Breast cancer is the most common type of cancer and is treated with surgical intervention, radiotherapy, chemotherapy, or a combination of these regimens. Despite its ample use, chemotherapy has limitations such as bioavailability, adverse side effects, high-dose requirements, low therapeutic indices, multiple drug resistance development, and non-specific targeting. Drug delivery vehicles or carriers, of which nanocarriers are prominent, have been introduced to overcome chemotherapy limitations. Nanocarriers have been preferentially used in breast cancer chemotherapy because of their role in protecting therapeutic agents from degradation, enabling efficient drug concentration in target cells or tissues, overcoming drug resistance, and their relatively small size. However, nanocarriers are affected by physiological barriers, bioavailability of transported drugs, and other factors. To resolve these issues, the use of external stimuli has been introduced, such as ultrasound, infrared light, thermal stimulation, microwaves, and X-rays. Recently, ultrasound-responsive nanocarriers have become popular because they are cost-effective, non-invasive, specific, tissue-penetrating, and deliver high drug concentrations to their target.
1. Nanocarriers for Breast Cancer Chemotherapy
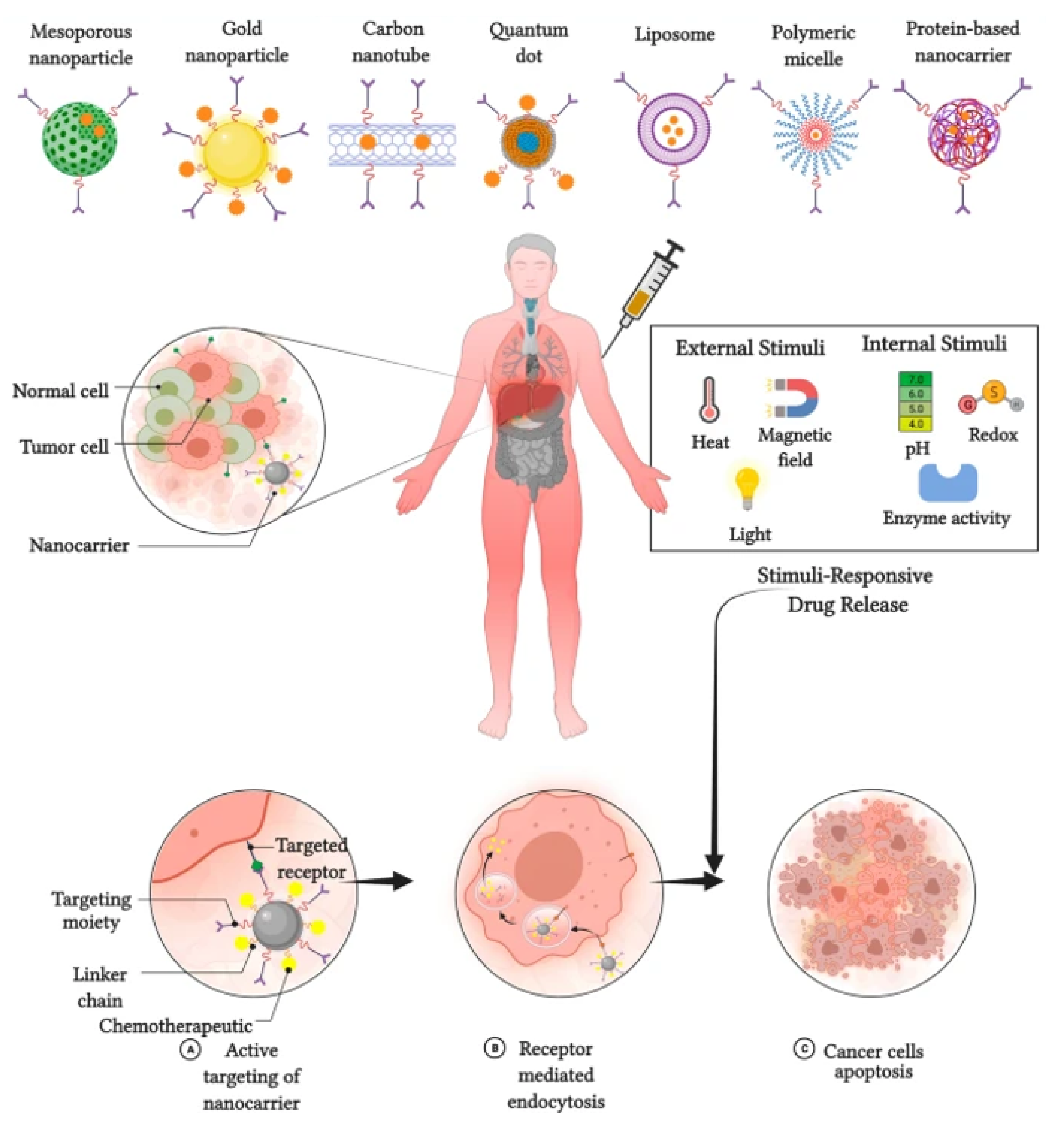
Nanoparticles designed for either targeted or non-targeted drug delivery have a small diameter (1–100 nm) and possess a large surface area to volume ratio
[1]. These properties allow them to bind, absorb, and carry therapeutic agents with high efficiency
[2]. Nanocarriers for breast cancer chemotherapy are broadly divided into two types: organic and inorganic (
Figure 1 [3]. Inorganic nanocarriers include quantum dots (QD), mesoporous silica nanoparticles (MSN), layered double hydroxide (LDH) nanoparticles, carbon nanotubes, and magnetic nanoparticles. Inorganic nanocarriers are preferred for their better anti-cancer agent-loading capacity, large surface area, reduced side effects, bioavailability, well-regulated drug release, and—most importantly—for their organic solvent tolerance. Organic nanocarriers, on the other hand, include polymeric nanoparticles, liposomes, micelles, protein nanoparticles, and dendrites. Organic nanocarriers are preferred for their easy synthesis and modification, enabling improved drug-loading efficacy, biodistribution, and therapeutic efficacy. Moreover, organic nanoparticles allow sustained drug release over a period of time and the use of organic solvents
[4].
Figure 1. Types of nanocarriers used in breast cancer chemotherapy.
The most commonly used nanocarriers in breast cancer chemotherapy include liposomes, dendrimers, micelles, carbon nanotubes, polymeric nanoparticles, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs)
