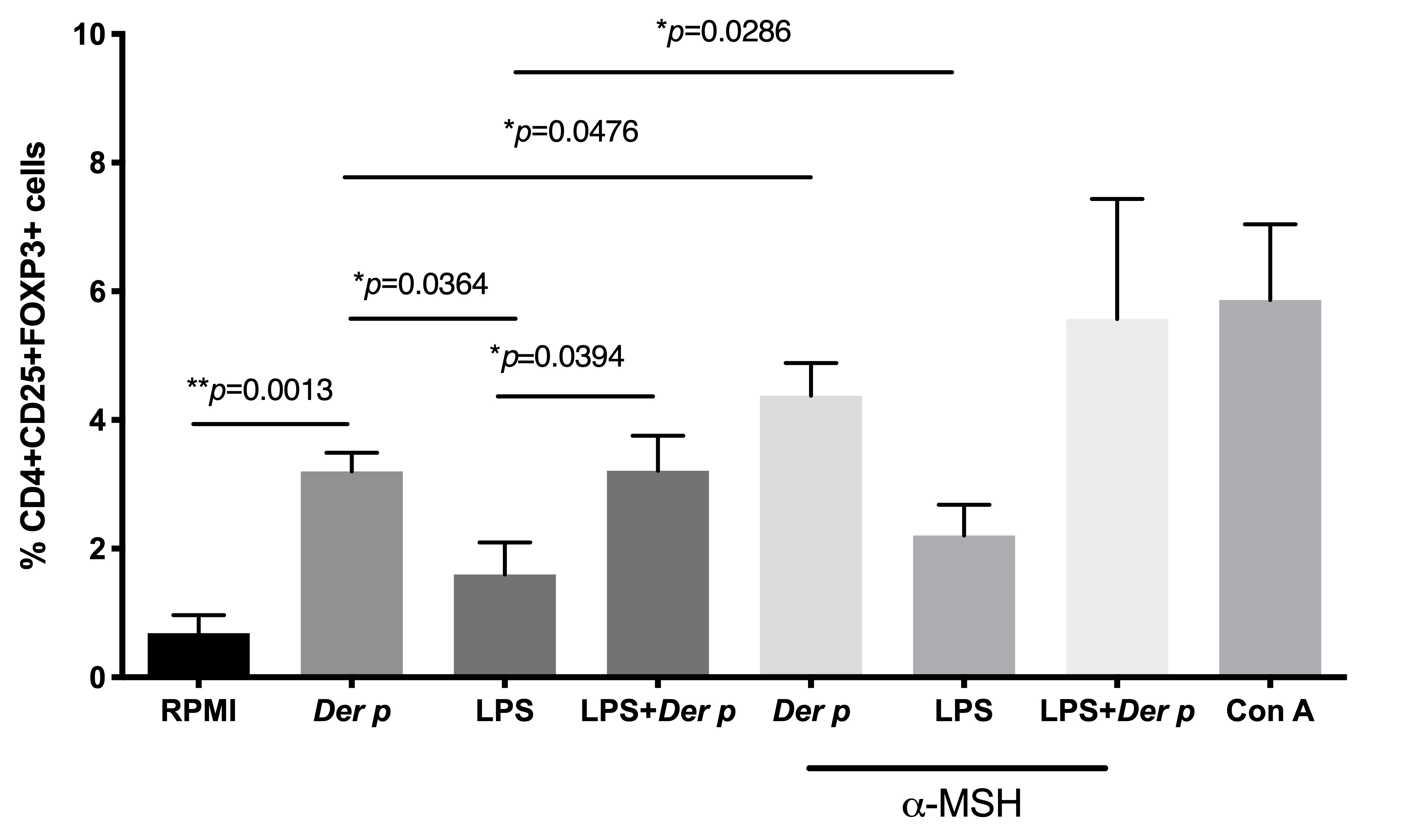
Figure 3. Frequency of T regulatory CD4+CD25+FOXP3+ cells in CD4+T cells after 72 h of culturing with Der p and LPS. *p≤0.005; **p≤0.01; ***p≤0.001.
