1. Introduction
With its many benefits, such as biodegradability, good material strength, and environmental friendliness, cellulose, which is a renewable resource found in crops such as straw, maize cobs, bagasse, and water hyacinth, has recently been used to create hydrogels on a large scale and at a low cost
[1][2]. However, its use is restricted because it involves a difficult but necessary dissolving procedure. Utilizing chemical processes to transform cellulose into specific derivatives is one way to increase the applicability of the substance
[3].
Crosslinked hydrophilic polymer structures called hydrogels have a high capacity for absorbing water and other biological fluids. Chemical linkage of polymer chains with an added crosslink agent affects the physical properties of the polymer depending on the degree of crosslinking and the crystallinity. If the amount of crosslinking agent used is too small, then the physical interaction between polymer bonds breaks easily, causing the hydrogel to become water soluble. However, if too much crosslinking agent is used, then a high crosslinking degree causes a low swelling degree of hydrogel. Crosslinking can make polymer elastic, reduce its viscosity, increase the thermal stability, increase the strength and toughness, lower the melting point (for crystalline polymer with a low degree of crosslinking), and transform thermoplastics into thermosets
[4].
Hydrogel can be formed using a variety of crosslinking agents depending on the cellulose derivative that is being used. The most often utilized crosslinking agents for cellulose are epichlorohydrin (ECH)
[5], aldehyde-based reagents
[6], urea derivatives
[7], and multifunctional carboxylic acids
[8]. Aldehydes are poisonous when left unreacted
[9]. The synthesis of hydrogels made from cellulose that have been crosslinked with citric acid results in hydrogels that are completely safe during the production process and have good swelling capabilities and biodegradability
[10].
2. Cellulose-Based Hydrogels
2.1. Cellulose
The fundamental structure of plant cell walls is cellulose, and in certain woods, cellulose accounts for about 40–50%
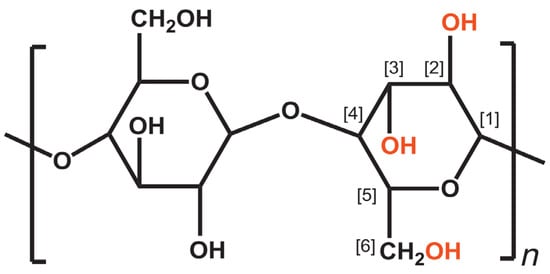
[11]. Cellulose is constructed from glucose chains linked via −1.4 glycosidic bonds formed between C
1 and C
4 of adjacent glucose groups. Each D-anhydroglucopyranose has three hydroxyl groups (OH) at positions C
2, C
3, and C
6, as shown in
Figure 1 [12].
Figure 1. Molecular structure of cellulose (
n = DP, degree of polymerization)
[13].
The OH group on C
1 is the OH found in aldehydes, referred to as reducing agents. This aldehyde group forms a pyranose ring through an intramolecular hemiacetal form. The OH groups on D-anhydroglucopyranose are one primary OH group and two secondary OH groups. In C
2 and C
6–OH groups, intermolecular hydrogen bonds form. In the C
3–OH group and oxygen on the pyranose ring, intramolecular hydrogen bonds form. Intramolecular and intermolecular hydrogen bonding occurs due to the large number of OH groups in cellulose
[14].
The use of cellulose as raw material is preferred in the manufacture of hydrogels based on natural polymers because of its inherent biocompatible and biodegradable properties, in addition to the excellent availability of various types of functional groups that can be used for modification, bio-adhesion, biocompatibility, accessibility, and affordability
[15][16].
2.2. Synthesis of Cellulose-Based Hydrogels
Several cellulose derivatives that have been developed to synthesize hydrogels include methylcellulose (MC)
[17], hydroxyethyl cellulose (HEC)
[18], hydroxypropyl cellulose (HPC)
[2], hydroxypropyl methylcellulose (HPMC)
[19], and carboxymethyl cellulose sodium (CMCNa)
[20]. These derivatives are known to be water-soluble cellulose derivatives.
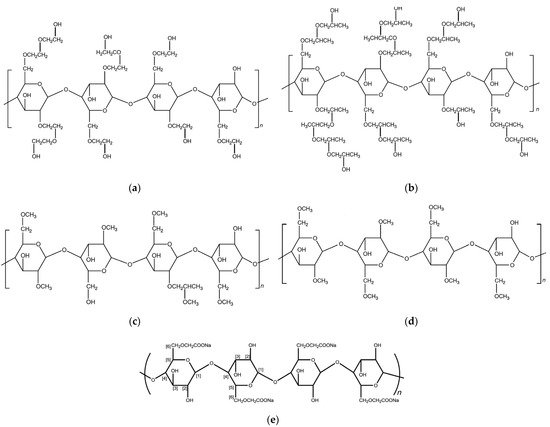
Figure 2 shows the molecular structure of HEC, HPC, HPMC, MC, and CMCNa.
