Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Theodora Nikou | -- | 2637 | 2022-09-27 15:16:19 | | | |
| 2 | Camila Xu | -165 word(s) | 2472 | 2022-09-28 02:51:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nikou, T.; Sakavitsi, M.E.; Kalampokis, E.; Halabalaki, M. Metabolism and Bioavailability of Olive Bioactive Constituents. Encyclopedia. Available online: https://encyclopedia.pub/entry/27715 (accessed on 07 February 2026).
Nikou T, Sakavitsi ME, Kalampokis E, Halabalaki M. Metabolism and Bioavailability of Olive Bioactive Constituents. Encyclopedia. Available at: https://encyclopedia.pub/entry/27715. Accessed February 07, 2026.
Nikou, Theodora, Maria Eleni Sakavitsi, Evangelos Kalampokis, Maria Halabalaki. "Metabolism and Bioavailability of Olive Bioactive Constituents" Encyclopedia, https://encyclopedia.pub/entry/27715 (accessed February 07, 2026).
Nikou, T., Sakavitsi, M.E., Kalampokis, E., & Halabalaki, M. (2022, September 27). Metabolism and Bioavailability of Olive Bioactive Constituents. In Encyclopedia. https://encyclopedia.pub/entry/27715
Nikou, Theodora, et al. "Metabolism and Bioavailability of Olive Bioactive Constituents." Encyclopedia. Web. 27 September, 2022.
Copy Citation
Consumption of olive products has been established as a health-promoting dietary pattern due to their high content in compounds with eminent pharmacological properties and well-described bioactivities. However, their metabolism has not yet been fully described.
metabolism
ADMET properties
in vitro assays
in vivo
human studies
hydroxytyrosol
tyrosol
oleuropein
oleocanthal
oleacein
1. Introduction
The consumption of olive tree (Olea europaea L.—Oleaceae) products, such as olive oil (OO) and table olives, key components of the Mediterranean diet (MD), is now synonymous with a healthy way of eating and living. The positive health impact of olive products is attributed to their exceptional chemical composition [1]. For instance, OO is characterized by a unique mixture of esters of unsaturated fatty acids, such as oleic, linoleic and linolenic acids. Furthermore, apart from the lipophilic fraction of OO, in recent years, a different group of constituents has been placed in the scientific spotlight due to its members’ significant biological activity—the so-called “olive oil polyphenols or biophenols (OBs)” [2]. It regards relatively polar compounds, which are found in small concentrations and belong to diverse chemical classes (e.g., phenylalcohols, phenolic acids, secoiridoids, flavonoids, terpenoids, lignans, hydroxy-isocromans, etc.) [3]. Amongst them, the phenylalcohols tyrosol (Tyr) and hydroxytyrosol (HTyr)—together with the secoiridoids oleacein (Olea) and oleocanthal (Oleo)—are so far the most characteristic and studied OBs for their pharmacological properties [1][4]. Moreover, the secoiridoid glucoside oleuropein (Oleu), which is abundant in olive drupes and leaves and found only in traces in OO, is considered to be a significant bioactive, since a plethora of studies for its benefits to human health are available [5][6][7][8][9].
The OBs, acting as extra nutrients, seem to be responsible for diverse health-promoting and disease-preventing properties and are genuinely associated with the beneficial health effects of the MD [10]. HTyr and Tyr, apart from olive products, are also present in other species of Oleaceae and in red wine, and they are also endogenously formed in humans from dopamine and tyramine metabolism, respectively [11][12]. The secoiridoids (Olea, Oleo, Oleu) penetrate to humans exclusively through consumption of olive products, expressing their protective and/or therapeutic effects. From a chemical point of view, HTyr is a hydroxylated derivative of Tyr, and their esters with elenolic acid (EA) form Oleu and Lig aglycones, respectively. The glycosylated derivatives of the later lead to Oleu and Lig, whereas the loss of carboxymethyl moiety of the aglycone forms with the simultaneous opening of the secoiridoid ring leads to Olea and Oleo (Figure 1).
