Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Haoyu Chen | -- | 2011 | 2022-09-28 09:25:05 | | | |
| 2 | Conner Chen | + 3 word(s) | 2014 | 2022-09-28 10:54:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lin, A.; Mai, X.; Lin, T.; Jiang, Z.; Wang, Z.; Chen, L.; Chen, H. Research Trends and Hotspots of Retinal OCT. Encyclopedia. Available online: https://encyclopedia.pub/entry/27797 (accessed on 07 February 2026).
Lin A, Mai X, Lin T, Jiang Z, Wang Z, Chen L, et al. Research Trends and Hotspots of Retinal OCT. Encyclopedia. Available at: https://encyclopedia.pub/entry/27797. Accessed February 07, 2026.
Lin, Aidi, Xiaoting Mai, Tian Lin, Zehua Jiang, Zhenmao Wang, Lijia Chen, Haoyu Chen. "Research Trends and Hotspots of Retinal OCT" Encyclopedia, https://encyclopedia.pub/entry/27797 (accessed February 07, 2026).
Lin, A., Mai, X., Lin, T., Jiang, Z., Wang, Z., Chen, L., & Chen, H. (2022, September 28). Research Trends and Hotspots of Retinal OCT. In Encyclopedia. https://encyclopedia.pub/entry/27797
Lin, Aidi, et al. "Research Trends and Hotspots of Retinal OCT." Encyclopedia. Web. 28 September, 2022.
Copy Citation
Optical coherence tomography (OCT) is a widely used technology for high-resolution and cross-sectional imaging of tissues by measuring backscattered light. The emergence of optical coherence tomography (OCT) has sparked great interest in retinal research.
retinal optical coherence tomography
trends
hotspots
1. Introduction
Optical coherence tomography (OCT) is a widely used technology for high-resolution and cross-sectional imaging of tissues by measuring backscattered light [1]. In 1991, the first report on retinal imaging by OCT in vitro was published [2]. Since then, OCT has undergone substantial progress. As the first generation of OCT systems, time-domain OCT has appeared as a diagnostic tool and revealed the pathogenesis of macular diseases [1]. Application of OCT for detecting and monitoring glaucoma was reported from the beginning of the retinal nerve fiber layer (RNFL) thickness measurements [3]. The advent of spectral-domain OCT (SD-OCT) has enabled the segmentation of selected layers and visualization of anatomic landmarks [4]. Moreover, various OCT signs may serve as indicators of disease severity, treatment response, and prognostic prediction. For example, the absence of intraretinal cysts has been proven to predict spontaneous closure of traumatic macular hole (MH) [5]. Recent advancements in OCT techniques, including enhanced depth imaging OCT and swept-source OCT, have facilitated in-depth analysis of the choroid. More recently, OCT angiography (OCTA) has been developed to generate high-resolution retinal microvasculature images [6]. The quantitative analysis of OCTA images enables the evaluation of vascular abnormalities in retinal vascular diseases [7].
The rapid development of OCT has sparked great interest in retinal research over the years. Thousands of publications have reported the advanced OCT technology and clinical applications, and systematic reviews and meta-analyses have focused on specific questions of retinal OCT research [8][9]. With the significant growth in the production of research literature, novel approaches are required to review and analyze the trends within the domain of retinal OCT knowledge [10].
2. Global Output on Retinal OCT Research
The number of publications on retinal OCT research has risen continually since 1991, and nearly half of the articles have been produced over the last five years. As of 2021, the number of publications exceeded 3000 per year. It is likely to keep rising according to the polynomial prediction function. More than 49,000 authors from 9030 institutions in 120 countries have published articles in this field. Among all the countries, the United States plays a leading role in the quantity and quality of the publications, showing the most publications, total citations, and H-index. Recently, China has presented the most rapid increase in annual publications (Figure 1), accounting for a gradual decrease in the gap between the United States and other countries.