Figure 2. Molecular structure of (
a) HEC (DS = 1.75); (
b) HPC (molar substitution [MS] = 4); (
c) HPMC (hydroxypropyl DS = 0.25 and methoxyl DS = 1.5); (
d) MC (DS = 1.75); (
e) CMCNa (DS = 1)
[13].
MC is a macromolecule of cellulose, with 27–32% of the hydroxyl group in the form of methyl ether. Various grades of MC with degrees of polymerization in the range of 50–1000, molecular weights in the range of 10,000–220,000 Da, and degree of substitution in the 1.64–1.92 range are commercially available
[21]. This methyl derivative of cellulose has the special property of forming a thermally reversible hydrogel upon heating, thus being classified as a polymer with a lower critical solution temperature
[22].
Bonetti et al.
[17] developed MC-based hydrogels with citric acid as a crosslinking agent. In the first 24 h, all hydrogels showed an increase in weight due to water absorption. Swelling balance is reached in the next 24 h. Increasing the degree of crosslinking of the sample causes a significant decrease in the swelling ratio. The equilibrium swelling degree of hydrogels prepared with a constant amount of MC is dependent on the amount of critic acid, with the average swelling values ranging from 800% for MCs with 5% citric acid to 3000% for MCs with 3% citric acid. Conversely, MC with 1% citric acid did not show significant differences in terms of swelling at the equilibrium compared with MC control. This finding indicates that the specimen’s swelling behavior is slightly affected by low crosslinking. In fact, an increase in the crosslinking degree causes an increase in crosslinking points, preventing crosslinked MC network expansion in the water environment.
In line with previous research, Quiroz et al.
[23] synthesized MC-based hydrogel with citric acid as a crosslinking agent. Citric acid functions as a crosslinking agent for MC hydrogels when used at low concentrations (5%
w/
w). The crosslinking decreased water vapor permeability and swelling, allowing good gas barrier properties to be obtained. The formulation of MC 1.5%, 0.25% sorbitol, and 5% citric acid (
w/
w MC) would allow reduced-affinity coating for water and oxygen to be obtained, which can be used to cover foods under low-humidity conditions and preserve nutrients susceptible to oxidation.
HEC is a partially substituted hydroxyethyl etherified cellulose. It is a hydrophilic polymer with a degree of substitution of at least 1.5. When the degree of substitution of HEC increases, the level of solubility in water will increase
[22]. With its biocompatibility and non-immunogenicity, HEC is often used as stabilizer, thickener, film, hydrogel, nanofiber in tissue engineering applications, and it can improve the quality of the resulting hydrogel both mechanically and rheologically
[24][25][26][27].
Fawal et al.
[18] developed an HEC-based hydrogel with citric acid as a crosslinking agent and tungsten trioxide (WO
3) as a support material for wound dressing applications. The FTIR analysis showed the presence of HEC and citric acid and that crosslinking had occurred. The gel fraction of hydrogel without WO
3 and with 0.02% WO
3 was 59.7% and 65.9%, respectively. Swelling or the highest water absorption was 300.1% without WO
3 and 165.6% with 0.02% WO
3, and decreased with increasing WO
3. The percent of water absorption decreased with increasing concentration of WO
3, because WO
3 consumes some hydrogen bonds.
In line with previous research, Wang et al.
[27] synthesized hydroxyethyl cellulose-g-poly(sodium acrylate)/medicinal stone (HEC-g-PNaA/medical stone)-based hydrogel with NMBA as a crosslinking agent. The addition of various amounts of medical stones can change the structure and composition of the hydrogel and affects the swelling capacity. With a medical stone of up to 10% by weight, the swelling capacity increased sharply by 400% and then decreased with further addition of medical stone. The addition of medical stone can decrease the degree of physical crosslinking and increase the swelling capacity because when NaA was grafted onto HEC and MS can participate in the polymerization reaction through its active silanol groups, contributing to the formation of ordinary polymer networks, preventing the intertwining of grafted polymer chains, and weakening hydrogen bonding interactions between groups. However, when the addition of medical stone exceeded 10% by weight, the swelling capacity decreased, because the tissue cavity for holding water was blocked and the hydrophilicity of the hydrogel decreased.