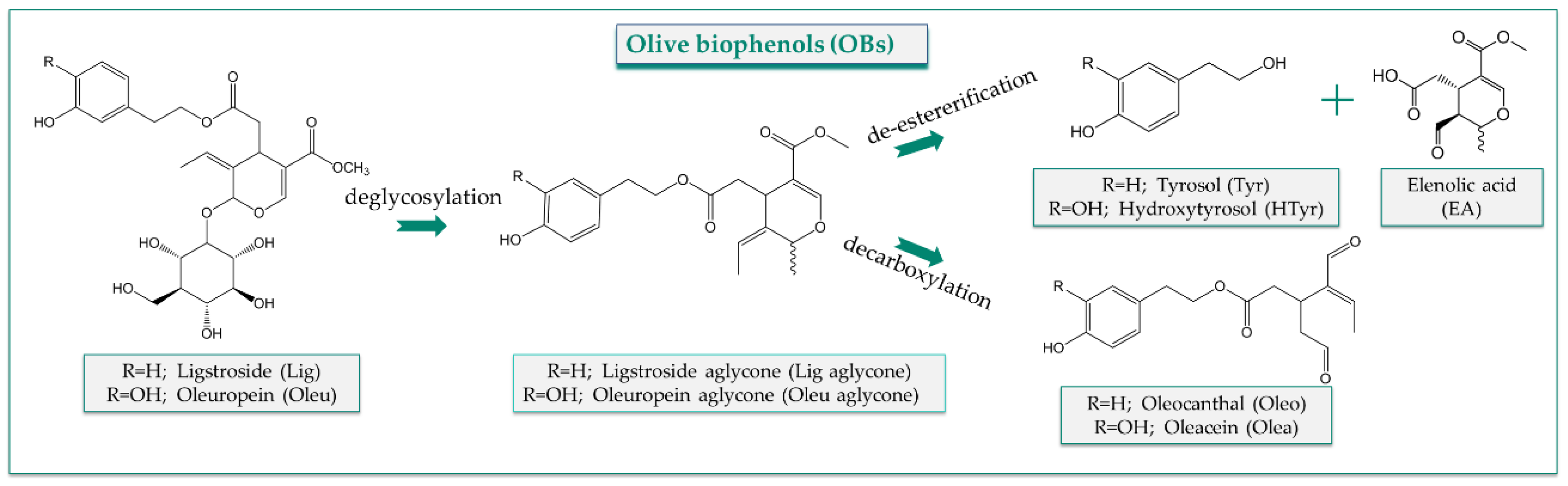
Figure 1. Chemical structures and formation of oleuropein (Oleu) and ligstroside (Lig) aglycones, oleocanthal (Oleo), oleacein (Olea), hydroxytyrosol (HTyr) and tyrosol (Tyr).
Numerous in vitro, in vivo and clinical studies have been performed over the last decade to determine the beneficial effects of OBs to human health [13][14][15]. Specifically, research on OBs has shown that there is a strong association of OBs with the prevention of certain pathologies in humans, placing them at the center of scientific interest. Research evidence has shown that OBs contribute to the improvement of human lipid profile and enhance high-density lipoprotein function [16], lower systolic pressure and prevent hypertension [17], regulate blood glucose [18] and reduce pro-inflammatory state [19] and oxidative stress [20]. These are just few of the studied beneficial effects of OBs to human health associated with several chronic and age-related disorders such as cancer, cardiovascular and neurodegenerative diseases [21][22][23]. Additionally, OBs have been found to protect against several metabolic disorders, such as insulin resistance [24] and obesity [25]. Based on experimental observations, OBs benefits are closely related to their chemical structure and their protective role against several diseases is attributed to their strong antioxidant and anti-inflammatory activity. They have been found to act as efficient free radical scavengers and metal ion chelators, counteracting the cytotoxic effects of oxidative stress in the organism [4][7][26]. In conjunction with the direct scavenging of reactive species, certain reports have demonstrated that the modulation of gene expression plays a crucial role in the antioxidant and anti-inflammatory properties of OBs [4]. Their anti-inflammatory properties are achieved by down-regulating inflammatory mediators via transcriptional or post-transcriptional mechanisms and by modulating the activation of kinases involved in the onset of inflammatory process at different levels [27][28]. Since oxidative stress pathways and inflammation are linked to numerous pathologies (neurodegenerative, cardiovascular and digestive disorders and cancer) the antioxidant and anti-inflammatory synergistic effects of OBs have been associated to the bioactivity of extra virgin OO against age-related and chronic diseases [1][4].