[5]. Liposomes are used for various purposes to increase drug-loading capacity while suppressing unnecessary drug effects. In contrast, lipids cause toxicity, and nanocarriers are quickly destroyed by phagocytes. Dendrimers have been commended for their higher loading capacity and bioavailability. However, dendrimers suffer from rapid clearance, organ accumulation, and synthesis variability. Micelles reduce toxicity and other side effects, but are used only for limited drugs and exhibit low drug-loading capacity
[6]. Carbon nanotubes are capable of penetrating and localizing at the cellular level, but as a material, they can be potentially toxic. Polymeric nanoparticles are biocompatible, degradable, non-toxic; however, they are less effective and susceptible to carrier degradation. SLNs have the advantage of being soluble and better controlled for drug release, despite their low drug-loading capacity and containing other complex structures
[7]. NLCs have multiple advantages compared to others, and their limitations include gelation of lipid dispersion and polymorphic transition. In general, nanocarriers for breast chemotherapy have their advantages and shortcomings. To improve their shortcomings while increasing treatment efficacy, different stimuli are utilized, which are designed to make the nanocarriers responsive.
2. Stimuli-Responsive Nanocarriers for Breast Cancer Chemotherapy
The application of stimuli to improve the efficacy of therapeutic agents delivered by nanocarriers has received considerable attention in recent years. Stimuli-responsive nanocarriers have been developed to compensate for the shortcomings of conventional nanocarrier-based chemotherapy
[8]. The delivery of therapeutic agents responsive to stimuli is based on both internal (endogenous) and external (exogenous) stimuli (
Figure 2).
Figure 2. Stimulus responsive nanocarriers. Adapted from Kaushik et al.
[9] with permission under the terms of the CC BY 4.0 License, Copyright 2022.
2.1. Internal Stimuli
Various internal stimuli are used for nanocarrier-based anti-cancer agent delivery to increase therapeutic efficacy and suppress adverse effects. Internal stimuli used with nanocarriers in breast cancer chemotherapy include pH, redox, and enzymatic stimuli
[10]. pH-responsive nanocarriers are internalized and dissociate, causing protonation and extracellular drug release. Subsequently, the nanoparticle is detached, which promotes endocytosis of nanocarriers and release of the drug
[11]. The redox-responsive nanocarrier system is the S–S bond that is chemically cross-linked as a gating or capping molecule on the surface of the nanoparticle and is cleaved upon the addition of agents, causing rapid drug release to the tumor cells
[12]. Drug release from NPs in an enzyme-responsive manner originates from specific enzyme-catalyzed chemical reactions that lead to the degradation, dissociation, or morphological transitions of the parent NPs
[13].
