Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dimitrios Giannopoulos | -- | 4039 | 2022-09-29 08:25:09 | | | |
| 2 | Catherine Yang | Meta information modification | 4039 | 2022-09-29 10:42:47 | | | | |
| 3 | Catherine Yang | Meta information modification | 4039 | 2022-10-08 08:42:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Giannopoulos, D.; Katsifis, I.; Katsourinis, D.; Rentizelas, A.; Founti, M. Heavy-Metal Contaminated Input along Liquid Biofuel Value Chains. Encyclopedia. Available online: https://encyclopedia.pub/entry/27946 (accessed on 08 February 2026).
Giannopoulos D, Katsifis I, Katsourinis D, Rentizelas A, Founti M. Heavy-Metal Contaminated Input along Liquid Biofuel Value Chains. Encyclopedia. Available at: https://encyclopedia.pub/entry/27946. Accessed February 08, 2026.
Giannopoulos, Dimitrios, Ilias Katsifis, Dimitrios Katsourinis, Athanasios Rentizelas, Maria Founti. "Heavy-Metal Contaminated Input along Liquid Biofuel Value Chains" Encyclopedia, https://encyclopedia.pub/entry/27946 (accessed February 08, 2026).
Giannopoulos, D., Katsifis, I., Katsourinis, D., Rentizelas, A., & Founti, M. (2022, September 29). Heavy-Metal Contaminated Input along Liquid Biofuel Value Chains. In Encyclopedia. https://encyclopedia.pub/entry/27946
Giannopoulos, Dimitrios, et al. "Heavy-Metal Contaminated Input along Liquid Biofuel Value Chains." Encyclopedia. Web. 29 September, 2022.
Copy Citation
The main types of contaminants include inorganic (mostly heavy metals—HMs) and organic pollutants (petroleum, pesticides, polycyclic aromatic hydrocarbons—PAHs, etc.). The presence of contamination in the value chains examined is assessed in order to identify the corresponding effects and implications. Their effect is studied in each of the typical stages of the existing value chains, including pre-processing, conversion and post-processing steps. Special focus is assigned to the gasification and pyrolysis conversion processes, reviewing the fate of contaminants along each process. In the gasification process, parameters such as temperature, pressure, HM type and concentration are evaluated. At the same time, in pyrolysis, the major parameters considered were residence time and reactor temperature.
liquid biofuels
heavy metals
contaminated biomass feedstock
1. Pre-Processing of Contaminated Feedstocks
Regarding bioethanol feedstocks, Asad et al. [1] examined pretreatment options of lignocellulosic and woody HMCB. Three widely used processes of chemical pretreatment (with dilute acid, alkali-catalyzed and ethanol organosolv) were investigated. The fractionation of phytoremediation biomasses for the production of bioethanol is also described. It was reported that in acidic conditions, pre-processing at a temperature of 170 °C with a sulfuric acid solution of 2% w/w, extracted up to 90% of metals (for Zn and Mn) recovered in the water effluent. As regards the intermediate product for the following process steps, a clean pulp was obtained. On the other hand, under alkaline conditions, low extraction of metals was observed. In a soda pretreatment of an HMCB at temperatures over 170 °C, the metal recovery was high, while a clean liquid stream and lignin were obtained. For organosolv, metal concentrations were mainly in the pulp and to a lesser extent in the water effluent and lignin. Wu et al. [2] implemented three chemical pretreatments. As a result, two optimal chemical pretreatments were identified (12% H2SO4, 4.0% NaOH + 2.0% H2SO4) that could capture 99% (of the total in raw stalk) Cd from the mature stalks.
Concerning feedstocks for gasification, dry pretreatment methods can be utilized. However, there have been no reports on how HMs affect the yield and quality of the syngas [3]. On the other hand, according to a study by Yu et al. [4], the wet pretreatment method of leaching with distilled de-ionized water can reduce the ash content of the product significantly. Although, with the extraction of contaminants (organic and inorganic), the composition of the residual solids can be changed, leading to complex properties.
The pretreatment of HM-contaminated feedstocks for pyrolysis has been intensively assessed. The relevant literature [3][5][6][7][8][9][10][11][12][13][14][15][16][17] distinguishes pre-processing methods in the dry and wet. Dry pretreatment methods include processes such as preheating, crushing and torrefaction, while wet methods incorporate the utilization of acids or solvents. Various studies have examined the influence of both dry and wet pre-processing on the yield and H content of bio-oil acquired from Heavy Metal-Contaminated Biomasses (HMCBs).
Regarding dry pre-processing, two relevant studies [5][6] chose to reduce the contaminated feedstock to a particle size of below 2 mm before the introduction to the pyrolysis stage. Nevertheless, according to the findings of Wiinikka et al. [7], enhanced conversion is achieved with finer particles (less than 0.25 mm in size). Lower conversion rates were reported in [7] for larger particle sizes (between 0.5 and 1.0 mm).
Wet pretreatment aims to demineralize the biomass feedstock before further processing, thus promoting the enhancement of bio-oil yield and quality. Heavy metal presence in biomass has been found to favor gas and bio-char production at the expense of lower liquid yield [8][9][10][11]. Wigley et al. [12] followed a combined treatment approach by applying both wet (acid leaching) and dry (torrefaction) treatments. Acid leaching was performed for 4 h in a solution containing 1% acetic acid at a temperature of 30 °C, while torrefaction took place for 20 min at 270 °C.
Alternative treatments have also been reported, which can be applied as a supplement to the original “decontamination” attribute of pyrolysis itself (transformation of toxic HM ions to an amorphous state in the bio-char [13][14]). Phosphate-assisted pyrolysis contains HMs in phosphate minerals [15][16], and other materials can be used to stabilize the heavy metal load of the biomass feedstock, such as FeCl3, Al2O3, NaOH and CaCO3 [17]. Nevertheless, the application of these alternative treatments is mostly advised in order to safely dispose of HMCB, and not when bio-oil production is the first priority [3].
2. Conversion of HM-Contaminated Feedstocks to Liquid Biofuel
2.1. Fermentation
Regarding the production of bioethanol, Asad et al. [1] examined enzymatic hydrolyses of HM-enriched and HM-free pulps. The influence of the metal load on the enzymatic hydrolysis into monomeric sugars was investigated, and little or no effect on polysaccharide hydrolysability was observed for metals such as Zn, Fe and Mn. Sayago [18] concludes that various cases of Cr-doped biomass can be used for bioethanol production since the HM load had an insignificant influence.
Nevertheless, the chromium samples produced 33% less ethanol than the “clean” hydrolyzed biomass of E. crassipes (8000 mg/L vs. 12,100 mg/L after 25 h). On the contrary, Wu et al. [2] claim that the Cd accumulation in rapeseed stalks could improve biomass enzymatic saccharification and consequent bioethanol production under two-step (4.0% NaOH + 2.0% H2SO4) chemical pretreatment. In particular, the ethanol yield (% dry matter) was increased by 8% and 12% in two respective Cd-contaminated samples. The positive impact of Cd in SSF (simultaneous saccharification and fermentation) is confirmed in Ko et al. [19], while Cr presence is also beneficial for the same process. On the other hand, negative impacts of biomass Zn and Cd contents on enzymatic hydrolysis were reported in the same paper, especially for lower enzyme dosages. In addition, the Cr contaminant in the biomass was shown to facilitate enzymatic hydrolysis at all three dosages. For Zn in biomass, a smaller impact was reported on bioethanol SSF processes.