At this point, it must be noted that pivotal role for the demonstration of a biological and/or pharmacological function for natural products—and so for food bioactive compounds, such as OBs—is their bioavailability in humans [29]. Assessment of bioaccessibility and/or bioavailability can be performed in vitro by using special cell line studies or in vivo with animal models and human biological fluids. The meaning of bioavailability describes a nutrient or food bioactive which is efficiently digested, absorbed and become available to provide its beneficial effect to the organism, while bioaccessibility is the amount of each food bioactive that is efficiently released from the respective food matrix, possibly permeates into the gastrointestinal (GI) tract and if so, it is eventually absorbed from the organism [30]. Thus, bioavailability includes bioaccessibility of orally administered food bioactives. Additionally, pharmacokinetics (PK) are incorporated into determining the fate of a compound in in vivo systems through the investigation of compound absorption, distribution, metabolism and excretion (and toxicity) or ADME(T) studies [31]. The key element in these studies is the investigation of metabolism, the process by which xenobiotics (in researchers' study’s case, OBs as pure chemical entities, food constituents, enriched extracts or supplements) and endogenous metabolites are converted enzymatically into more hydrophilic and easily excreted compounds [32]. Formed or biotransformed metabolites of the parent molecule might possess similar or different activity or even potential toxicity [32]. Such studies are fundamental in a drug’s development process. However, they are sporadic in the area of food bioactives and rather rare in the area of natural products [33].
To that end, it is vital to understand the biotransformation of a parent compound into other analogues or derivatives that could possibly act as accomplices and/or competitors in the demonstration of a biological function; thus, acquaintance with the metabolism of a food bioactive compound is highly important [34]. Metabolism takes place in various tissues, of which liver and intestine are the main sites for orally administered compounds. Metabolism transformations can be divided into phase I and phase II reactions. Oxidation, hydrolysis and reduction are common phase I reactions mainly occurring in the stomach, liver and gut wall. Phase II reactions, also called conjugation reactions, occur mostly in the liver, but also in the small intestine and involve the addition of glucuronic, sulfate, glutathionyl, acetyl and methyl moieties on the molecule, catalyzed by the respective enzymes [35]. A compound often goes through phase I before phase II transformation. The phase III detoxification system must be mentioned as a part of a compound’s metabolism process since it has the important task of eliminating toxic metabolites from cells [36]. Finally, human gut microbiota makes key contributions to metabolism, transforming hundreds of food bioactives into metabolites with altered activity, toxicity and lifetime within the body [37].
Several scientific approaches have been proposed for the investigation of the metabolism of food bioactives, though is still remains mostly unexplored. This is especially true for OBs; despite the plethora of bibliographical data concerning their biological activity in vitro, in vivo and in human trials, the studies focusing on their bioavailability and metabolism are quite restricted. Routinely, in vitro assays are used to establish the metabolic profile of a moiety, incorporating microsomes, supersomes, cytosol, S-9 fraction and cell-based models (primary hepatocytes, liver slices and perfused liver), which contain metabolism enzymes or are tested for evaluation of compounds’ intestinal permeability [35]. Additionally, complementary in silico models are used to assist in vitro screenings and the prediction of the metabolism catalyzed by the respective enzymes [38]. In vitro bioaccessibility experiments are usually employed as an important first step in studying the influence of gastrointestinal digestion and food matrices on the bioavailability of compounds. The succeeding step is in vivo studies conducted to evaluate PK parameters. In vivo PK studies are performed with animals, such as mice and rats, to generate PK data and determine compounds’ clearance, bioavailability, exposure, half-life, distribution volume and toxicity [39][40]. As expected, the final go/no-go decision will be made after studies in humans. For food bioactives, such as OO and OBs, usually dietary interventions are designed monitoring Cmax and Tmax in human biological fluids (blood plasma) and excretions (urine, feces), which are investigated to detect the existence of possible metabolic derivatives of the administered compounds [41].