Figure 1. Number of publications of the top 10 productive countries/regions.
3. Trends and Hotspots in Retinal OCT Research
The keyword co-occurrence analysis (Figure 2) has favored classifying the knowledge structure and hotspots.
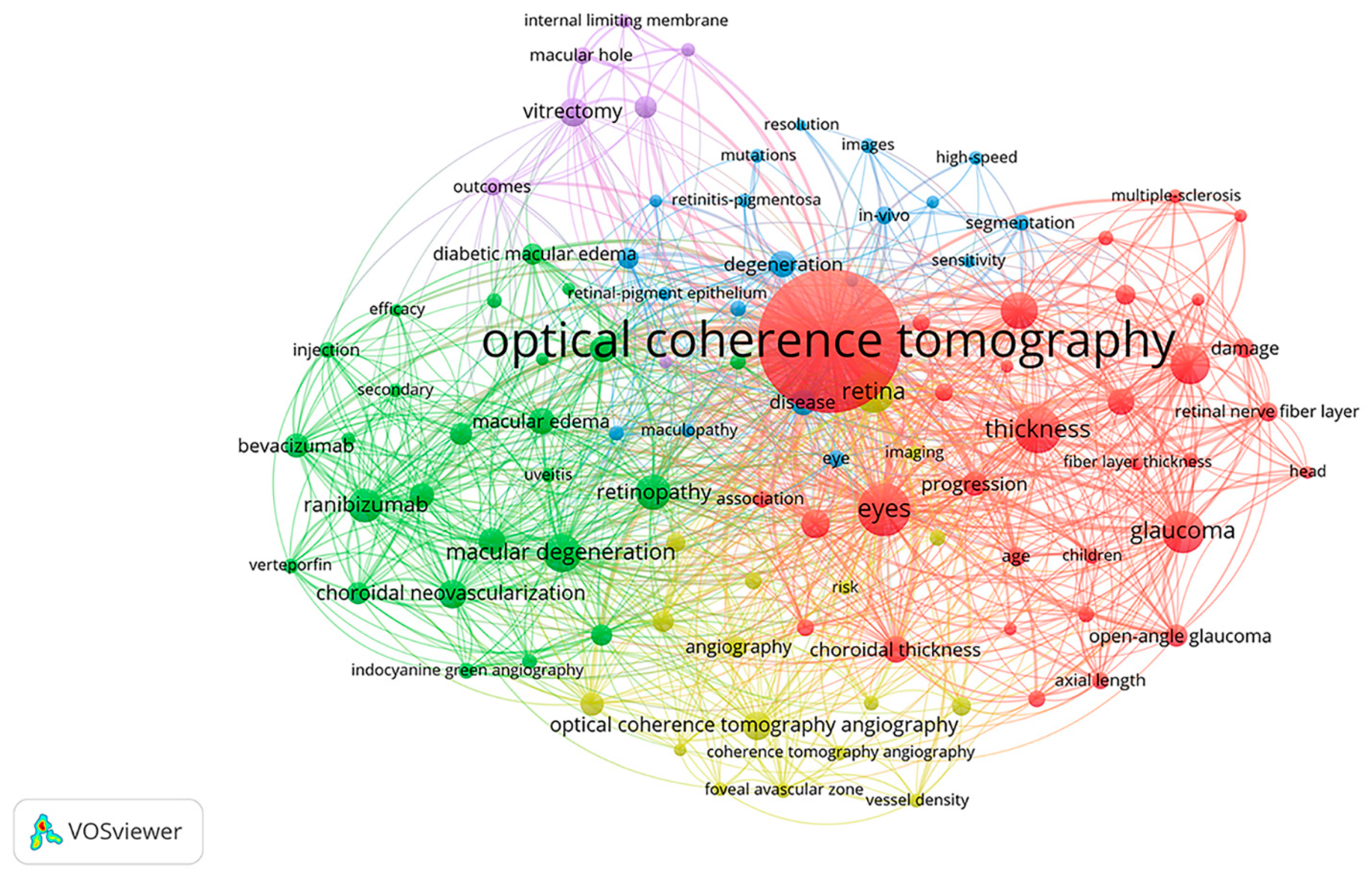
Figure 2. Co-occurrence analysis of the top 100 keywords.