HPMC is a propyleneglycol ether of methylcellulose, described by the PhEur as a partly O-methylated and O-(2-hydroxypropylated) cellulose. HPMC is a water-soluble polymer that is available in several grades with different viscosities and substitution rates. HPMC hydrogel has high levels of transparency, stability, and viscosity because of its good biocompatibility and thermosensitive natural polymers
[22][28][29].
Seyedlar et al.
[19] developed HPMC-based hydrogels with biphasic calcium phosphate (BCP) that were applied to tissue engineering. HPMC-based hydrogels can reduce the invasiveness of osteoplasty surgery, shorten the operating time, and cause homogeneous cell distribution. Incorporation of hydroxyapatite (HAp) and β-tricalcium phosphate (TCP) nanoparticles on BCP in an HPMC aqueous solution increased the viscosity of injection scaffold but decreased the gelation temperature.
In line with previous research, Bashir et al.
[30] synthesized HPMC hydrogel with HPMC-pectin-co-acrylic acid as a polymer and NMBA as a crosslinking agent. PAA containing COOH group is the reason for the increase in the swelling pattern, which has a greater tendency to ionize as the high porosity of hydrogel increases at pH 7.4. The HPMC formulation gradually increased from 0.5 g to 1.5 g, causing the percentage of drug release to also increase simultaneously from 75.36% to 87.62% at pH 7.4, because HPMC has higher swellability and hydrophilic properties at pH 7.4.
HPC is a polymer in which some of the hydroxyl groups of cellulose have been hydroxypropylated, forming -OCH
2CH(OH)CH
3 groups. During the HPC manufacturing process, the added hydroxypropyl group can be esterified, having a mole substitution value (number of moles of hydroxypropyl groups per glucose ring) greater than 3. Therefore, HPC must have a degree of substitution (DS) value of 2.5 and a molarity of substitution (MS) of 4 to have good water solubility
[21][31][32][33].
Chen et al.
[2] developed HPC-based hydrogels made by modifying HPC to alkynyl-HPC as a polymer and molybdenum disulfide (MoS
2) as a crosslinking agent. The hydrogels produced from this research had high water absorption capabilities and thicker pore walls. The addition of MoS
2 with HPC can make the hydrogel to be effective in removing methylene blue dyes. The addition of MoS
2 into HPC can induce a reduction in the swelling ratio of the hydrogel because the addition of MoS
2 into HPC weakens the effect of the volume phase transition of hydroxypropyl cellulose, which causes an increase in crosslinking.
In line with previous research, Yan et al.
[34] synthesized HPC hydrogel with ECH as a crosslinking agent, and ammonia as a co-crosslinking agent. It was found that the adsorption ability of the resin had a strong relationship with the pH value. The microporous structure and the chemical structure of the prepared crosslinked HPC resin are the key factors in producing hydrogels with high adsorption capacity of anionic dyes. The resin can also be used in neutral conditions with a high adsorption capacity for anionic dyes.
CMCNa is a hydrophilic polymer prepared by partial substitution of OH groups in the second, third, and sixth positions of cellulose by carboxymethyl groups. The DS value varies in the range of 0.6–1, affecting several physicochemical properties of the polymer. Therefore, due to the higher DS value, the water solubility and sodium content of CMCNa increase and the polymer tolerance for other components in the solution improves
[22].
Alam et al.
[20] developed a CMCNa-based hydrogel with ECH as a crosslinking agent. FTIR analysis showed the presence of CMCNa and ECH, as well as the fact that crosslinking had occurred. The hydrogel with the highest water absorption or water retention value (WRV) was obtained with a composition of 3% of CMCNa and 4% of ECH.
In line with previous research, Astrini et al.
[35] synthesized CMCNa hydrogel with divinyl sulfone as a crosslinking agent. The weight loss of CMCNa and crosslinked CMCNa/HEC hydrogels indicated a loss of moisture in the samples when the temperature increased (100–170 °C). The TD was 285.5 °C (68.2% weight loss) for CMCNa and 276.6 °C (56.8% weight loss) for crosslinked CMCNa/HEC (5/1). The peak temperature of the main degradation step of CMCNa/HEC (5/1) shifted to a lower temperature compared with pure CMCNa. The crosslinked structure plays an important role in thermal decomposition and indicates that CMCNa is more stable than CMCNa/HEC. With increasing synthesis temperature and reaction time, water absorption capacity also increased.
As a polyelectrolyte, CMCNa is sensitive to pH and ionic strength. Therefore, the compatibility of CMCNa in a solution with other components is an important characteristic. CMCNa is highly compatible with most 10% and 50% monovalent inorganic salt solutions of the cations that form CMCNa soluble salts. Crosslinked CMCNa is capable of absorbing large amounts of water and swells to form superabsorbent hydrogels that exhibit superior mechanical and viscoelastic properties compared with other crosslinked cellulose derivatives hydrogels
[36].