2.2. Transesterification
Unfortunately, no papers examined biodiesel production from HM-contaminated feedstocks. Therefore, the content of HMs in biodiesel, obtained from vegetable oil derived from HMCB, remains an underexplored topic. In a study by Angelova et al. [20] on the deposition and allocation of HMs in oil crops, the content of Cd, Cu and Pb in plant organs and in the oil of rapeseed (Brassica napus L.) grown in a polluted area, is reported. HMs were distributed in the following order (in decreasing value): leaves > stems > roots > fruit shell > seeds. In the seeds, which contain pure plant oil, the concentration effect of HMs was the lowest. However, the quantities of Pb, Cu and Cd in the rapeseed oil were higher than the accepted thresholds for human consumption. However, it has not been specified yet if the biodiesel exhaust fumes from rapeseed plants are associated with hazardous metal emissions [21].
2.3. Gasification
On the other hand, various studies have examined the fate and effect of HM through the gasification of contaminated biomass feedstocks. HMs can contribute to corrosion, fouling and erosion of the gasification facilities. Another common issue is catalyst deactivation [22][23]. Moreover, during the gasification process, many unwanted products are formed, such as ash and tar [24][25]. The tar, which sometimes contains HMs, can cause significant problems, such as plugging and corrosion and filter blockage [24].
Studies have heavily focused on the different parameters of the gasification process that contribute to the transfer and distribution of HM compounds into the gas phase. Many experiments and modeling simulations have been implemented that deduced different results. A simulation from Jiang et al. [22] concluded that temperature and HM content of the biomass are critical parameters that influence the fate of HMs in the gasification process. Some elements (As, Cd, Zn, Pb) volatized at temperatures above 600 °C, others (Ni, Cu, Mn, Co) transferred to the syngas at temperatures 1000–1200 °C and Cr, Al and Mg remained in the solid phase even at temperatures higher than 1200 °C. As for the pressure, it was found that it increases product yield and raises the HM transition temperature by 100–200 °C [22]. According to the bench scale experiments performed by Cui et al. [26], most HMs volatized into the gas phase, except for Al and Fe. It must be noted that Pb and Zn were the most abundant in the gas phase [26]. Syc et al. [27] studied the distribution of HMs in the gasification system. The experiment focused on the gasification of energy crops (flax, mixed hardwood). The results showed that the HMs Cd, Zn, Pb, Ni and Cu were found in the bed ash, cyclone ash and downstream syngas. Further, the HM content in the syngas was found to be in the range of 0.37–4.2 mg/m3 [3].
In another experiment by Pudasainee et al. [23], which involved the introduction of a slurry composed of glycol and straw char in a gasifier reactor, it was deduced that the heavy metal load was allocated in many parts of the gasification systems, such as the cooler, boiler and the syngas cleaning section. Furthermore, Ni had the largest concentration in the syngas (53.2 μg/Nm3), followed by Cr, Pb, As, V and Cd. Hg has the lowest concentration (24 μg/Nm3) [23].
The volatilization temperatures of HMs in the gasification process, according to empirical and theoretical studies, can be categorized as follows:
-
Certain HMs (Mn) may be entirely condensed in gasification gas.
-
Others (Hg and Cd) are mostly expected to be present in the syngas.
-
Co can be totally or partially volatilized at hot gas cleaning systems temperatures ranging between 500 and 800 °C
-
A considerable group of HM species As, Cd, Zn, Cr, Pb, Cr, Sb and Ni will also be present in syngas, even at temperatures lower than 500 °C [3].
From the simulations and experiments that are mentioned above, it is apparent that the HΜs that are contained in the contaminated biomass are volatilized. However, the type and amount of heavy metals that transfer to the gas phase are heavily dependent on temperature and pressure. The most common HMs that can be found in the syngas are Zn, Cu, Pb and Cd. Generally, a temperature lower than 1000 °C and a pressure of up to 30 atm are considered optimal conditions for gasification in terms of limiting the transfer of many volatile HMs [22].
It is important to note that when it comes to liquid biofuels (and bio-oil in particular), benchmark values have not been set for the maximum accepted level, as regards both inorganic compounds and heavy metal contents [3]. An upper limit <0.10 wt% for particles as well as for HMs has been recommended [28].
In addition, the distribution of HMs depends on [3]:
-
Chemical speciation of metals and dynamics of fluidization.
-
Reaction temperature and pressure.
-
Nature of the heavy metal-contaminated biomass.
-
Application of upstream or downstream treatment processes.
-
Reactor type (fluidized, fixed or entrained bed).
-
Effect of the materials contained in the fluidized bed.
-
What type of gas (air, oxygen, steam, etc.) is the gasification agent.
The type of gasifier that is used is an important parameter. It has been observed that the countercurrent downdraft fixed-bed gasifier is more efficient compared to the fluidized bed gasifier. The emissions from the first were almost one order of magnitude lower than those of a fluidized bed gasifier [29][30]. Additionally, the steam-fluidized is better than the air-fluidized bed gasification when it comes to the volatilization of HMs [31][32]. The steam-fluidized bed not only reduces the HM emission but also has a better catalytic role and produces syngas that has a higher LHV. However, it has been observed that the type of the gasification agent (such as H2O, O2, CO2) is not the most important parameter that influences HM transfer to the syngas [33].
In order to acquire an overview of the HM load ratio that is carried from the feedstock to syngas after the completion of the conversion stage, Figure 1 and Figure 2 have been elaborated. This ratio is important since syngas will be subsequently transformed to liquid biofuel through the Fischer–Tropsch process. For better distinguishing of the reported data points, Figure 1 and Figure 2 cover the HM load ranges of 0–2570 and 0–7250 mgHM·kg−1, respectively. The x-axis contains the feedstock HM loads, while the respective part that was carried by the syngas (also in mg of HM contained in syngas per kg of feedstock) is represented in the y-axis.