2.4. α-MSH Inhibits the Expression of CD69 in CD4+T Cells and Decreases IL-4 and IL-6 after Der p Stimulation
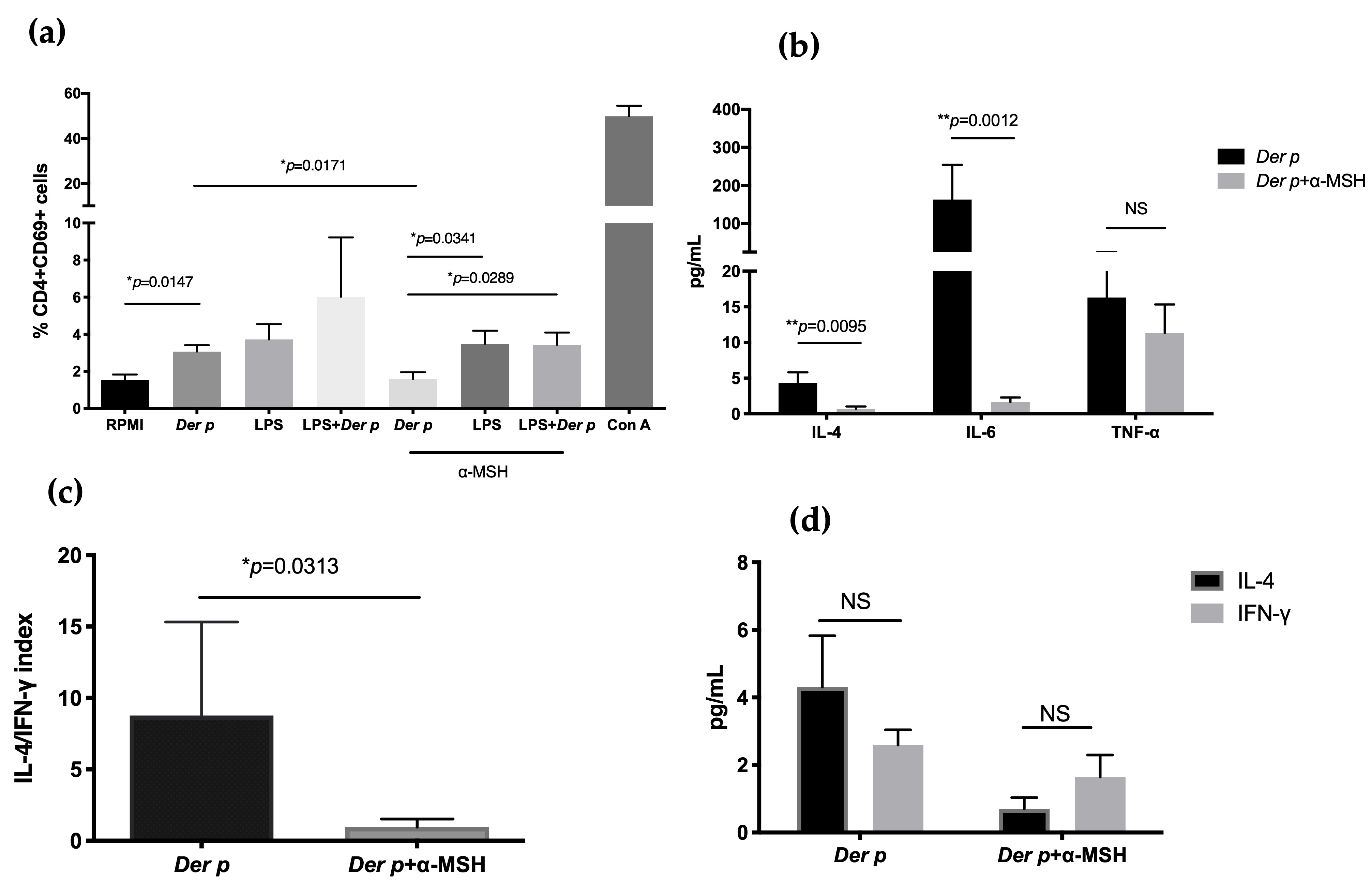
Figure 4. Expression of CD69 in CD4+T cells and decreases in IL-4 and IL-6 after Der p stimulation. (a) CD69 expression in CD4+T cells after 72 h of culturing with Der p, LPS, and α-MSH; (b) IL-4, IL-6, and TNF-α in supernatant after 72 h of culturing with Der p, LPS, and α-MSH; (c) IL-4/IFN-γ index; (d) IL-4 and IFN-γ comparison after culturing with Der p and α-MSH. *p≤0.005; **p≤0.01; ***p≤0.001.