3.1. Cluster 1 (Figure 2, Red Cluster): Thickness Measurements by OCT
This cluster describes the thickness measurements as the first major trend in retinal OCT research. Retinal thickness has been considered a marker for disease severity [1], defined as the distance between the internal limiting membrane (ILM) and the retinal pigment epithelium (RPE). This metric has been commonly used to evaluate the retinal morphological changes after the treatments. The reproducibility of thickness measurements has been well investigated since it is an essential quality to determine the utility of a device for clinical use [11].
The success of OCT in detecting glaucomatous structural damage began with the measurements of RNFL thickness by taking glaucoma from a primarily subjectively evaluated disease to an objectively assessed disease [3]. In 2005, the first report to measure longitudinal changes of RNFL thickness in glaucoma was published using time-domain OCT [12]. Later, the emergence of SD-OCT can achieve higher reproducibility of RNFL measurements and improve the ability to detect glaucoma progression [13]. Besides, other retinal layers, such as the ganglion cell-inner plexiform layer, have been measured for early glaucoma detection.
In 2008, Spaide et al., proposed the enhanced depth imaging OCT to obtain detailed choroidal images and measure the choroidal thickness, defined as the vertical distance from the posterior edge of the RPE to the choroid/sclera junction [14]. Choroidal thickness has become a quantitative biomarker for choroidal tissues suggestive of different pathogenesis in various diseases. For example, choroidal thickening has been observed in polypoidal choroidal vasculopathy, in contrast with choroidal thinning in exudative age-related macular degeneration (AMD) [15].
3.2. Cluster 2 (Figure 2, Green Cluster): Therapies for the Treatments of Macular Degeneration and Macular Edema
This cluster displays the management of macular degeneration and macular edema, emerging as the second major trend in retinal OCT. Macular degeneration, also denominated AMD, is a leading cause of blindness among the aging population in developed countries. The advances in chemistry and pharmacology have allowed for the effective treatments of the neovascular AMD, characterized by the formation of choroidal neovascularization. Verteporfin was the first agent for photodynamic therapy in AMD with predominantly classic subfoveal choroidal neovascularization [16]. In 2006, ranibizumab was approved and proved superior to verteporfin with low rates of severe ocular adverse events [17][18]. After that, other anti-vascular endothelial growth factor (anti-VEGF) agents, including bevacizumab and aflibercept, have shown similar treatment efficacy to ranibizumab [19][20]. With the anti-permeability effects, intravitreal anti-VEGF has become an effective treatment for neovascular AMD, and OCT has been extensively performed to detect retinal changes in therapeutic follow-up. Moreover, an OCT-guided, variable-dosing regimen with the intravitreal injection can be provided if retreatment with anti-VEGF is necessary [21].
Macular edema manifests as abnormal macular swelling and thickening associated with the accumulation of intra- or subretinal fluid [22]. It can occur in various pathologic conditions, including diabetic retinopathy, retinal vein occlusion, uveitis, and postsurgical inflammation. As a multifactorial pathologic example, diabetic macular edema (DME is primarily due to the increased retinal capillary permeability in diabetic patients. Triamcinolone acetonide has been used as a corticosteroid agent for treating DME patients unresponsive to laser photocoagulation [23]. Later, the intravitreal anti-VEGF injection provided superior visual acuity gain over standard laser in DME [24]. However, the relative effects are dependent on baseline vision. In eyes with retinal vein occlusion, intravitreal anti-VEGF therapy has become the current standard of care in macular edema though photocoagulation, and corticosteroid therapies are reasonable in certain circumstances [25]. In pseudophakic macular edema, topical steroidal or nonsteroidal anti-inflammatory drugs, either separately or combined, have been demonstrated to be effective [26]. Remarkably, various OCT biomarkers have been extensively used to evaluate the severity of macular edema and treatment responses [27][28].