CMCNa-based hydrogels can be used in enzyme immobilization, wound healing, drug delivery, and adsorbents. They can be made into materials for applications involving anti-bacterial activity, drug delivery, wound healing, and tissue engineering
[37][38][39]. CMCNa is easily synthesized from cellulose derived from waste biomass extraction, such as oil palm empty fruit bunches and bagasse because it provides unique CMCNa properties, such as good adsorption, high swelling capacity, and good optical properties (i.e., how it interacts with light, focusing on biomedical applications). The high methylation group in the biomass waste is also an advantage for the production of CMCNa-based hydrogels.
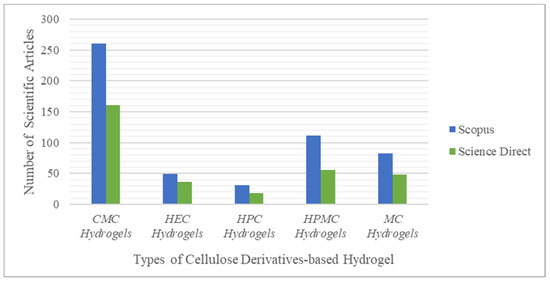
Among the five cellulose derivatives mentioned above, CMCNa remains a favorite raw material for developing hydrogel materials. This is supported by the statistics shown in Figure 3, obtained from Scopus and ScienceDirect.
Figure 3. Statistics of the search results for scientific articles on Scopus and ScienceDirect 2.
The statistical data in
Figure 3 were collected by searching for related articles using several keywords, such as “MC hydrogel,” “HPMC hydrogel,” “HEC hydrogels,” “HPC hydrogel,” and “CMCNa hydrogel,” in the years ranging from 2011–2021. The data indicate that Scopus and ScienceDirect had 260 and 161 scientific articles on the topic of CMCNa-based hydrogels, respectively. This result may be due to the nature of CMCNa itself; CMCNa exhibits a relatively constant level of viscosity over a wide temperature range. The carboxyl group present in CMCNa is the reason for this advantage, because the addition of the carboxyl group to cellulose can adjust the properties and allow the end user to obtain a certain texture beyond the thickness. CMCNa also has high water absorption
[20] and swelling ratio
[40] when used for hydrogel materials.
Most CMCNa that is used as a raw material in hydrogel synthesis is made from natural materials. Research on manufacturing CMCNa with natural ingredients has been conducted in the past. Rachtanapun et al.
[41] reported cellulose from durian rind isolated with NaOH and bleached with hydrogen peroxide. The cellulose was converted to CMCNa using various NaOH concentrations for carboxymethylation. The best results showed that the DS values increased with increasing NaOH concentrations.
Recently, Phan and Thi
[42] synthesized CMCNa from another natural material, namely, passion fruit peel cellulose. Passion fruit peel has excellent potential with a dry weight of cellulose of about 42%
[42] and high cellulose content of about 86.2 g/kg
[43]. They conducted an experiment to extract the cellulose from passion fruit peel, which was then synthesized into CMCNa. The highest cellulose extraction yield was 32.13% at 1 M NaOH and 1.25 M HNO
3. The obtained cellulose was then characterized using FTIR; several peaks were observed, indicating that the cellulose produced was pure cellulose and showing the presence of β-(4, 17)-glycosidic linkages between the glucose units in cellulose. This cellulose was synthesized into CMCNa, with a maximum CMCNa yield of 79.5% and a degree of substitution of 0.78, which were achieved at 20% NaOH concentration and 2 g monochloroacetic acid (MCA). The functional groups of CMCNa were analyzed using FTIR. The presence of –COO and –COONa groups was observed, indicating that cellulose etherification was successful.
Many studies have been conducted on the manufacture of CMCNa from various natural materials, with good and high yields; therefore, CMCNa from natural materials has the potential to be used as a raw material in the manufacture of hydrogels. In particular, passion fruit peel has been used only as a feed mixture
[44] and in the manufacture of pectin extracts
[33]. In the material sector, passion fruit peel is only used as a film
[45], activated carbon
[46], and microcrystalline cellulose
[47].
In recent decades, crosslinked CMCNa networks have been obtained by applying crosslinking technology chemically and physically. Chemical crosslinking involves the use of bifunctional crosslinkers such as ECH, multifunctional carboxylic acid, and PEGDE. However, some diglycidyl ethers produce large amounts of toxic by-products under crosslinking conditions that require elimination by extensive washing, thereby affecting the hydrogel biocompatibility and environmental safety of the production process.