However, exploration of the metabolism of food bioactives is hindered by the existence of many complications. The use of low dosages, the complexity of the tested active entity in cases of extract supplementation (e.g., OBs) or foodstuffs (enriched with OBs OO) as well as unavailability of reference standards are regarded as critical complications in the field of natural product bioavailability and metabolism studies [40]. Therefore, the analytical platform which prescribes the limits of detection and sensitivity is of great importance as well as the preparation of samples prior analysis for the recovery of target compound(s) is used. For instance, OBs bind strongly to plasma proteins, hampering recovery prior to analysis, detection efficiency and the establishment of appropriate quantitation protocols [42]. For this purpose, alternative scientific approaches and more sophisticated methodologies are gradually incorporated to address these constraints. For example, metabolomics, which propose a holistic data mining and interpretation, have recently offered new opportunities to investigate metabolism patterns and pathways [43].
Additionally, the large interindividual variability affecting ADMET parameters, such as age, sex, dietary habits, microbiome composition, genetic variation, drug exposure and many other factors, complicate food bioactive evaluation further. Regarding OBs, few studies have reported individual data on their ADMET properties and on determinants such as the aforementioned factors [36][44]. Additionally, human gut microbiota complicates more the monitoring of OBs’ metabolic fate. OBs known to survive phase I and II metabolism finally undergo colonic metabolism by human gut microbiota. It has long been speculated by data in the literature that the microbial population of the human gut is a major contributor to the overall metabolism of not only orally submitted bioactive compounds but also of phase I and II metabolites that have been excreted back into the intestine via enterohepatic circulation [45][46]. In addition, the normal levels of endogenously produced metabolic derivatives in human biological fluids, such as the phenylalcohols HTyr and Tyr, should be taken into consideration in the design of such studies, and their PK parameters should be more carefully evaluated to reach reasonable conclusions.
More specifically, the information concerning the bioavailability of most OBs is limited despite the intensive research that has been devoted the past two decades to the investigation of their biological properties. This fact is reflected in the number of review papers published from 2002 to 2022 [29][47][48][49][50][51]. Regarding OBs’ metabolism, sporadic reviews have been published focusing either on specific compounds [12] or specific approaches [52], or—in most cases due to the high variety of chemical categories and extremely high number of different compounds included in each olive product—they are investigated and discussed separately [34][50]. Additionally, although over the past decade, the studies on colonic metabolism and modulation of gut microbiota by dietary compounds have gained scientific attention, bibliographical data that cover the colonic biotransformation of OBs are limited so far [52]. Finally, the data regarding humans are limited to the study of metabolism after HTyr or OO supplementation [53].
2. Metabolism and Bioavailability of Olive Bioactive Constituents
Currently, the bioavailability of food bioactives is studied as an integral part to the demonstration of a health benefit. Olive products, rich in OBs, are among the most studied foodstuffs in terms of their chemical composition and health beneficial effects, whereas limited and scattered data exist for their bioavailability. In the current research, the researchers attempted to gather all the recent information regarding the bioavailability and metabolism of OBs. The effort targeted the most studied chemical groups of compounds regarding their pharmacological properties, especially phenylalcohols and secoiridoids, namely HTyr, Tyr, Oleo, Olea and Oleu. The aim of the current approach was to highlight the basic metabolic differences of OBs according to their chemical structure. Special attention was also given on human gut microbiome metabolism, which currently prevails in contemporary research design. Based on the first scrutiny of the used literature, the majority of publications concentrate on HTyr, followed by Oleu, due to their commercial availability. As expected HTyr, phenolic extracts and enriched OO expose plentiful data in humans and less in vitro and in vivo due to the ease of their administration. However, this fact is not usually found in the study of natural products’ ADME properties. Researchers investigation revealed that regarding phenylalcohols, there is strong evidence for ADME(T) properties and there is a large amount of information regarding the bioavailability and metabolism of these compounds. Both HTyr and Tyr have been found to be absorbed in a dose-dependent manner in humans. A variation on results given for HTyr and Tyr is linked with the quantitation methodologies and their endogenous biosynthesis. At least 16 metabolic derivatives have been identified so far, while excretion levels Tmax and Cmax have been determined. Bioavailability is the only parameter under question due to analytical challenges existing for their detection in biological matrices.