3.3. Cluster 3 (Figure 2, Blue Cluster): Degenerative Retinal Diseases
Degenerative retinal diseases are heterogeneous and multi-etiological groups of disorders that will result in irreversible visual damage and compromised life quality [29]. The depth-resolved OCT allows people to identify the tissue loss layer by layer because the changes may vary among layers in these atrophic diseases.
Retinitis pigmentosa (RP) is one of the most common degenerative retinal diseases characterized by the degeneration of photoreceptor cells and RPE. The progressive loss of outer retinal layers has been demonstrated on OCT. At the early stage of RP, the optical intensity of the ellipsoid zone has proved to be an indicator of retinal degeneration [30]. As RP progresses, the thinning or loss of the outer segments may happen [31].
Geographic atrophy (GA) is an advanced form of AMD with the degeneration of photoreceptors and RPE. SD-OCT has become the most recent reference standard for GA assessment among the existing imaging modalities. An OCT-based classification system has been proposed to define atrophy. It can help recognize the biomarkers at different stages of atrophy, including incomplete and complete RPE and outer retinal atrophy [32]. To optimize the diagnosis and prognosis of AMD patients, automated segmentation and quantification of GA from OCT have been well investigated [33].
3.4. Cluster 4 (Figure 2, Yellow Cluster): OCTA Technique
In 2012, a novel OCTA technique, namely the split-spectrum amplitude-decorrelation angiography, was developed with an improved signal-to-noise ratio of flow detection than other amplitude-decorrelation algorithms [34]. Though fluorescein angiography has been traditionally used for retinal vasculature evaluation, the imaging of OCTA shows the advantages of capturing all retinal vascular layers without dye injection [35]. Recently, OCTA has become a noninvasive and convenient technique for detailed imaging and quantitative evaluation of vascular abnormalities [7][36].
Vessel density was calculated as the percentage area occupied by blood vessels measured by OCTA. It has been found that vessel density was associated with the severity of visual field damage in glaucoma. This association was generally stronger than standard structural measures such as RNFL [37]. The quantification of vessel density has facilitated the understanding of the vasculature involved in the pathophysiology and improved the ability of disease monitoring [38].
Given that previous imaging techniques have limited the choriocapillaris imaging, the advent of OCTA is vital to present the choriocapillaris as a granular appearance [39]. The choriocapillaris enface images have demonstrated what appear to be areas of missing flow signal, known as signal voids or flow voids. Interestingly, the choriocapillaris signal voids have shown to follow a power law distribution, the alterations of which offer diagnostic possibilities and impact theories of disease pathogenesis [40]. Besides, choriocapillaris signal voids have appeared to be a valuable parameter for evaluating eyes with AMD. With deeper penetration than spectral-domain OCTA, swept-source OCTA has achieved reproducible imaging of the choriocapillaris and associated signal voids in eyes with drusen [41]. However, the choriocapillaris flow speeds or capillary leakage are still not provided by the current OCTA techniques, which may be promising to reveal the further pathogenesis in diseases affected by the choroid [42].
3.5. Cluster 5 (Figure 2, Purple Cluster): Vitrectomy for MH and ERM
Pars plana vitrectomy is the primary treatment option for patients with MH. Recently, vitrectomy with ILM peeling has been recommended due to the improved visual and anatomic success compared with no ILM peeling [43]. For symptomatic epiretinal membrane (ERM), vitrectomy with membrane peeling remains the mainstay of treatment, and sometimes additional ILM peeling is performed to reduce recurrence [44].