3. Types of Crosslinking Agents in the Synthesis of Cellulose-Based Hydrogels
A crosslinking agent is used in hydrogel synthesis to form a three-dimensional network of hydrogels through the process of chemical crosslinking, physical linkage, ionic, and hydrogen bonding
[48]. Chemical crosslinking is the formation of chemical bonds between molecular chains to form a three-dimensional network that connects molecules
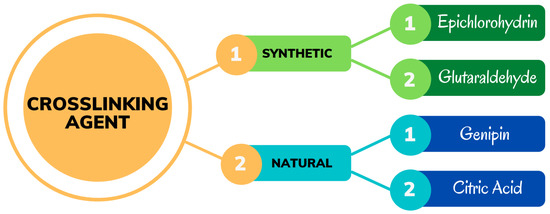
[49]. To synthesize the hydrogels, the crosslinking agent can be derived from natural materials and synthetic materials, as shown in
Figure 4.
Figure 4. Types of crosslinking agents for cellulose-based hydrogels.
Depending the cellulose derivative used, several crosslinking agents can be used to form hydrogels, including ECH
[5], aldehyde-based reagents
[6], urea derivatives
[7], and multifunctional carboxylic acids
[8]. However, some reagents, such as aldehydes, are toxic in their unreacted state
[9]. Even though the unreacted chemical is usually removed after crosslinking by extensive washing with distilled water, as a rule, toxic crosslinking should be avoided to maintain the biocompatibility of the final hydrogel to ensure environmentally friendly production
[10].
3.1. Synthetic Crosslinking Agent for Cellulose-Based Hydrogels
Previous research on the development of CMCNa-based hydrogels with synthetic crosslinking agents is shown in Table 1. This table describes the synthetic crosslinking agents used in the synthesis of CMCNa-based hydrogels, which are ECH and GA.
Table 1. Research on the types of synthetic crosslinking agent for cellulose-based hydrogels.
On the basis of
Table 1, Zhang and Qiao
[50] successfully synthesized hydrogel with CMCNa as a polymer and ECH as a crosslinking agent. In soil, the addition of superabsorbent polymers (SAPs) can lower water evaporation and percolation. However, the repeating water absorbency (RWA) and salt tolerance of prepared SAPs do not meet the requirements for their use. This research examined the effect of valence cations (Na
+, Ca
2+, and Al
3+) on the structural variations of CMCNa-based hydrogels crosslinked with ECH. The results showed that, because of the existence of more carboxyl groups, the higher addition of NaOH resulted in a higher water absorbency (WA). It was found that the sample with 5% CMCNa and 3% NaOH was a qualified hydrogel with WA of 969.0 g/g in deionized water. In the solution, the hydrophilicity and the salt resistance of the sample decreased with increasing cation valence. In the sample, the introduction of Na
+ resulted in the replacement of H
+ from the carboxyl group. The coordination of the Ca
2+ and carboxyl group was tridentate bridging and bidentate chelating for the Al
3+ and the carboxyl group. The introduction of polyvalent cations benefited the stabilization of the carboxyl group, but resulted in lower WA because of the hindered swelling ability of the CMCNa sample.
In line with previous research, Peptu et al.
[51] synthesized alginate (AG)/CMCNa-based hydrogel with ECH as a crosslinking agent. It was found that high superabsorbent properties, indicated by a maximum swelling ratio of 1273%, were observed for the sample with 1:1 AG:CMC molar ratio, 6.6% polymer concentration, and 0.75 mL of ECH. This result was expected, because the SEM showed a porous structure. Despite its porous structure, another sample with a 1:1 AG:CMC molar ratio, 6.6% polymer concentration, and 3 mL of ECH had a swelling ratio of only 362%. This condition can be explained by the higher ECH concentration of the sample before, which defined a wider network of crosslinked polymers. The number of crosslinking agents affected the swelling ratio, and with few crosslinking agents, higher swelling ratios were obtained compared with the samples with a high number of crosslinking agents that showed low swelling ratios. These results indicate that the swelling ratio is dependent on both the crosslinking agent and the polymer concentration.
Khabibi et al.
[52] successfully synthesized CS/CMCNa-based hydrogel with GA as a crosslinking agent. This CS/CMCNa-based hydrogel has a higher swelling ability. Additionally, the higher addition of GA causes a decrease in water adsorption. The decrease in membrane swelling is possibly due to the CS and CMC hydrophilic groups binding with GA during the crosslinking reaction.