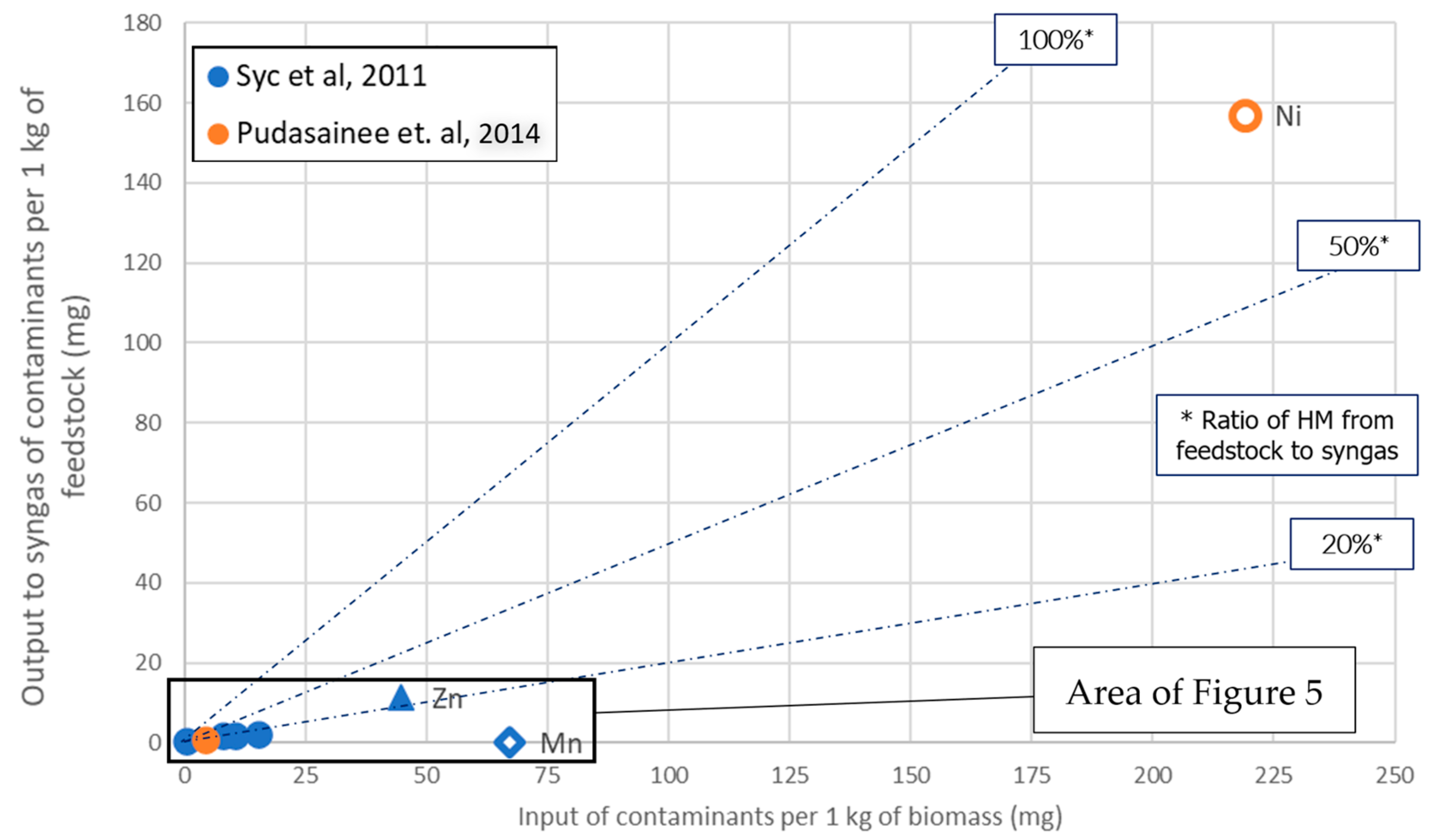
Figure 1. Gasification: Output to syngas vs. input with feedstock of HM species. All available input loads shown.
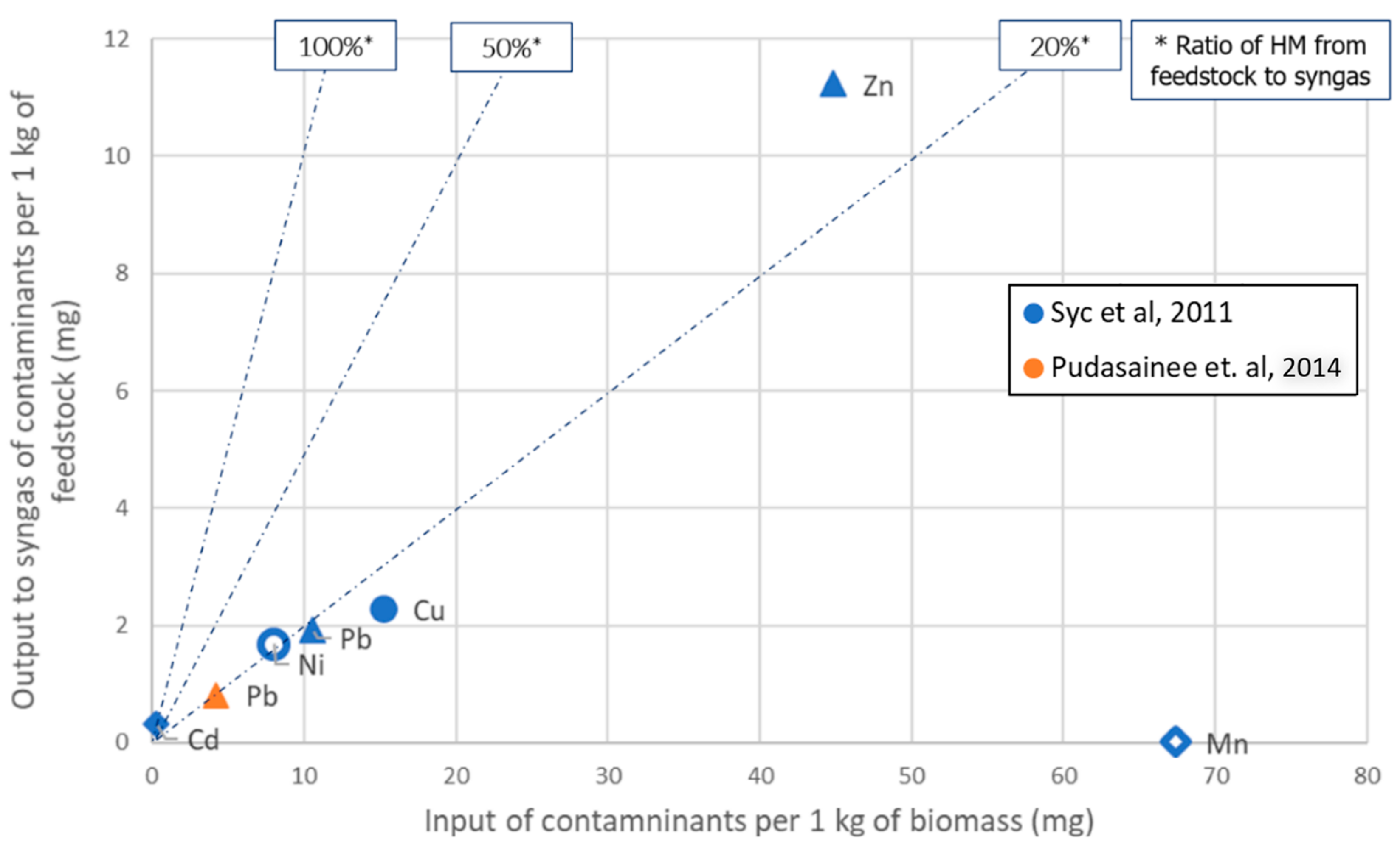
Figure 2. Gasification: Output to syngas vs. input with feedstock of HM species. Focus on low-input loads.
The data are distinguished in terms of the specific HM species and the source they have been retrieved from. In particular, Ni data are shown by a hollow circle, Pb by a triangle, while the rest of the HM species have a corresponding representing shape. Accordingly, the color of each point corresponds to the respective literature source. The diagonal lines with the indications “100%”, “50%” and “20%”, represent cases in which the produced syngas carries the corresponding ratio of the HM load. This means that the worst cases are closer to the “100%” line.