2.2. External Stimuli
External stimuli originate from outside of the body to initiate anti-cancer agent delivery. External stimuli used in breast cancer chemotherapy include magnetic fields, ultrasound, and light
[14]. In contrast to the internal stimuli, the external stimuli would introduce contrast agents to the image—that of nanoparticles located in the target tissues, cells, or organelles. This further triggers nanocarriers from outside the body through particular stimuli at a specific time. Magnetic systems are widely utilized for targeting and imaging
[15]. As magnetic-responsive nanotherapeutics are non-invasive signals, an externally applied magnetic field can damage the moving particles and increase the accumulation of therapeutic agents in tumors. A magnetic field could be employed for in vivo applications, and could have greater advantages for targeted cancer therapy as compared with intrinsic stimuli-responsive nanotherapeutics. Ultrasound is one of the most commonly used exogenous stimuli in cancer therapy
[16]. The unique advantages of ultrasound responsiveness include safety, non-invasiveness, and deeper penetration into the tissue. Many exogenous stimuli are used for drug delivery systems, among which temperature-responsive drug delivery systems offer potential advantages compared to other counterparts. This is due to their flexible design, regulation of phase transition temperatures, and passive targeting capability. The localized hyperthermia from 42.5 to 43.5 °C helps to evade cancer cells by inducing high temperatures in tumor tissues. However, these hyperthermic stimuli would enlarge the blood vessels and modify the perforation of tumor cell membranes, thereby enhancing anti-tumor drug delivery
[17].
2.3. Internal vs. External Stimuli
Both internal and external stimuli have their own advantages and disadvantages, as presented in Table 1. Internal stimuli are safe and provide efficient and controllable drug release without compromising cell and site specificity. Internal stimuli have the disadvantage of not being controlled manually. External stimuli have the advantage of being manually controlled and modulated based on individual requirement making it vital in personalized treatment. They also provide upgraded site-specific drug delivery and enable regulated and payload release. However, external stimuli need more sophisticated equipment and normal cell injury may happen. Nevertheless, compared to internal stimuli, external stimuli are preferred for nanocarrier based chemotherapy.
Table 1. Advantages and disadvantages of internal and external stimuli.
3. Therapeutic Agents in Ultrasound-Responsive Breast Cancer Treatment
Therapeutic agents are chemical substances that are delivered to the body for the treatment or mitigation of disease conditions or ailments. These substances can be drugs, proteins, genes, compounds, or other pharmaceutically active ingredients. As the human genome has been sequenced and genetic technology has advanced, there is a growing body of knowledge on genetic changes, initiation and proliferation, therapeutic mechanisms, and novel treatment targets for cancer therapy. Understanding the pathophysiology of the disease, human gene sequences, and discovery of novel molecular targets is the core of modern medicine to conquer cancer therapy. Numerous noteworthy advances have been made in the development of targeted therapy. These targeted therapies are designed to attack cancer cells while causing less damage to normal healthy cells. Targeted therapies are drugs or other substances that block the growth and spread of cancer by interfering with specific molecules or targets that are involved in the growth, spread, and progression of cancer. Targeted therapies are currently at the center of anti-cancer drug development; hence, they are the cornerstone of precision medicine. Similarly, in breast cancer, many drugs are being developed and integrated with nanocarriers. Table 2 lists some of the drugs used in breast cancer treatment along with nanocarriers responsive to ultrasound.
Table 2. Drugs used in ultrasound-responsive nanocarrier breast cancer treatment.