In line with previous research, Sritweesinsub and Charuchinda
[53] synthesized AG/CMCNa-based hydrogel with GA as a crosslinking agent. The increase in the CMC-to-AG ratio on the crosslinked hydrogel with GA alone improves its swelling ratio. GA was suggested to be able to effectively crosslink at hydroxyl groups of CMC. The swelling ratio of crosslinked hydrogel with Cu
2+ alone could be slightly improved when the AG increased due to the crosslink interaction between the carboxylate group in AG and Cu
2+. However, when GA and Cu
2+ were employed, it took a greater swelling time than the crosslinking agent alone (40 times). The time to reach the maximum swelling value was extended due to the formation of crosslinks between copper ion and carboxylate groups in AG similar to the formation of crosslinks between the hydroxyl group of CMC and GA. This finding shows that an increase in the AG ratio caused a decrease in the swelling ratio because of enhanced crosslink density.
3.2. Natural Crosslinking Agent for Cellulose-Based Hydrogels
Previous research on the development of CMCNa-based hydrogels with natural crosslinking agents is shown in Table 2. This table describes the natural crosslinking agents used in the synthesis of CMCNa-based hydrogels, which are genipin and citric acid.
Genipin (from the fruit of gardenia) is widely used as a alternative crosslinking agent to dialdehydes because of its biocompatibility. Genipin can bind polymers with biological tissues covalently, such as CS and gelatin
[56]. Genipin is a natural crosslinking agent and is 10,000 times less toxic than the GA crosslinking agent, which is commonly utilized to crosslink the hydrogel with a minimum toxic effect
[54].
Based on
Table 2, Muhamad et al.
[54] synthesized kappa-carrageenan (kC)/CMCNa hydrogel with genipin crosslinking agent. The mixture hydrogel beads of kC: CMCNa with a ratio of 90:10 swelled the fastest, followed by 80:20, 70:30, and 60:40. When the weight fraction of carrageenan increases at 90:10, the counterions in the solution (SO
3−) also increase. The increases in the SO
3− ion resulted in a stronger electrostatic repulsion between the SO
3− groups and increased the osmotic pressure, thereby increasing the polymer swelling.
To determine the swelling response of the hydrogel to pH, a swelling test of beads was conducted in an acidic medium of pH 1.2 and a medium of pH 7.4. Most mixture ratios of beads exhibit better swelling in pH 7.4 than in pH 1.2. In the mixture ratio of 70:30 beads, the swelling degree is 109% and 100% at pH 7.4 and 1.2, respectively. The carboxylate COONa changes to COOH (acid form) at low pH. Therefore, most of the carboxymethyl groups in the form of COOH are less ionized. As the pH increases, the carboxylic groups become ionized, and the resulting repulsion in the network will cause the beads to swell. As a result, beads with a mixture ratio of 70:30 were chosen. Although beads with a mixture ratio of 80:20 and 90:10 had a better degree of swelling than beads with a mixture ratio of 70:30, they were not suitable for the formation of beads because they did not produce spherical beads. Beads with a mixture ratio of 60:40 were not chosen because their structure was not strong and could be dissolved in the pH medium.
Beads crosslinked with the highest concentration of genipin (1.5 mM) show lower swelling than 0.5 mM. A high concentration of genipin could result in a great amount of chemical crosslinking of the C/CMCNa chains. This condition could restrict the mobility and hydration of the macromolecular chain in the beads and lead to less swelling in terms of diameter.
In recent years, citric acid has served as a non-toxic crosslinking agent for hydrogel synthesis. Demitri et al.
[8] successfully synthesized CMCNa and HEC-based hydrogel as a polymer and created hydrogels with citric acid crosslinker. The SR analysis indicated that at the same citric acid concentration, the swelling of CMCNa crosslinked with 10% citric acid was higher than that of HEC. The swelling of HEC-based hydrogel was the same as that of CMCNa-based hydrogel with citric acid concentration of 20%, thereby showing that the reaction rate between citric acid and HEC was higher than the reaction rate between citric acid and CMCNa at a citric acid concentration of 20%. This condition may have occurred because HEC is less sterically obstructed than CMCNa and can react faster than the CMCNa chain.
However, CMCNa/HEC with weight ratio of 3/1 showed that at a citric acid concentration of 3.75%, an SR of 900% can be reached. These hydrogels, once swollen, were characterized by good rigidity and the ability to maintain the same form. With this finding, it can be concluded that the use of citric acid as a crosslinking agent in hydrogel synthesis is not only environmentally friendly, but also gives a higher SR. However, at citric acid concentrations lower than 1.75%, weak crosslinking between cellulose and citric acid will form, thereby producing hydrogels with an insufficient mechanical properties.