The abovementioned qualitative findings of the literature review are confirmed in Figure 1 and Figure 2. A high risk of Cd presence in the syngas is demonstrated; nevertheless, there is only one data point with a very low Cd load (~1 ppm). The relevant risk for Ni and Zn also seems higher than the rest of the HM species. All species examined show at least a 20% incorporation to syngas, except for Mn. Only two cases of syngas showed contamination levels higher than 10 ppm: Ni (~160 ppm) in [23] and Zn (11 ppm) in [27].
2.4. Pyrolysis
Flash pyrolysis generally meets the requirements of accomplishing the research goals of HMCB utilization: the rather low pyrolysis temperature (as opposed to combustion and gasification) inhibits metals from volatilization, and valuable bio-oil is obtained [34].
Mineral matter is typically carried by biomass. Generally, the major biomass minerals are composed of Ca, Si, K, Mg and Na, with smaller amounts of P, S, Mn, Al and Fe and trace amounts of heavy metals. Inorganic compounds are found in the form of silicates, oxides, sulfates, phosphates, carbonates and chlorides. The ash (mineral) content has an influence on the liquid, gaseous and solid fraction production rates and properties (adsorptive properties of char, heating value and the elemental composition) [9][35][36].
The most influential process parameters in terms of quantity and quality of the products obtained are: (a) reactor temperature, (b) residence time, (c) heating rate, (d) physicochemical pretreatment, (e) particle size and (f) geometrical configuration of the reactor and solid heat carrier [36][37][38].
There are mainly three goals in the pyrolytic conversion of contaminated biomass [9]:
-
to acquire a final product (bio-oil) with zero (or negligible) heavy metal load,
-
to minimize the emission of any gaseous compound containing heavy metals (such as free ions, hydroxides or carbonates) and
-
to capture heavy metals in the structure of the bio-char.
A variety of factors (i.e., plant type, operating conditions [39], pretreatment [29][40], as well as downstream processes [29][34]) have been shown to affect the characteristics of both the liquid and the solid output in various ways. Zhong et al. [41] have pyrolyzed a hyperaccumulating plant carrying high HM loads, both under slow and fast conditions. The HM concentrations for 1 kg of biomass feedstock were reported to have the following values: Al: 13,976 mg; Zn: 9838 mg; Fe: 642 mg; Cd: 560 mg; Cu: 77.6 mg; Pb: 62.5 mg and Cr: 45.4 mg. It was shown that high reaction temperatures and heating rates favored the increase in HM concentration in the liquid product (e.g., Cd and Zn showed higher concentrations in bio-oil produced at 750 °C than 650 °C). The temperature of 650 °C was actually optimal in terms of bio-oil quality (low HM concentrations) and yield.
Another experiment was performed by Lievens et al. [38], studying the lab-scale pyrolysis process of treating a sunflower feedstock with Cd, Cu, Zn and Pb. The volatilization of Cd and Zn was conveniently prevented due to non-favorable temperature levels of 623 K. Cu and Pb were correspondingly bound in the biochar within a temperature range between 623 and 873 K [38]. Moreover, a related study [42] showed that the pH of the liquid fraction increases with temperature raise, therefore, reducing the solubility of heavy metals.
The flash pyrolysis of HM-rich willow was the topic of [34]. It was thereby shown that a temperature rise from 350 to 550 °C facilitated Cd volatilization, while Zn remained in the bio-oil. Only traces of Pb were found in the bio-oil, even at the maximum temperatures examined. The study determined the optimal temperature range in terms of bio-oil yield and corresponding HM load, in the range of 350–450 °C. It was not advised to opt for lower operating temperatures, despite more intense HM incorporation in the biochar. The reason for that was the worse properties of the bio-oil obtained.
Leijenhorst et al. [29] applied fast pyrolysis on agricultural waste at a process temperature between 400 and 600 °C. The inorganic elements were almost entirely (>95% wt.) absent from the bio-oil, while heavy metals were contained in the bio-char in particular. Higher reaction temperatures and the respective volatilization of heavy metals under these conditions have also been identified by [34][41] to facilitate the gaseous phase transfer of heavy metals. Additional affecting parameters include the structural bond between HMs and biomass [10][43][44] and between HMs and the organic vapors produced [33].
HMCB pyrolysis was also the core process of the work by Dilks et al. [42]. The reactor was coupled with a downstream cyclone, achieving less HM loads in the bio-oil, while no upstream pretreatment was applied. The biochar obtained was reported as not suitable to be disposed of in the environment, as well as inappropriate to be used in terms of metal extraction/recovery. The same negative assessment regarding the biochar attributes was shared with [34].
Focusing on fluidized beds, Koppolu et al. [45] showed that it is possible to operate at higher temperatures (~600 °C) without any adverse effects regarding the incorporation rates of HM into biochar. However, Stals et al. [34] propose quite a lower temperature (around 350 °C) for a fluidized bed pyrolysis reactor coupled with a hot gas filter.
The issue of the influence of pyrolysis temperature is also assessed in [38][46][47][48], where higher oxygen bio-oil content and increased tar cracking were observed at 700 °C, leading to a decrease in terms of the bio-oil heating value (∼16.8–19.0 MJ·kg−1). On the other side, lower temperatures are assigned to less HM in the bio-oil [49][50] and higher biochar output [41][49]. A quite important side-effect of choosing a pyrolysis temperature on a scale of 350–450 °C is the minimum HM leachability of the biochar obtained. This finding has been confirmed in the cases of Cd and Pb contamination [34][51].
The optimum operating temperature for HMCBs pyrolysis is 350–450 °C in terms of the maximum HMs removal and acceptable bio-oil yield, which can be extended by 150–250 °C (i.e., threshold of up to 600 °C) mainly depending on the type and concentration of HMs inside plants’ organs, reactor configuration and pre/post-treatment techniques. Sun et al. [52] showed that operating conditions, including temperature, processing method and feedstock type, influence the physicochemical and biological properties of bio-chars and hydro-chars obtained from the pyrolysis of biomass [3].
Apart from the influence of temperature, the bio-oil yield and contents are heavily affected by the lignocellulosic composition of the contaminated feedstock [8]. As demonstrated by Lievens et al. [38], pyrolysis of two different contaminated biomass samples (sunflower and birch) provided a variety of outputs in terms of corresponding fraction yields, attributes and energy contents. In addition, heavy metals tend to show various concentrations and respective accumulating trends in different parts of the feedstocks harvested (e.g., leaves, branches, stems, etc.) [10][14][43][53]. As a consequence, a difference may be observed regarding the yield, composition and heating value of the bio-oil obtained after pyrolyzing different parts of the contaminated biomass [38].
Similar to the previous gasification section, all of the literature cases that provided enough data to be represented in an HM input-output diagram were elaborated upon, and the corresponding figures (Figure 3 and Figure 4) were obtained. In the pyrolysis case, the important ratio is how much of the HM load is contained in the bio-oil, since out of this intermediate product, the final biofuel will be eventually acquired. In parallel to the gasification process, fast pyrolysis is expected not only to provide a good yield of bio-oil but also to capture the HM load in the biochar. Due to the better distinguishing of data points, two figures are presented, covering the HM load ranges of 0–16,000 and 0–100 mgHM·kg−1, respectively.