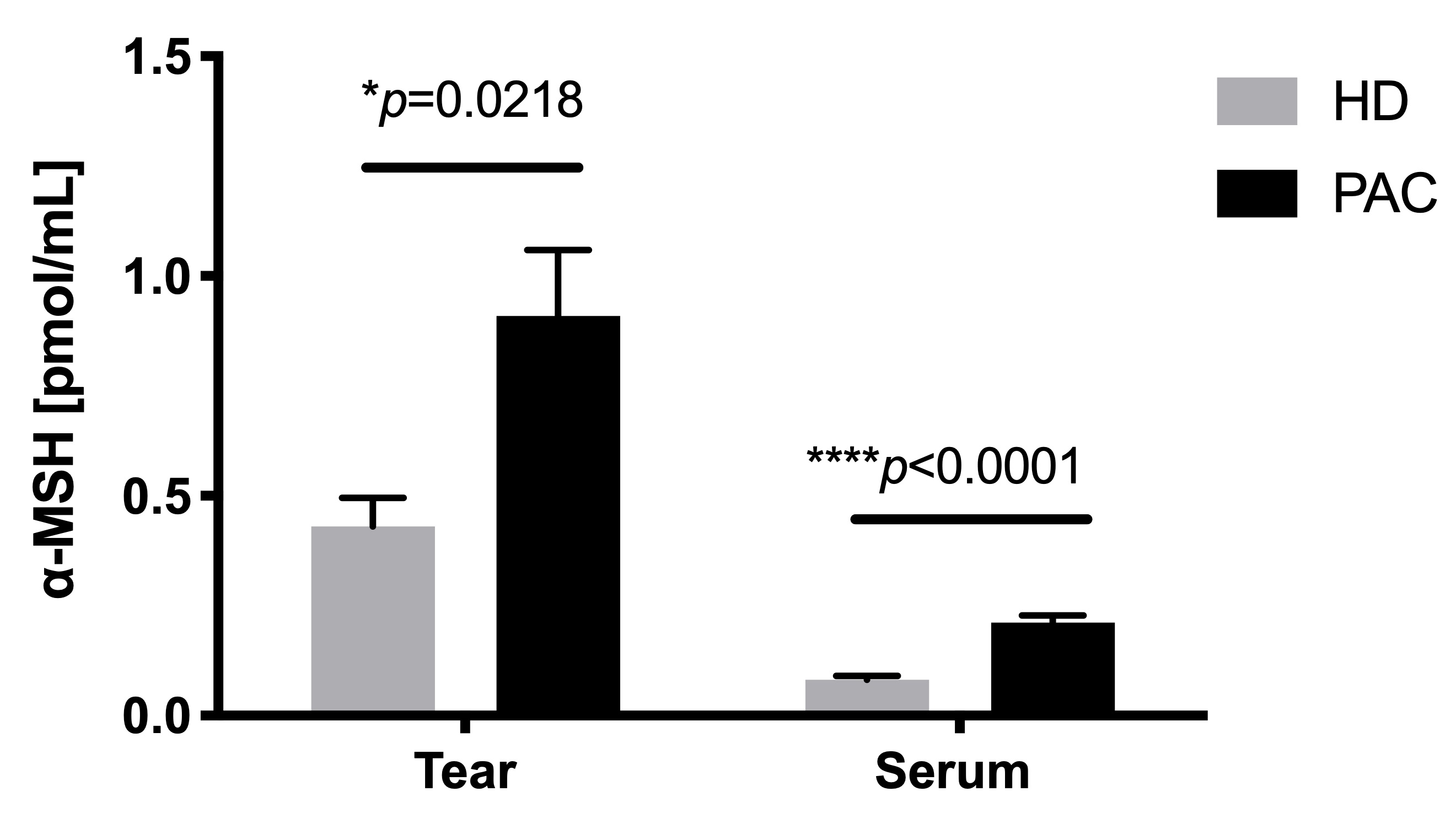
2.5. Increased Concentration of α-MSH in the Tears of Patients with Allergic Conjunctivitis
Figure 5. Concentration of α-MSH in the tears of patients with allergic conjunctivitis. α-MSH was measured through an ELISA sandwich of tear and serum samples from healthy donors (HD) and perennial allergic conjunctivitis (PAC) patients. *p≤0.005; **p≤0.01; ***p≤0.001.