OCT imaging is essential in the preoperative and postoperative management of MH and ERM. Preoperatively, OCT has been utilized to identify the vitreomacular interface disorders, including MH and ERM. Moreover, multiple OCT parameters have prognostic value in the anatomic and visual outcomes. In eyes with MH, preoperative hole diameter, such as the base and minimum diameters determined by OCT, can predict the postoperative success rate of surgery [45]. For ERM, OCT biomarkers that were suggestive of a worse prognosis included the presence of cystoid macular edema, ectopic inner foveal layers, and cone outer segment termination defects [44]. Postoperatively, the anatomic outcomes can be evaluated using OCT, including the hole closure, membrane removal, traction relief, and retinal reattachment. Furthermore, the presence of subretinal fluid postoperatively can be detected by OCT, which may probably account for poor vision despite successful surgery.
4. Emerging Frontiers in Retinal OCT Research
In the past 31 years, the emerging frontiers of retinal OCT are OCTA, vessel density, choriocapillaris, central serous chorioretinopathy, Alzheimer’s disease, and deep learning. The emerging trend of the OCTA technique has developed the subtopics of vessel density and choriocapillaris. Central serous chorioretinopathy is considered one of the pachychoroid spectrum disorders, characterized by the increased choroidal thickness and dilated outer choroidal vessels on OCT [42]. The emerging research on Alzheimer’s disease has benefited from the quantitative OCT/OCTA analyses that have extended into neurodegenerative disorders. Evidence has shown the retinal thickness and microvascular abnormalities associated with Alzheimer’s disease [9]. Nowadays, OCT/OCTA-based deep learning algorithms have been applied in the classification tasks for various diseases and segmentation tasks, including the delineation of macular edema [46]. The emerging frontiers of Alzheimer’s disease and deep learning suggest a multidisciplinary trend that may engage in retinal OCT research.
5. Limitations
Bibliometric analysis is relatively comprehensive and objective for exploring the scientific activities in a particular knowledge domain, but it has some limitations. Firstly, it only extracted articles and reviews from 1991 to 2021; thus, those crucial articles with other document types or published in 2022 may be neglected. Secondly, it did not evaluate the quality of publications, then the articles with high and low qualities were given the same weight. Thirdly, the information from the WOS database was downloaded as “full records and cited references,” which may omit some valuable details or opinions. Although the WOS database is the most commonly used and recommended database for bibliometric analysis [47], some vital publications may not be included in this database. Therefore, other databases such as PubMed or Scopus should be adopted for further investigations.
References
- Geitzenauer, W.; Hitzenberger, C.K.; Schmidt-Erfurth, U.M. Retinal optical coherence tomography: Past, present and future perspectives. Br. J. Ophthalmol. 2011, 95, 171–177.
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181.
- Schuman, J.S.; Hee, M.R.; Puliafito, C.A.; Wong, C.; Pedut-Kloizman, T.; Lin, C.P.; Hertzmark, E.; Izatt, J.A.; Swanson, E.A.; Fujimoto, J.G. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch. Ophthalmol. 1995, 113, 586–596.
- Staurenghi, G.; Sadda, S.; Chakravarthy, U.; Spaide, R.F. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: The IN•OCT consensus. Ophthalmology 2014, 121, 1572–1578.
- Chen, H.; Chen, W.; Zheng, K.; Peng, K.; Xia, H.; Zhu, L. Prediction of spontaneous closure of traumatic macular hole with spectral domain optical coherence tomography. Sci. Rep. 2015, 5, 12343.
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55.
- Jia, Y.; Bailey, S.T.; Hwang, T.S.; McClintic, S.M.; Gao, S.S.; Pennesi, M.E.; Flaxel, C.J.; Lauer, A.K.; Wilson, D.J.; Hornegger, J.; et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc. Natl. Acad. Sci. USA. 2015, 112, E2395–E2402.