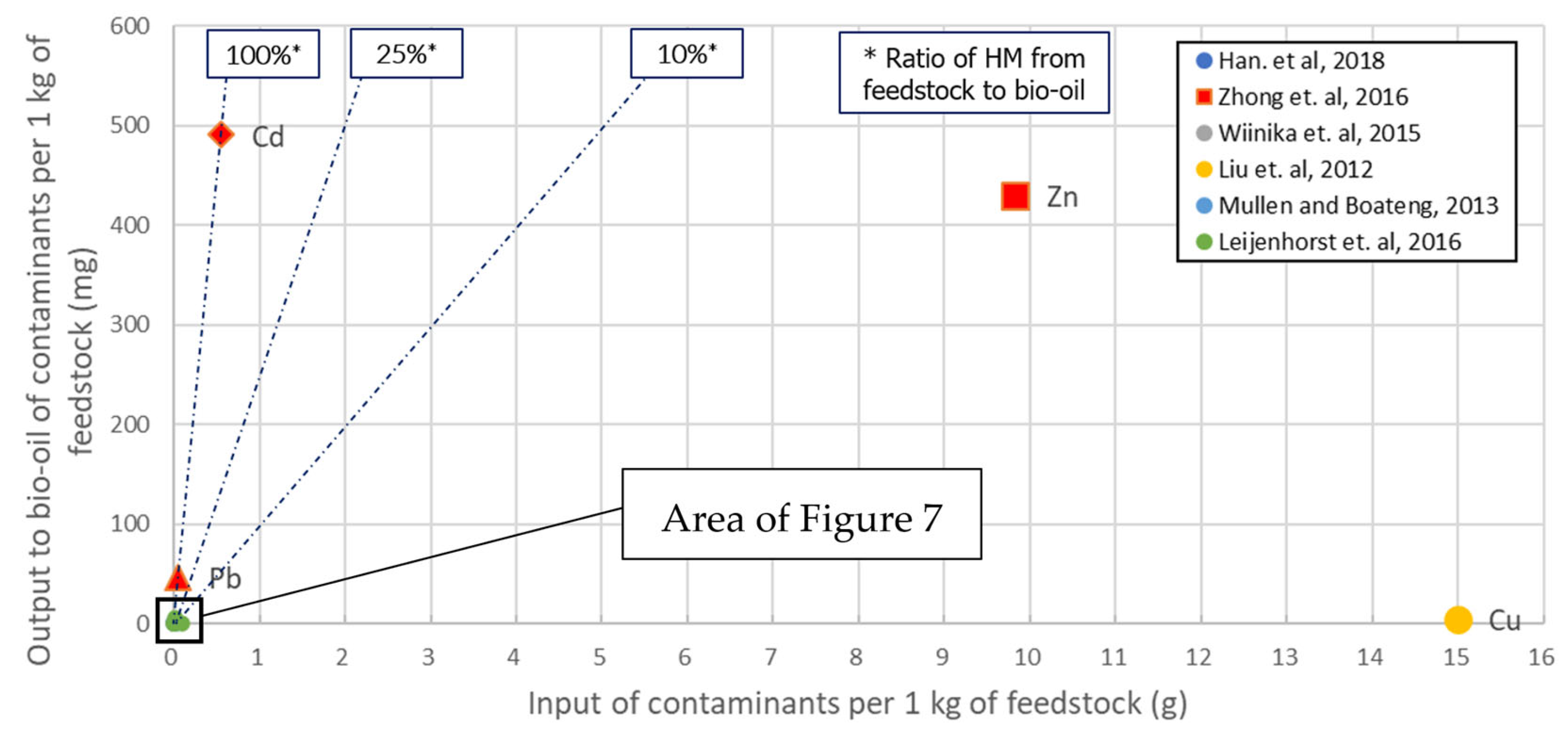
Figure 3. Pyrolysis: Output to bio-oil vs. input with feedstock of HM species. All available input loads are shown.
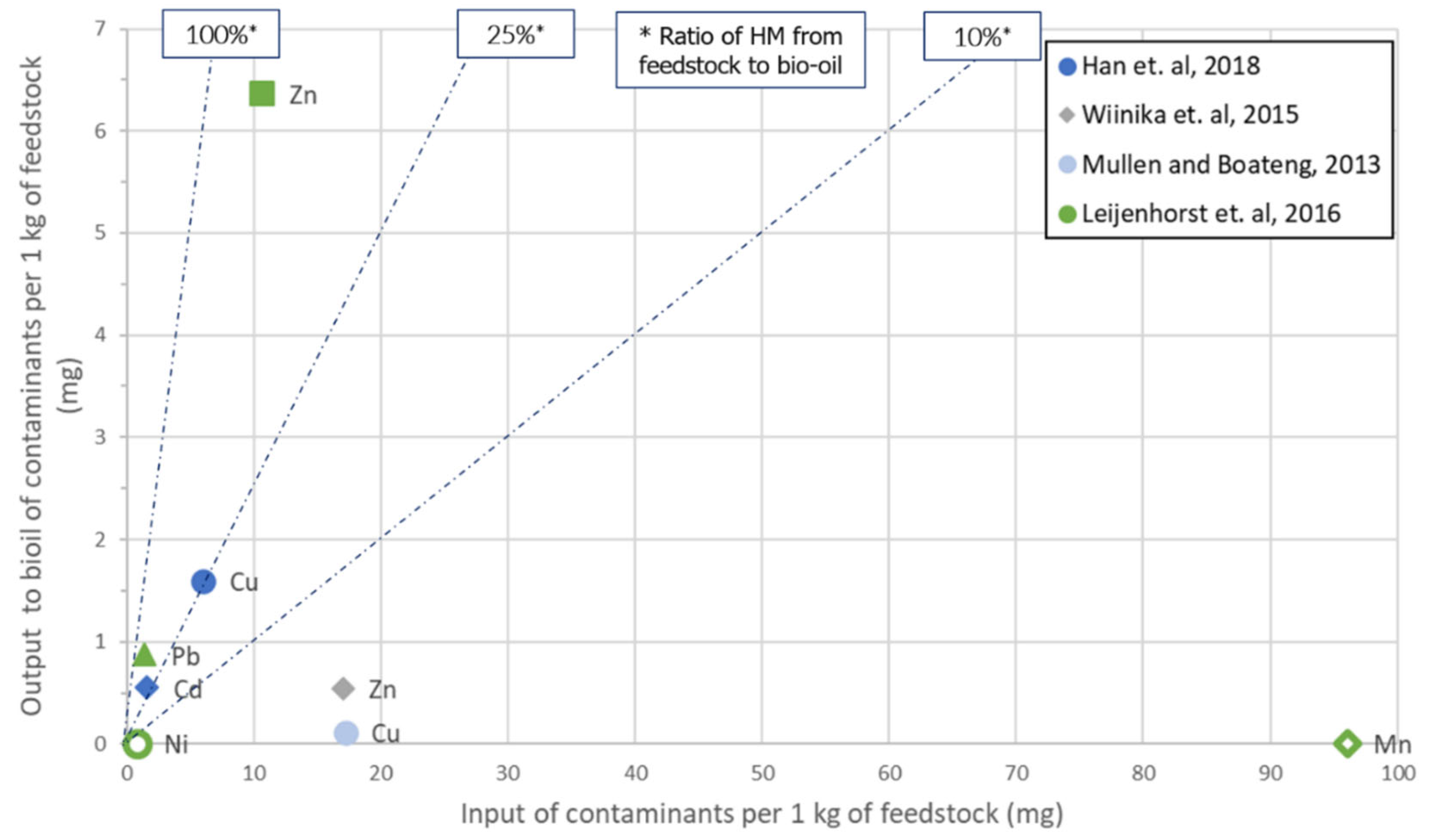
Figure 4. Pyrolysis: Output to bio-oil vs. input with feedstock of HM species. Focus on low input loads.