3. Discussion
Conjunctival inflammation in acute forms of ocular allergy has increased CCR4+CCR9+ effector T cells, with a diminished frequency of CD4+CD25+FOXP3+ regulatory T cells in peripheral blood [10]. Bonini et al. [15] reported an infiltration of CD4+TLR4+ cells in the conjunctiva of VKC patients, suggesting a significant role of these cells in the chronic forms of AC. Interestingly, Taylor et al.[17] reported, in the macrophage cell line, J774, and adherent spleen macrophages of mice, that alpha-MSH was able to inhibit LPS-stimulated cells. Thus, this study aimed to evaluate the potential involvement of TLR4/α-MSH in CD4-activated cells in patients with perennial allergic conjunctivitis.
In this work, we observed an increased expression of TLR4 in the CD4 T+ cells of patients with perennial allergic conjunctivitis (PAC). TLR4 is a pattern-recognition receptor (PRR) that recognizes bacterial LPS and the Der p2 allergen [19]. Der p2 is a major allergen found in Dermatophagoides pteronyssinus (Der p) [20]. Stimulation of TLR4 trough Der p2 induces IgE secretion and an inflammatory response characterized by eosinophils and lymphocytes in experimental allergic asthma [20]. The function of human CD4+TLR4+T cells has been studied by other authors, showing that LPS stimulation increases cell adhesion to fibronectin by CD4+T cells [21]. Remarkably, when we stimulated PBMC in patients with perennial allergic conjunctivitis with Der p, we observed that TLR4 and CD69 were increased in CD4+T cells, suggesting that allergen-specific stimulation also induces TLR4. Remarkably, when we stimulated the PBMC of patients with perennial allergic conjunctivitis with Der p, we observed an increased TLR4 and CD69 in CD4+T cells, suggesting that allergen-specific stimulation also induces TLR4. In line with our results, Sahoo et al. showed, in a mouse model, that the neutralization of TLR4 in splenic T cells using a peptide antagonist and stimulating with anti-CD3 and anti-CD28 decreased the CD69 expression, suggesting a role of TLR4 in acute effector responses induced by T cell receptor (TCR) [22]. On the other hand, TLR4 signalization on mice dendritic cells inhibits Treg cell differentiation through IL-6 lacking immune regulation [23][24]. Further studies isolating CD4+TLR4+ cells from blood samples from allergic conjunctivitis patients and stimulating them with Der p are needed to determine if CD4+TLR4+ cells have a role in the induction of the IL-6 pro-inflammatory microenvironment and the inhibition of Treg cells.
Treg cell differentiation is directed by the recognition of self-antigen-MHC complexes in the thymus [25]and in the periphery through the cytokines, TGF-β and IL-2, and TCR-Ag-MHC [26]. The implication of other soluble molecules, such as α-melanocyte-stimulating hormone (α-MSH), has been linked to the induction of the Treg differentiation in a mouse model [18]. In this work, we observed that the addition of α-MSH to Der p-stimulated cells induced CD4+CD25+FOXP3+ cells and also diminished the CD69 expression in CD4+T cells. CD69 is an early activation marker expressed transiently in lymphocytes, and its function is related to proinflammation, engaging blocking antibodies against CD69, which inhibits the ability of T cells to activate macrophages by contact and diminish the activation of effector T cells [27]. Similar to us, Fang et al. showed that SVα-MSH, an analog of α-MSH, down-regulated the CD69 expression in autoreactive cells and induced CD4+CD25+FOXP3+T cells in a mouse model of autoimmune encephalomyelitis [28]. On the one hand, in a mouse model of allergic airway inflammation, α-MSH decreases the production of IL-4 and IL-13 from a bronchoalveolar lavage [29]. In this work, we observed the down-regulation of IL-4 and IL-6 when α-MSH was added to cells stimulated with Der p. Our results suggest that α-MSH induces Treg-differentiation and also diminishes the Th2 inflammatory microenvironment. We did not observe IL-10 or TGF-β secretion, supporting that α-MSH suppression could be cell-dependent and lead to the differentiation of effector or regulatory activity. To understand the differentiation of Treg cells induced by α-MSH in allergic conjunctivitis, cAMP, CREB, and ERK need to be explored, which will lead to the uncovering of the mechanism involved in the regulatory activity induced by α-MSH.