- Castillo, M.M.; Mowatt, G.; Elders, A.; Lois, N.; Fraser, C.; Hernández, R.; Amoaku, W.; Burr, J.M.; Lotery, A.; Ramsay, C.R.; et al. Optical coherence tomography for the monitoring of neovascular age-related macular degeneration: A systematic review. Ophthalmology 2015, 122, 399–406.
- Katsimpris, A.; Karamaounas, A.; Sideri, A.M.; Katsimpris, J.; Georgalas, I.; Petrou, P. Optical coherence tomography angiography in Alzheimer’s disease: A systematic review and meta-analysis. Eye 2021, 36, 1419–1426.
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377.
- Paunescu, L.A.; Schuman, J.S.; Price, L.L.; Stark, P.C.; Beaton, S.; Ishikawa, H.; Wollstein, G.; Fujimoto, J.G. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1716–1724.
- Wollstein, G.; Schuman, J.S.; Price, L.L.; Aydin, A.; Stark, P.C.; Hertzmark, E.; Lai, E.; Ishikawa, H.; Mattox, C.; Fujimoto, J.G.; et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch. Ophthalmol. 2005, 123, 464–470.
- Langenegger, S.J.; Funk, J.; Töteberg-Harms, M. Reproducibility of retinal nerve fiber layer thickness measurements using the eye tracker and the retest function of Spectralis SD-OCT in glaucomatous and healthy control eyes. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3338–3344.
- Spaide, R.F.; Koizumi, H.; Pozzoni, M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2008, 146, 496–500.
- Chung, S.E.; Kang, S.W.; Lee, J.H.; Kim, Y.T. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology 2011, 118, 840–845.
- Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: One-year results of 2 randomized clinical trials—TAP report. Arch. Ophthalmol. 1999, 117, 1329–1345.
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431.
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444.
- Martin, D.F.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908.
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548.
- Fung, A.E.; Lalwani, G.A.; Rosenfeld, P.J.; Dubovy, S.R.; Michels, S.; Feuer, W.J.; Puliafito, C.A.; Davis, J.L.; Flynn, H.W., Jr.; Esquiabro, M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am. J. Ophthalmol. 2007, 143, 566–583.
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.R.; Omri, S.; Gélizé, E.; Jonet, L.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68.
- Martidis, A.; Duker, J.S.; Greenberg, P.B.; Rogers, A.H.; Puliafito, C.A.; Reichel, E.; Baumal, C. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 2002, 109, 920–927.
- Mitchell, P.; Bandello, F.; Schmidt-Erfurth, U.; Lang, G.E.; Massin, P.; Schlingemann, R.O.; Sutter, F.; Simader, C.; Burian, G.; Gerstner, O.; et al. The RESTORE study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011, 118, 615–625.
- Ip, M.; Hendrick, A. Retinal Vein Occlusion Review. Asia-Pac. J. Ophthalmol. 2018, 7, 40–45.
- Holló, G.; Aung, T.; Cantor, L.B.; Aihara, M. Cystoid macular edema related to cataract surgery and topical prostaglandin analogs: Mechanism, diagnosis, and management. Surv. Ophthalmol. 2020, 65, 496–512.
- Zur, D.; Iglicki, M.; Busch, C.; Invernizzi, A.; Mariussi, M.; Loewenstein, A. OCT Biomarkers as Functional Outcome Predictors in Diabetic Macular Edema Treated with Dexamethasone Implant. Ophthalmology 2018, 125, 267–275.
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Gerendas, B.S.; Midena, E.; Sivaprasad, S.; Tadayoni, R.; Wolf, S.; Loewenstein, A. Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2019, 242, 123–162.
- Kaur, G.; Singh, N.K. The Role of Inflammation in Retinal Neurodegeneration and Degenerative Diseases. Int. J. Mol. Sci. 2021, 23, 386.
- Gong, Y.; Chen, L.J.; Pang, C.P.; Chen, H. Ellipsoid zone optical intensity reduction as an early biomarker for retinitis pigmentosa. Acta Ophthalmol. 2021, 99, e215–e221.