The high risk previously identified regarding the presence of Cd in the syngas is also demonstrated in the case of bio-oil. This risk is additionally observed for Pb, while Zn shows both very high and very low cases of incorporation in bio-oil. Cu also demonstrates a mixed behavior, in two cases with a very low incorporation ratio and one with a medium ratio. As an overview, at least 25% of most of the HM species examined ended up in the bio-oil, except Mn. A specific source [41] contained the cases featuring a high HM concentration in bio-oil: ~500 ppm of Cd, >400 ppm of Zn and ~40 ppm of Pb.
3. Post-Processing
3.1. Post-Processing of Bioethanol
Sayago [18] reported that there is no contamination present in the produced ethanol, but chromium remains in the waste and is, therefore, disposed of. In addition, a desorption process with chemical reagents (e.g., sodium hydroxide (NaOH)) for chromium recovery and reuse was proposed.
3.2. Post-Processing of Syngas
As it was mentioned above, by increasing the operating temperature of the gasification process, the volatilization rate of HMs and the ability of applied filters to capture elements with fine particulates in the syngas are negatively affected [26][53]. Many HMs (Cu, Pb, Zn) are abundant in the gaseous stream, and as a result, the syngas requires conditioning and clean-up before any further usage or syngas upgrading with catalysts [3]. According to another report, cyclones were not effective in capturing some HMs such as Cd, Zn, Pb and Cu because of the high temperature of syngas and their high volatilization [25].
It was also observed in other studies that the hot-gas filter is more capable of reducing the transfer of heavy metals into the syngas compared to the cyclone [29][54]. Stals et al. [34] reported that the reduction induced by gas filtration is observed for all three tested temperatures (623, 723, 823 K), and the volatilization increase with increasing temperature is only present for the case of cadmium.
Electrostatic precipitators have been used for the collection of solid particles from the gaseous phase. They were shown to have a collection efficiency of 98–99% for solid particles with diameters of 0.3–20 μm. On the contrary, the electrostatic precipitators had lower efficiency (75%) for other particle sizes such as 4–400, 1.80–309 and 3.90–375 μm. HMs (such as Cu, Zn, Cr and Ni) accumulated inside the electrostatic precipitators and the cyclone upstream and not in the cyclone downstream of the gasifier [55]. Finally, the ash, which is a by-product of the gasification process, needs to be managed or disposed of. If the ash from the gasification of contaminated biomass from HMs cannot be reused, landfilling seems to be a promising disposal method [27].
3.3. Post-Processing of Bio-Oil
It is important to distinguish the downstream impact of the HM load from the positive catalytic effect of heavy metals in terms of bio-oil energy content and yield by enhancing thermo-decomposition and activation energy during the core process [5][56][57][58][59]. The post-processing stage expected to be the most affected by the HM load of the bio-oil is catalytic upgrading [29]. Relevant problems focus on catalyst poisoning and deactivation, subsequently degrading the process efficiency. Additional detrimental impacts can be correspondingly expected from water content and coke presence, always depending on the attributes of the catalyst used [29][60][61][62]. In particular, the Fe and Cu from HMCB pyrolysis have been found to accumulate in the active areas of catalyst HZSM-5 [60], while coke is reported to have poisonous effects on catalysts thereby lowering useful product yields [63][64]. On the contrary, a situation of benefits caused by the presence of coke has also been reported by [65], where ZnO2 poisoning by CO2 and water is correspondingly impeded.
Nevertheless, various literature sources claim the primary production of HM-free bio-oil from contaminated biomass feedstocks [5][6][38][66]. In a pilot-scale experiment, Wiinikka et al. [7] demonstrated, on the one hand, the feasibility of HM-free bio-oil and, on the other hand, the concentration of heavy metals in the biochar.
It seems, however, that post-treatment processes should be eventually applied, at least for cases of high-HM concentrations in specific types of heavy metal hyperaccumulating species. Relevant options would include separation methods before bio-oil condensation [7][29], or even considering cyclones, as recommended and tested by [41]. Moreover, as concluded by [3], downstream upgrading would be facilitated by adopting an additional treatment process, such as the adsorption or filtration of the bio-oil produced by fast pyrolysis.
References
- Asad, M.; Menana, Z.; Ziegler-Devin, I.; Bert, V.; Chalot, M.; Herzig, R.; Mench, M.; Brosse, N. Pretreatment of trace element-enriched biomasses grown on phytomanaged soils for bioethanol production. Ind. Crop. Prod. 2017, 107, 63–72.
- Wu, Y.; Wang, M.; Yu, L.; Tang, S.-W.; Xia, T.; Kang, H.; Xu, C.; Gao, H.; Madadi, M.; Alam, A.; et al. A mechanism for efficient cadmium phytoremediation and high bioethanol production by combined mild chemical pretreatments with desirable rapeseed stalks. Sci. Total Environ. 2019, 708, 135096.
- Dastyar, W.; Raheem, A.; He, J.; Zhao, M. Biofuel Production Using Thermochemical Conversion of Heavy Metal-Contaminated Biomass (HMCB) Harvested from Phytoextraction Process. Chem. Eng. J. 2018, 358, 759–785.
- Yu, C.; Thy, P.; Wang, L.; Anderson, S.; VanderGheynst, J.; Upadhyaya, S.; Jenkins, B. Influence of leaching pretreatment on fuel properties of biomass. Fuel Process. Technol. 2014, 128, 43–53.
- Liu, W.-J.; Tian, K.; Jiang, H.; Zhang, X.-S.; Ding, H.-S.; Yu, H.-Q. Selectively Improving the Bio-Oil Quality by Catalytic Fast Pyrolysis of Heavy-Metal-Polluted Biomass: Take Copper (Cu) as an Example. Environ. Sci. Technol. 2012, 46, 7849–7856.
- Lievens, C.; Carleer, R.; Cornelissen, T.; Yperman, J. Fast pyrolysis of heavy metal contaminated willow: Influence of the plant part. Fuel 2009, 88, 1417–1425.
- Wiinikka, H.; Carlsson, P.; Johansson, A.-C.; Gullberg, M.; Ylipää, C.; Lundgren, M.; Sandström, L. Fast Pyrolysis of Stem Wood in a Pilot-Scale Cyclone Reactor. Energy Fuels 2015, 29, 3158–3167.
- Balsamo, R.A.; Kelly, W.J.; Satrio, J.A.; Ruiz-Felix, M.N.; Fetterman, M.; Wynn, R.; Hagel, K. Utilization of Grasses for Potential Biofuel Production and Phytoremediation of Heavy Metal Contaminated Soils. Int. J. Phytoremediation 2014, 17, 448–455.