Although we used high LPS doses, we observed effector Th2 responses in cultured cells from PAC patients. High LPS concentrations induce an IL-10 enriched microenvironment [30]; thus, once differentiated CD4+TLR4+ cells are committed with effector functions activating a Th2 inflammatory response independently of LPS concentration. Similarly, the α-MSH dose evaluated in this work was not at physiological concentration but was able to down-regulate the activation of effector cells. Hence, the results presented here show a biological phenomenon observed in cells isolated from allergic patients that could be used as a starting point to evaluate the pharmacological development of immune-modulators based in α-MSH to regulate the allergic process.
α-MSH is a neuropeptide produced by the hypothalamus, keratinocytes, and lachrymal glands[31]. At the ocular level, α-MSH is involved in the homeostasis of the ocular surface in a mouse model of dry eye [32]. Previous studies have demonstrated higher concentrations of neuropeptides in ocular allergic reactions, including substance P (SP), calcitonin gene-related peptide (CGRP), and vasoactive intestinal peptide (VIP), after allergenic challenges, suggesting their participation as inflammatory mediators. Nevertheless, the involvement of α-MSH has not been fully explored in relation to allergic conjunctivitis. Kleiner et al. showed that the basophils from allergic rhinitis patients decreased CD203 after stimulation with α-MSH basophils and allergens or anti-IgE, suggesting that α-MSH suppressed the proinflammatory effector function in human basophils [33]. In our study, PAC patients showed an increased α-MSH concentration in tears and serum, compared with healthy controls. It is well known that proinflammatory cytokines induce α-MSH as a neuroendocrine axis in order to down-regulate inflammation[31]. Thus, it would be necessary to determine the expression of the melanocortin receptors in T cells and ocular surface cells, since it is possible that these cells may not respond to α-MSH or lack immune regulation, despite the fact that α-MSH is increased.
Schirmer’s test without anesthesia measures both basal and reflex tearing, and tear break up time measures the tear film stability of the ocular surface, thus providing a functional measure of mucin, aqueous, and lipid layer. Tearing, itching, foreign body sensation, and photophobia are all common symptoms of allergic conjunctivitis in which inflammation and mechanical irritation alter the density and function of goblet cells and accessory lacrimal glands. Long-term consequences of allergic conjunctivitis may result in fibrotic changes in the ocular surface, thus significantly altering Schirmer´s test and TBUT. However, in the acute stages, Schirmer´s test may be greater than expected due to increased sensibility of the ocular surface, and TBUT may also be altered even in young allergic patients. [34] Although mostly used in dry eye syndrome, both tests are clinically relevant to explore the function of the ocular surface unit, together with the clinical examination under the biomicroscope. Meibomian glands produce the lipids that form the surface lipid layer, [35] and secretion of meibum could be influenced by the melanocortin 5 receptor (MCR5) expressed on gland cells. [36][37] Furthermore, α-MSH down-regulates MUC5AC in cultured epithelial cells from the nasal mucosa through TNF-α inhibition; [38] thus, anti-inflammatory actions of α-MSH could be contributing to change the tear stability. Whether tear α-MSH is a local compensation mechanism to increase the meibomian gland’s function or is a neuroendocrine-immune altered pathway contributing to changes in the mucin secretion at the ocular surface is not known and needs further investigation.
4. Conclusions
Our results suggest a novel mechanism involved in controlling ocular allergic diseases in which α-MSH diminishes the concentration of IL-6 and IL-4, restoring the frequency of Tregs and down-regulating the CD4 activation. We also demonstrated the involvement of CD4+TLR4+ cells as an effector cell subset in ocular allergy.
The entry is from 10.3390/ijms21217861
References
- Miraldi Utz, V.; Kaufman, A.R. Allergic eye disease. Pediatr. Clin. N. Am. 2014, 6, 607–620.
- Gomes, P.J. Trends in prevalence and treatment of ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 451–456.
- Calderon-Ezquerro, M.C.; Guerrero-Guerra, C.; Galán, C.; Serrano-Silva, N.; Guidos-Fogelbach, G.; Jiménez-Martínez, M.C.; Larenas-Linnemann, D.; López-Espinosa, E.D.; Ayala-Balboa, J. Pollen in the atmosphere of Mexico City and its impact on the health of the pediatric population. Atmos. Envrion. 2018, 186, 198–208.