- Hood, D.C.; Lazow, M.A.; Locke, K.G.; Greenstein, V.C.; Birch, D.G. The transition zone between healthy and diseased retina in patients with retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2011, 52, 101–108.
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology 2018, 125, 537–548.
- Zhang, G.; Fu, D.J.; Liefers, B.; Faes, L.; Glinton, S.; Wagner, S.; Struyven, R.; Pontikos, N.; Keane, P.A.; Balaskas, K. Clinically relevant deep learning for detection and quantification of geographic atrophy from optical coherence tomography: A model development and external validation study. Lancet Digit. Health 2021, 3, e665–e675.
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 2012, 20, 4710–4725.
- Spaide, R.F.; Klancnik, J.M., Jr.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50.
- Jia, Y.; Bailey, S.T.; Wilson, D.J.; Tan, O.; Klein, M.L.; Flaxel, C.J.; Potsaid, B.; Liu, J.J.; Lu, C.D.; Kraus, M.F.; et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology 2014, 121, 1435–1444.
- Yarmohammadi, A.; Zangwill, L.M.; Diniz-Filho, A.; Suh, M.H.; Yousefi, S.; Saunders, L.J.; Belghith, A.; Manalastas, P.I.; Medeiros, F.A.; Weinreb, R.N. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology 2016, 123, 2498–2508.
- Liu, L.; Jia, Y.; Takusagawa, H.L.; Pechauer, A.D.; Edmunds, B.; Lombardi, L.; Davis, E.; Morrison, J.C.; Huang, D. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol. 2015, 133, 1045–1052.
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image artifacts in optical coherence tomography angiography. Retina 2015, 35, 2163–2180.
- Spaide, R.F. Choriocapillaris Flow Features Follow a Power Law Distribution: Implications for Characterization and Mechanisms of Disease Progression. Am. J. Ophthalmol. 2016, 170, 58–67.
- Zhang, Q.; Zheng, F.; Motulsky, E.H.; Gregori, G.; Chu, Z.; Chen, C.L.; Li, C.; de Sisternes, L.; Durbin, M.; Rosenfeld, P.J.; et al. A Novel Strategy for Quantifying Choriocapillaris Flow Voids Using Swept-Source OCT Angiography. Investig. Ophthalmol. Vis. Sci. 2018, 59, 203–211.
- Borrelli, E.; Sarraf, D.; Freund, K.B.; Sadda, S.R. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog. Retin. Eye Res. 2018, 67, 30–55.
- Brooks, H.L., Jr. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology 2000, 107, 1939–1948; discussion 1948–1949.
- Fung, A.T.; Galvin, J.; Tran, T. Epiretinal membrane: A review. Clin. Exp. Ophthalmol. 2021, 49, 289–308.
- Ullrich, S.; Haritoglou, C.; Gass, C.; Schaumberger, M.; Ulbig, M.W.; Kampik, A. Macular hole size as a prognostic factor in macular hole surgery. Br. J. Ophthalmol. 2002, 86, 390–393.
- Ting, D.S.W.; Peng, L.; Varadarajan, A.V.; Keane, P.A.; Burlina, P.M.; Chiang, M.F.; Schmetterer, L.; Pasquale, L.R.; Bressler, N.M.; Webster, D.R.; et al. Deep learning in ophthalmology: The technical and clinical considerations. Prog. Retin. Eye Res. 2019, 72, 100759.
- Peng, C.; Kuang, L.; Zhao, J.; Ross, A.E.; Wang, Z.; Ciolino, J.B. Bibliometric and visualized analysis of ocular drug delivery from 2001 to 2020. J. Control. Release 2022, 345, 625–645.
More
Information
Subjects:
Ophthalmology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
859
Revisions:
2 times
(View History)
Update Date:
28 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