- Raveendran, K.; Ganesh, A.; Khilart, K.C. Influence of Mineral Matter on Biomass Pyrolysis Characteristics. Fuel 1995, 74, 1812–1822.
- Mayer, Z.A.; Apfelbacher, A.; Hornung, A. A comparative study on the pyrolysis of metal- and ash-enriched wood and the combustion properties of the gained char. J. Anal. Appl. Pyrolysis 2012, 96, 196–202.
- Agblevor, F.A.; Besler, S. Inorganic Compounds in Biomass Feedstocks. 1. Effect on the Quality of Fast Pyrolysis Oils. Energy Fuels 1996, 10, 293–298.
- Wigley, T.; Yip, A.C.; Pang, S. A detailed product analysis of bio-oil from fast pyrolysis of demineralised and torrefied biomass. J. Anal. Appl. Pyrolysis 2017, 123, 194–203.
- Lin, H.-J.; Rong, C.-X.; Jiu, B.-B.; Li, B.-X.; Yu, Q.-J.; Gan, L.-H.; Zhang, Z.-Y. Effects of chromium on pyrolysis characteristic of water hyacinth (Eichornia crassipes). Renew. Energy 2018, 115, 676–684.
- Wang, S.; Gao, B.; Li, Y.; Ok, Y.S.; Shen, C.; Xue, S. Biochar provides a safe and value-added solution for hyperaccumulating plant disposal: A case study of Phytolacca acinosa Roxb. (Phytolaccaceae). Chemosphere 2017, 178, 59–64.
- Li, S.; Zhang, T.; Li, J.; Shi, L.; Zhu, X.; Lü, J.; Li, Y. Stabilization of Pb(II) accumulated in biomass through phosphate-pretreated pyrolysis at low temperatures. J. Hazard. Mater. 2017, 324, 464–471.
- Shi, L.; Wang, L.; Zhang, T.; Li, J.; Huang, X.; Cai, J.; Lü, J.; Wang, Y. Reducing the bioavailability and leaching potential of lead in contaminated water hyacinth biomass by phosphate-assisted pyrolysis. Bioresour. Technol. 2017, 241, 908–914.
- Liu, Y.-N.; Guo, Z.-H.; Sun, Y.; Shi, W.; Han, Z.-Y.; Xiao, X.-Y.; Zeng, P. Stabilization of heavy metals in biochar pyrolyzed from phytoremediated giant reed (Arundo donax) biomass. Trans. Nonferrous Met. Soc. China 2017, 27, 656–665.
- Sayago, U.F.C. Design of a sustainable development process between phytoremediation and production of bioethanol with Eichhornia crassipes. Environ. Monit. Assess. 2019, 191, 221.
- Ko, C.-H.; Yu, F.-C.; Chang, F.-C.; Yang, B.-Y.; Chen, W.-H.; Hwang, W.-S.; Tu, T.-C. Bioethanol production from recovered napier grass with heavy metals. J. Environ. Manag. 2017, 203, 1005–1010.
- Angelova, V.; Ivanova, R.; Ivanov, K. Heavy Metal Accumulation and Distribution in Oil Crops. Commun. Soil Sci. Plant Anal. 2004, 35, 2551–2566.
- Van Ginneken, L.; Meers, E.; Guisson, R.; Ruttens, A.; Elst, K.; Tack, F.M.G.; Vangronsveld, J.; Diels, L.; Dejonghe, W. Phytoremediation for heavy metal-contaminated soils combined with bioenergy production. J. Environ. Eng. Landsc. Manag. 2007, 15, 227–236.
- Jiang, Y.; Ameh, A.; Lei, M.; Duan, L.; Longhurst, P. Solid–gaseous phase transformation of elemental contaminants during the gasification of biomass. Sci. Total Environ. 2016, 563–564, 724–730.
- Pudasainee, D.; Paur, H.-R.; Fleck, S.; Seifert, H. Trace metals emission in syngas from biomass gasification. Fuel Process. Technol. 2014, 120, 54–60.
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An Overview of Advances in Biomass Gasification. Energy Environ. Sci. 2016, 9, 2939–2977.
- Tafur-Marinos, J.A.; Ginepro, M.; Pastero, L.; Torazzo, A.; Paschetta, E.; Fabbri, D.; Zelano, V. Comparison of inorganic constituents in bottom and fly residues from pelletised wood pyro-gasification. Fuel 2014, 119, 157–162.
- Cui, H.; Turn, S.Q.; Keffer, V.; Evans, D.; Tran, T.; Foley, M. Study on the fate of metal elements from biomass in a bench-scale fluidized bed gasifier. Fuel 2011, 108, 1–12.
- Šyc, M.; Pohořelý, M.; Jeremiáš, M.; Vosecký, M.; Kameníková, P.; Skoblia, S.; Svoboda, K.; Punčochář, M. Behavior of Heavy Metals in Steam Fluidized Bed Gasification of Contaminated Biomass. Energy Fuels 2011, 25, 2284–2291.
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl. Energy 2014, 116, 178–190.
- Leijenhorst, E.; Wolters, W.; van de Beld, B.; Prins, W. Inorganic element transfer from biomass to fast pyrolysis oil: Review and experiments. Fuel Process. Technol. 2016, 149, 96–111.
- Vervaeke, P.; Tack, F.; Navez, F.; Martin, J.; Verloo, M.; Lust, N. Fate of heavy metals during fixed bed downdraft gasification of willow wood harvested from contaminated sites. Biomass-Bioenergy 2006, 30, 58–65.
- Liao, C.; Wu, C.; Yan, Y. The characteristics of inorganic elements in ashes from a 1 MW CFB biomass gasification power generation plant. Fuel Process. Technol. 2007, 88, 149–156.
- Poole, D.J.; Sharifi, V.; Swithenbank, J.; Kilgallon, P.; Simms, N.; Oakey, J.; Ardelt, D. Continuous analysis of elemental emissions from a biofuel gasifier. J. Anal. At. Spectrom. 2007, 22, 532–539.
- Nzihou, A.; Stanmore, B. The fate of heavy metals during combustion and gasification of contaminated biomass—A brief review. J. Hazard. Mater. 2013, 256–257, 56–66.
- Stals, M.; Thijssen, E.; Vangronsveld, J.; Carleer, R.; Schreurs, S.; Yperman, J. Flash pyrolysis of heavy metal contaminated biomass from phytoremediation: Influence of temperature, entrained flow and wood/leaves blended pyrolysis on the behaviour of heavy metals. J. Anal. Appl. Pyrolysis 2010, 87, 1–7.
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Pyrolysis Characteristics of Biomass and Biomass Components. Fuel 1996, 75, 987–998.
- Piskorz, J.; Majerski, P.; Radlein, D.; Scott, D.S.; Bridgwater, A.V. Fast Pyrolysis of Sweet Sorghum and Sweet Sorghum Bagasse. J. Anal. Appl. Pyrolysis 1998, 46, 15–29.
- Demirbas, A. Effect of initial moisture content on the yields of oily products from pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2004, 71, 803–815.