- Rosario, N.; Bielory, L. Epidemiology of allergic conjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 471-476.
- Reyes, N.; Saban, D. T helper Subsets in Allergic Eye Disease. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 477–484.
- Shaker, M.; Salcone, E. An update on ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 505–510.
- Segundo, G.R.; Sopelete, M.C.; Terra, S.A.; Pereira, F.L.; Justino, C.M.; Silva, D.A.; Taketomi, E.A. Diversity of allergen exposure: Implications for the efficacy of environmental control. Braz. J. Otorhinolaryngol. 2009, 75, 311–316.
- Leonardi, A.; Motterle, L.; Bortolotti, M. Allergy and the eye. Clin. Exp. Immunol. 2008, 153 (Suppl. 1), 17 21.
- Magaña, D.; Aguilar, G.; Linares, M.; Ayala-Balboa, J.; Santacruz, C.; Chávez, R.; Estrada-Parra, S.; Garfias, Y.; Lascurain, R.; Jiménez-Martínez, M.C. Intracellular IL-4, IL-5 and IFN-g as the main characteristic of CD4+CD30+T cells after allergen-stimulation, in patients with vernal keratoconjunctivitis. Mol. Vis. 2015, 21, 443–450.
- Galicia-Carreón, J.; Santacruz, C.; Ayala-Balboa, J.; Robles-Contreras, A.; Perez-Tapia, S.M.; Garfias, Y.; Hong, E.; Jiménez-Martínez, M.C. An imbalance between frequency of CD4+CD25+FOXP3+ regulatory T cells and CCR4+ and CCR9+ circulating helper T cells is associated with active perennial allergic conjunctivitis. Clin. Dev. Immunol. 2013, 2013, 919742.
- Reithofer, M.; Jahn-Schmid, B. Allergens with Protease Activity from House Dust Mites. Int. J. Mol. Sci. 2017, 18, 1368.
- Shakib, F.; Ghaemmaghami, A.M.; Sewell, H.F. The molecular basis of allergenicity. Trends Immunol. 2008, 29, 633–642.
- Redfern, R.L.; McDermott, A.M. Toll-like receptors in ocular surface disease. Exp. Eye Res. 2010, 90, 679–687.
- Perros, F.; Lambrecht, B.N.; Hammad, H. TLR4 signalling in pulmonary stromal cells is critical for inflammation and immunity in the airways. Respir. Res. 2011, 12, 125
- Bonini, S.; Micera, A.; Iovieno, A.; Lambiase, A.; Bonini, S. Expression of Toll-like receptors in healthy and allergic conjunctiva. Ophthalmology 2005, 112, 1528, discussion 1548–1549
- Nishida, T.; Miyata, S.; Itoh, Y.; Mizuki, N.; Ohgami, K.; Shiratori, K.; Ilieva, I.B.; Ohno, S.; Taylor, A.W. Anti-inflammatory effects of alpha-melanocyte-stimulating hormone against rat endotoxin-induced uveitis and the time course of inflammatory agents in aqueous humor. Int. Immunopharmacol. 2004, 4, 1059–1066.
- Taylor, A.W. The immunomodulating neuropeptide alpha-melanocyte-stimulating hormone (alpha-MSH) suppresses LPS-stimulated TLR4 with IRAK-M in macrophages. J. Neuroimmunol. 2005, 162, 43–50.
- Taylor, A.; Namba, K. In vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH). Immunol. Cell Biol. 2001, 79, 358–367.
- Trompette, A.; Divanovic, S.; Visintin, A.; Blanchard, C.; Hegde, R.S.; Madan, R.; Thorne, P.S.; Wills-Karp, M.; Gioannini, T.L.; Weiss, J.P.; et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 2009, 457, 585–588.
- Thomas, W.R.; Hales, B.J.; Smith, W.-A. House dust mite allergens in asthma and allergy. Trends Mol. Med. 2010, 16, 321–328.
- Zanin-Zhorov, A.; Tal-Lapidot, G.; Cahalon, L.; Cohen-Sfady, M.; Pevsner-Fischer, M.; Lider, O.; Cohen, I.R. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J. Immunol. 2007, 179, 41–44.