In line with previous research, Gorgieva and Kokol
[24] created CMCNa/HEC hydrogel with citric acid crosslinker and found that increasing the CMCNa concentration increased the swelling capacity of the hydrogel, with an increase of 10–20% for the hydrogels made from CMCNa/HEC 3:1 compared to the hydrogels made from CMCNa/HEC 1:1. Moreover, the hydrogels made with higher HEC content were less stable because of their low crosslinking ability, as influenced by their higher substitution degree (fewer −OH groups) compared to CMCNa.
At pH 6.25 ± 0.25 (pH of distilled water), the carboxylic acid groups should be ionized (COO−), because the pKa of the carboxylic acid in the polysaccharide is 4.6. At this pH, the hydrogen bonds will be broken, thereby resulting in electrostatic repulsion between macromolecules, and water will be taken up. The hydrogel made from CMCNa/HEC 1:1 has fewer hydrogen bonds than the hydrogel from CMCNa/HEC 3:1. Moreover, the CMCNa/HEC 3:1 hydrogel crosslinked with higher (5.75%, w/w) citric acid concentration formed fewer hydrogen bonds compared with the hydrogels with 3.75% (w/w) of citric acid, and the response to changes in pH was immediate. Using 3.75% (w/w) citric acid resulted in higher and more intensive swelling in alkaline medium than in acidic medium, thereby indicating that the gels, being weakly acidic, have more ionized carboxylic groups in alkaline pH. Thus, greater electrostatic repulsion occurred between COO− groups, thereby opening the network and increasing the water uptake of the gels.
Durpekova et al.
[55] studied CMCNa/HEC-based hydrogel with citric acid as a crosslinking agent and acid whey as polymeric solution. They found that the mixture CMCNa/HEC hydrogel has a higher swelling capacity than just CMCNa or HEC, with the same citric acid concentration and swelling in distilled water. The HEC-based hydrogel was less stable and showed a lower potential for absorption once citric acid was introduced. It is caused by its low crosslinking capability, which is due to a higher degree of substitution (fewer –OH groups) than that of CMCNa. CMCNa is a polyelectrolyte compound that shows ionic strength and sensitivity to pH. CMCNa increases the swelling capacity of a hydrogel as a consequence of the Gibbs–Donnan effect, thereby increasing the osmotic pressure. An increase in osmotic pressure can force water to enter the hydrogel and inhibit any rise in the ionic strength of the external solution. However, poor crosslinking efficiency has been reported when only CMCNa is utilized because of electrostatic repulsion between the charged macromolecules of polyelectrolyte chains. Thus, in hydrogels, HEC promotes the formation of intermolecular rather than intramolecular crosslinks.
As confirmed in other research, the swelling is not only dependent on the ratio of the polymer, but can also be modified by varying the amount of the crosslinking agents. When a higher concentration of citric acid was present in the polymer solution (caused by an increase in crosslinking density), lower uptake of water was observed. Moreover, hydrogels with a low concentration of citric acid were not sufficiently formed due to limited crosslinking.
Although a higher absorption capacity was observed from CMCNa/HEC (3/1) with 5.75% w/w citric acid for the samples prepared from water, the sample prepared from whey showed similar values at the citric acid concentration of 5% wt. The CMCNa/HEC hydrogel crosslinked by 5% of citric acid with 0.5% of acid whey solution (pH 4.5) showed the best swelling values. Low-protein acid whey can be used to replace the distilled water that is commonly used to synthesize hydrogels and to effectively utilize the waste product of the dairy industry. The swelling results of the whey/cellulose-based hydrogels showed high swelling capacities (1000–1700%), comparable to that of the other synthesized hydrogels from water.
Swelling media of different pH were utilized to confirm the effect of pH on the swelling capacity of cellulose/whey hydrogel. Hydrogel reached the maximum swelling capacities after it had been soaked in distilled water at pH 7.2 (1115%) and saline solution at pH 10.0 (994%). A significant decrease in swelling capacity occurred in acidic media at pH 2.5, which was caused by the protonation of the carboxyl groups. At pH values higher than the pKa of carboxylic groups (pKa 4–5), the carboxylic acid groups became deprotonated. Electrostatic repulsive forces between the negatively charged sites (COO−) can lead to enhanced water uptake capability.
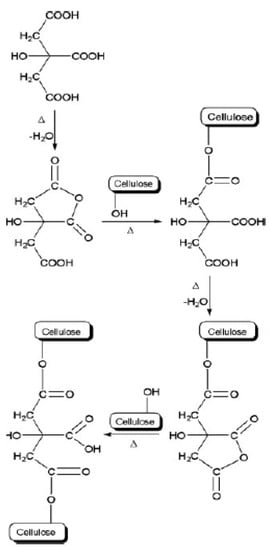
The working mechanism of citric acid is that when it is heated, the carboxylic acid group in citric acid will be dehydrated, thus forming a cyclic anhydride. Then, the cyclic anhydride of citric acid crosslinks with the hydroxyl groups on the cellulose through an esterification reaction. Demitri et al.