- Lievens, C.; Yperman, J.; Vangronsveld, J.; Carleer, R. Study of the potential valorisation of heavy metal contaminated biomass via phytoremediation by fast pyrolysis: Part I. Influence of temperature, biomass species and solid heat carrier on the behaviour of heavy metals. Fuel 2008, 87, 1894–1905.
- Debela, F.; Thring, R.W.; Arocena, J.M. Immobilization of Heavy Metals by Co-pyrolysis of Contaminated Soil with Woody Biomass. Water Air Soil Pollut. 2011, 223, 1161–1170.
- Oasmaa, A.; Peacocke, C. Properties and Fuel Use of Biomass-Derived Fast Pyrolysis Liquids A Guide; EU Biocoup View Project; VTT Publications: Espoo, Finland, 2018.
- Zhong, D.; Zhong, Z.; Wu, L.; Ding, K.; Luo, Y.; Christie, P. Pyrolysis of Sedum plumbizincicola, a zinc and cadmium hyperaccumulator: Pyrolysis kinetics, heavy metal behaviour and bio-oil production. Clean Technol. Environ. Policy 2016, 18, 2315–2323.
- Dilks, R.; Monette, F.; Glaus, M. The major parameters on biomass pyrolysis for hyperaccumulative plants—A review. Chemosphere 2016, 146, 385–395.
- Mayer, Z.A.; Apfelbacher, A.; Hornung, A. Effect of sample preparation on the thermal degradation of metal-added biomass. J. Anal. Appl. Pyrolysis 2012, 94, 170–176.
- Raveendran, K.; Ganesh, A. Heating Value of Biomass and Biomass Pyrolysis Products. Fuel 1996, 75, 1715–1720.
- Koppolu, L.; Agblevor, F.A.; Clements, L. Pyrolysis as a technique for separating heavy metals from hyperaccumulators. Part II: Lab-scale pyrolysis of synthetic hyperaccumulator biomass. Biomass-Bioenergy 2003, 25, 651–663.
- Liu, W.-J.; Li, W.-W.; Jiang, H.; Yu, H.-Q. Fates of Chemical Elements in Biomass during Its Pyrolysis. Chem. Rev. 2017, 117, 6367–6398.
- Taarning, E.; Osmundsen, C.M.; Yang, X.; Voss, B.; Andersen, S.I.; Christensen, C.H. Zeolite-catalyzed biomass conversion to fuels and chemicals. Energy Environ. Sci. 2010, 4, 793–804.
- Şensöz, S.; Kaynar, I. Bio-oil production from soybean (Glycine max L.); fuel properties of Bio-oil. Ind. Crop. Prod. 2006, 23, 99–105.
- Hornung, A.; Apfelbacher, A.; Sagi, S. Intermediate Pyrolysis: A Sustainable Biomass-to-Energy Concept-Biothermal Valorisation of Biomass (BtVB) Process. J. Sci. Ind. Res. 2011, 70, 664–667.
- Wright, M.M.; Daugaard, D.E.; Satrio, J.A.; Brown, R.C. Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 2010, 89, S2–S10.
- Song, X.; Xue, X.; Chen, D.; He, P.; Dai, X. Application of biochar from sewage sludge to plant cultivation: Influence of pyrolysis temperature and biochar-to-soil ratio on yield and heavy metal accumulation. Chemosphere 2014, 109, 213–220.
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578.
- Werle, S.; Bisorca, D.; Katelbach-Woźniak, A.; Pogrzeba, M.; Krzyżak, J.; Ratman-Kłosińska, I.; Burnete, D. Phytoremediation as an effective method to remove heavy metals from contaminated area—TG/FT-IR analysis results of the gasification of heavy metal contaminated energy crops. J. Energy Inst. 2017, 90, 408–417.
- Han, Z.; Guo, Z.; Zhang, Y.; Xiao, X.; Peng, C. Potential of Pyrolysis for the Recovery of Heavy Metals and Bioenergy from Contaminated Broussonetia papyrifera Biomass. Bioresources 2018, 13, 2932–2944.
- Díaz-Somoano, M.; Martínez-Tarazona, M.R. Trace Element Evaporation during Coal Gasification Based on a Thermodynamic Equilibrium Calculation Approach. Fuel 2003, 82, 137–145.
- Poškas, R.; Sirvydas, A.; Poškas, P.; Jouhara, H.; Striugas, N.; Pedišius, N.; Valinčius, V. Investigation of warm gas clean-up of biofuel flue and producer gas using electrostatic precipitator. Energy 2018, 143, 943–949.
- Kinata, S.E.; Loubar, K.; Paraschiv, M.; Bouslamti, A.; Belloncle, C.; Tazerout, M. Slow pyrolysis of CCB-treated wood for energy recovery: Influence of chromium, copper and boron on pyrolysis process and optimization. J. Anal. Appl. Pyrolysis 2013, 104, 210–217.
- Harumain, Z.A.S.; Parker, H.L.; García, A.M.; Austin, M.J.; McElroy, C.R.; Hunt, A.J.; Clark, J.H.; Meech, J.A.; Anderson, C.W.N.; Ciacci, L.; et al. Toward Financially Viable Phytoextraction and Production of Plant-Based Palladium Catalysts. Environ. Sci. Technol. 2017, 51, 2992–3000.
- Lin, Y.-C.; Huber, G.W. The critical role of heterogeneous catalysis in lignocellulosic biomass conversion. Energy Environ. Sci. 2008, 2, 68–80.
- Kunkes, E.L.; Simonetti, D.A.; West, R.M.; Serrano-Ruiz, J.C.; Gärtner, C.A.; Dumesic, J.A. Catalytic Conversion of Biomass to Monofunctional Hydrocarbons and Targeted Liquid-Fuel Classes. Science 2008, 322, 417–421.
- Mullen, C.A.; Boateng, A.A. Accumulation of Inorganic Impurities on HZSM-5 Zeolites during Catalytic Fast Pyrolysis of Switchgrass. Ind. Eng. Chem. Res. 2013, 52, 17156–17161.
- Jacobson, K.; Maheria, K.C.; Kumar Dalai, A. Bio-Oil Valorization: A Review. Renew. Sustain. Energy Rev. 2013, 23, 91–106.
- Iliopoulou, E.; Stefanidis, S.; Kalogiannis, K.; Delimitis, A.; Lappas, A.; Triantafyllidis, K. Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B Environ. 2012, 127, 281–290.
- Kabir, G.; Hameed, B. Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew. Sustain. Energy Rev. 2017, 70, 945–967.
- Payormhorm, J.; Kangvansaichol, K.; Reubroycharoen, P.; Kuchonthara, P.; Hinchiranan, N. Pt/Al2O3-catalytic deoxygenation for upgrading of Leucaena leucocephala-pyrolysis oil. Bioresour. Technol. 2013, 139, 128–135.
- Cutrufello, M.G.; Ferino, I.; Monaci, R.; Rombi, E.; Solinas, V. Acid-Base Properties of Zirconium, Cerium and Lanthanum Oxides by Calorimetric and Catalytic Investigation. Top. Catal. 2002, 19, 225–240.
More
Information
Subjects:
Energy & Fuels
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
640
Revisions:
3 times
(View History)
Update Date:
08 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