| Drug |
Product/Platform |
Type of Nanocarrier |
Reference |
| Doxorubicin |
Perfluoropropane |
Liposome |
[18][19] |
| Doxorubicin |
Polyethylene glycol |
Liposome |
[20] |
| Doxorubicin |
Polyethylene glycol |
Liposome |
[21] |
| Cisplatin |
Soy phosphatidyl choline (SPC-3), cholesterol, dipalmitoyl phos-phatidyl glycerol (DPPG), and methoxy-polyethylene glycol-distearoyl phosphatidylethanolamine (mPEG 2000-DSPE) |
Liposome |
[22] |
| EndoTAG-1 and paclitaxel |
Cationic |
Liposome |
[23] |
| Paclitaxel |
1,2-dioleoyl-sn-glycero-3-phosphocholine |
Liposome |
[24] |
| Resveratrol |
Chloroform solutions of cadmium oxide and sucrose laurate |
Liposome |
[25] |
| Cisplatin |
Distearoyl phosphoethanolamine-polyethylene glycol and phosphatidylcholine |
Liposome |
[26] |
| Paclitaxel |
Polyethyleneglycol (PEG)-phosphatidylethanolamine (PE) (PEG-PE) |
Liposome |
[27] |
| Doxorubicin and silymarin |
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) |
Liposome |
[28] |
| Epirubicin-hydrochloride |
Phosphatidylcholines with thin film hydration using egg yolk |
Liposome |
[29] |
| Curcumin |
Polyethylene glycol (PEG) |
Liposome |
[30] |
| A7R-cysteine peptide |
Distearoylphosphosphatidyl-ethanolamine
(DSPE-PEG2000) |
Liposome |
[31] |
| Raloxifene |
Methanol-ethyl acetate |
Liposome |
[32] |
| Artemisinin |
Polyethylene glycol 2000 (PEG 2000) |
Liposome |
[33] |
| Thymoquinone |
Thymoquinone (2-isopropyl-5-methyl-1,4-benzoquinone) and Triton X-100; 1,2-dipalmitoyl-sn-glycero-3-phospho-choline (DPPC) |
Liposome |
[34] |
| Doxorubicin |
Lipoic acid, hyaluronic acid, L-lysine methyl ester |
Polymer nanoparticles |
[35] |
| Doxorubicin |
Chitosan and pluronic F127 |
Polymer nanoparticles |
[36] |
| Cisplatin |
Luteinizing hormone-releasing hormone (LHRH)-modified dextran |
Polymer nanoparticles |
[37] |
| Tamoxifen citrate |
Polylactide-co-glycolide |
Polymer nanoparticles |
[38] |
| Paclitaxel |
Albumin nanoparticle |
Polymer nanoparticles |
[39] |
| Paclitaxel |
Folic acid Polylactic-co-glycolic acid, polyethylene glycol succinate |
Polymer nanoparticles |
[40] |
| Paclitaxel |
Montmorillonite and Poly(D, L-lactide-co-glycolide) |
Polymer nanoparticles |
[41] |
| Paclitaxel and ceramide |
Poly(beta-amino ester) and poly(D,L-lactide-co-glycolide) |
Polymer nanoparticles |
[42] |
| Docetaxel |
Albumin nanoparticle |
Polymer nanoparticles |
[43] |
| Quercetin |
Polylactic-co-glycolic acid, polyethylene glycol 1000 succinate |
Polymer nanoparticles |
[44] |
| Doxorubicin and Salinomycin |
Polyacrylic acid and Polyethylene glycol |
Micellar nanoparticle |
[45] |
| Paclitaxel |
Polyethylene glycol succinimidyl succinate |
Micellar nanoparticle |
[12] |
| Doxorubicin and Paclitaxel |
Lauryl carbamate derivative of plant-based polymer inulin |
Micellar nanoparticle |
[46] |
| Paclitaxel |
Polyethylene glycol-b-polylactide |
Micellar nanoparticle |
[11] |
| Fisetin |
Pluronic127 folic acid |
Micellar nanoparticle |
[47] |
| Paclitaxel |
Dextran-g-indomethacin |
Micellar nanoparticle |
[48] |
| Aminoflavone |
Anti-epidermal growth factor receptor |
Micellar nanoparticle |
[49] |
| Paclitaxel |
PEG-block-poly[(1,4-butanediol)-diacrylate-β-5-amino-1-pentanol] polyethyleneimine-block-PDHA |
Micellar nanoparticle |
[50] |
| Aminoflavone |
Poly(amidoamine) dendrimer, polyethylene glycol derivatives |
Micellar nanoparticle |
[51] |
| Doxorubicin |
Pluronic copolymer P123 polyethylene glycol-block-poly (di-isopropanolamino ethyl methacrylate) diblock copolymer |
Micellar nanoparticle |
[52] |
| Paclitaxel |
Methoxy polyethylene glycol-polylactide (mPEG-PLA) |
Micellar nanoparticle |
[53] |
| Paclitaxel |
polyethylene glycol (PEG)-polyacrylic acid (PAA) (PEG-PAA) |
Micellar nanoparticle |
[54] |