[8] explained in detail that at 60 °C, the carboxylic acid in citric acid begins to dehydrate to cyclic anhydride, and at 160 °C, the citric acid is already degraded. The thermal stability of CMCNa is observed at a temperature below 100 °C and is degraded above 100 °C. Therefore, the right temperature for the crosslinking process of CMCNa with citric acid is 80 °C.
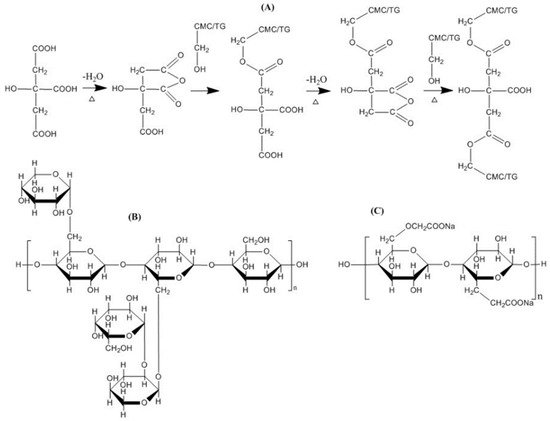
The following figures illustrate the crosslinking mechanism by citric acid that occurs in cellulose (Figure 5) and CMCNa (Figure 6).
Figure 5. Mechanism of crosslinking of cellulose with citric acid (adapted with permission from reference
[8]).
Figure 6. Mechanism of crosslinking of CMC/TG with citric acid
[16]. Possible crosslinking reaction between citric acid, TG and CMC (
A), structure of tamarind gum (
B) and structure of carboxymethyl cellulose (
C).
According to previous research, cellulose-based hydrogels that are crosslinked with citric acid produced a hydrogel with good swelling capability, biodegradability, and safe production process
[10]. However, lower water absorption was observed when higher concentrations of citric acid were present in the polymer solution. Likewise, if the concentration of citric acid is low, then crosslinking in the hydrogel is not sufficient to form a hydrogel. In addition, a trend toward the use of citric acid in hydrogel synthesis from year to year has not been observed since Demitri et al.
[8] developed a citric acid crosslinked cellulose derivative-based hydrogel. This situation is evidenced by the fact that citric acid is still not frequently used as a crosslinking agent in natural hydrogels, especially in CMCNa-based hydrogels, when compared with other synthetic hydrogels that are chemically more dangerous, as shown in
Figure 7.
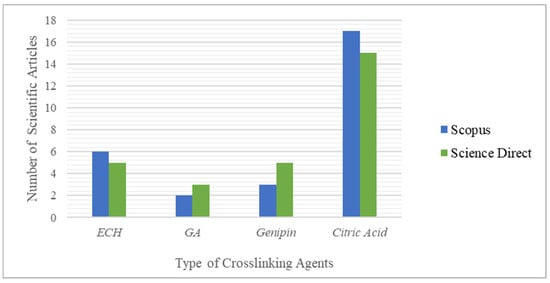
Figure 7 describes the statistics of the search results on the types of crosslinking agents.
Figure 7. Statistics of the search results for scientific articles on Scopus and ScienceDirect 3.
Figure 7 was prepared using the Scopus and ScienceDirect search engines based on keywords such as “ECH crosslinked CMCNa hydrogel”, “GA crosslinked CMCNa hydrogel”, “genipin crosslinked CMCNa hydrogel” and “citric acid crosslinked CMCNa hydrogel”. The above graph shows that the use of citric acid as a crosslinking agent in the synthesis of CMCNa-based hydrogels has come a long way, and is more desirable than ECH, GA, and genipin, as evidenced by the greater number of scientific articles published on the synthesis of hydrogels based on CMCNa with citric acid as a crosslinking agent (17 and 15 articles published in Scopus and ScienceDirect, respectively, in 2011–2021).
A greater amount of research is available because the synthesis of CMCNa-based hydrogel with citric acid as a crosslinking agent can produce higher swelling properties
[8][24], offers stability on different pH swelling media
[54], biodegradability, and ensures much better safety
[10] than other crosslinking agents such as ECH, GA, and genipin. Another reason is that GA is more often used as a crosslinking agent for CS-based hydrogel
[57][58] and genipin
[59][60].