- Sahoo, S.S.; Pratheek, B.M.; Meena, V.S.; Nayak, T.K.; Kumar, P.S.; Bandyopadhyay, S.; Maiti, P.K.; Chattopadhyay, S. VIPER regulates naive T cell activation and eff ector responses: Implication in TLR4 associated acute stage T cell responses. Sci. Rep. 2018, 8, 7118.
- Jung, M.K.; Jeong-Eun, K.; Eui-Cheol, S. IL-17A-producing Foxp3+ regulatory T cells and human diseases. Immune Netw. 2017, 17, 276–286.
- Pasare, C.; Ruslan, M. Toll pathway-dependent blockade of CD4+ CD25+T cell-mediated suppression by dendritic cells. Science 2003, 299, 1033–1036.
- Hsieh, C.S.; Liang, Y.; Tyznik, A.J.; Self, S.G.; Liggitt, D.; Rudensky, A.Y. Recognition of the peripheral self by naturally arising CD25+CD4+T cell receptors. Immunity 2004, 21, 267–277.
- La Cava, A. Tregs are regulated by cytokines: Implications for autoimmunity. Autoimmun. Rev. 2008, 8, 83–87.
- Sancho, D.; Gómez, M.; Sánchez-Madrid, F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005, 26, 136–140.
- Fang, J.; Han, D.; Hong, J.; Zhang, H.; Ying, Y.; Tian, Y.; Zhang, L.; Lin, J. SV α-MSH, a novel α-melanocyte stimulating hormone analog ameliorates autoimmune encephalomyelitis through inhibiting autoreactive CD4+T cells activation. J. Neuroimmunol. 2014, 269, 9–19.
- Raap, U.; Brzoska, T.; Sohl, S.; Päth, G.; Emmel, J.; Herz, U.; Braun, A.; Luger, T.; Renz, H. α-Melanocyte-stimulating hormone inhibits allergic airway inflammation. J. Immunol. 2003, 171, 353–359.
- Xu, H.; Liew, L.N.; Kuo, I.C.; Huang, C.H.; Goh, D.L.M.; Chua, K.Y. The modulatory effects of lipopolysaccharide- stimulated B cells on differential T-cell polarization. Immunology 2008, 125, 218–228
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020
- Ru, Y.; Huang, Y.; Liu, H.; Du, J.; Meng, Z.; Dou, Z.; Liu, X.; Wei, R.H.; Zhang, Y.; Zhao, S. α-Melanocyte-stimulating hormone ameliorates ocular surface dysfunctions and lesions in a scopolamine-induced dry eye model via PKA-CREB and MEK-Erk pathways. Sci. Rep. 2015, 5, 1–14.
- Kleiner, S.; Braunstahl, G.J.; Rüdrich, U.; Gehring, M.; Eiz-Vesper, B.; Luger, T.A.; Steelant, B.; Seys, S.F.; Kapp, A.; Beohm, M.; et al. Regulation of melanocortin 1 receptor in allergic rhinitis in vitro and in vivo. Clin. Exp. Allergy 2016, 46, 1066–1074.
- Chidi-Egboka, N.C.; Briggs, N.E.; Jalbert, I.; Golebiowski, B. The ocular surface in children: A review of current knowledge and meta-analysis of tear film stability and tear secretion in children. Ocul. Surf. 2019, 17, 28–39.
- Arita, R.; Fukuoka, S.; Morishige, N. New Insights into the Lipid Layer of the Tear Film and Meibomian Glands. Eye Contact Lens. 2017, 43, 335–339.
- Zhang, L.; Li, W.H.; Anthonavage, M.; Pappas, A.; Rossetti, D.; Cavender, D.; Seiberg, M.; Eisinger, M. Melanocortin-5 receptor and sebogenesis. Eur. J. Pharmacol. 2011, 660, 202–206.
- House, J.S.; Zhu, S.; Ranjan, R.; Linder, K.; Smart, R.C. C/EBPalpha and C/EBPbeta are required for Sebocyte differentiation and stratified squamous differentiation in adult mouse skin. PLoS ONE 2010, 5, e9837.
- Lee, S.N.; Ryu, J.H.; Joo, J.H.; Choi, Y.H.; Lee, H.J.; Kim, Y.J.; Kim, K.B.; Yoon, J.H. α-Melanocyte-stimulating hormone inhibits tumor necrosis factor -stimulated MUC5AC expression in human nasal epithelial cells. Am. J. Respir. Cell Mol. Biol. 2011, 44, 716–724.