On the other hand, fewer information was found for secoiridoids. The peculiar and sensitive chemical structure of secoiridoids hinders the design of metabolism studies due to isolation and detection difficulties of these compounds. The bioavailability of Olea and Oleo has been scarcely studied either in in vitro studies or in preclinical and clinical trials. Most research in this field regarding secoiridoids has been focused on Oleu and its aglycone forms. Oleu showed most of the data and witness possible biotransformations of the rest secoiridoids, yet all studies are focused on the phenylalcohol pathway, totally neglecting the elenolic part of the secoiridoids. Additionally, the lack of standard compounds has limited human intervention and is usually investigated as an extract or enriched OO. Generally, it can be presumed that in vitro outcomes can be used as first observations for the design and performance of in vivo experimentations, while the consequent human studies could give the go/no-go decision for the use of such compounds as nutraceuticals. However, more research is required to draw conclusions about the bioavailability and metabolism of such sensitive and unstable compounds. Additionally, this could provide insight regarding extra virgin OO as a more complex food mixture with multiple beneficial health effects. Regarding OBs’ colonic metabolism, limited studies were found and preliminary results around OBs’ metabolism by human gut microbiota could inspire the design of future experimental approaches. Hence, the metabolism studies of OO and OBs specifically is a contemporary subject of research that could contribute significantly to the general understanding of dietary interventions to prevent or even cure human malfunctions, guide personalized nutrition, inform toxicology risk assessment and improve drug discovery and development.
References
- Visioli, F.; Davalos, A.; de las Hazas, M.C.L.; Crespo, M.C.; Tomé-Carneiro, J. An overview of the pharmacology of olive oil and its active ingredients. Br. J. Pharmacol. 2020, 177, 1316–1330.
- Nikou, T.; Witt, M.; Stathopoulos, P.; Barsch, A.; Halabalaki, M. Olive Oil Quality and Authenticity Assessment Aspects Employing FIA-MRMS and LC-Orbitrap MS Metabolomic Approaches. Front. Public Health 2020, 8, 534.
- Angelis, A.; Hamzaoui, M.; Aligiannis, N.; Nikou, T.; Michailidis, D.; Gerolimatos, P.; Termentzi, A.; Hubert, J.; Halabalaki, M.; Renault, J.-H.; et al. An integrated process for the recovery of high added-value compounds from olive oil using solid support free liquid-liquid extraction and chromatography techniques. J. Chromatogr. A 2017, 1491, 126–136.
- Rodríguez-López, P.; Lozano-Sanchez, J.; Borrás-Linares, I.; Emanuelli, T.; Menéndez, J.A.; Segura-Carretero, A. Structure–biological activity relationships of extra-virgin olive oil phenolic compounds: Health properties and bioavailability. Antioxidants 2020, 9, 685.
- Acar-Tek, N.; Ağagündüz, D. Olive Leaf (Olea europaea L. folium): Potential Effects on Glycemia and Lipidemia. Ann. Nutr. Metab. 2020, 76, 10–15.
- Silenzi, A.; Giovannini, C.; Scazzocchio, B.; Varì, R.; D’Archivio, M.; Santangelo, C.; Masella, R. Extra virgin olive oil polyphenols: Biological properties and antioxidant activity. In Pathology; Academic Press: Cambridge, MA, USA, 2020; pp. 225–233.
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686.
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219.
- Carrera-González, M.P.; Ramírez-Expósito, M.J.; Mayas, M.D.; Martínez-Martos, J.M. Protective role of oleuropein and its metabolite hydroxytyrosol on cancer. Trends Food Sci. Technol. 2013, 31, 92–99.
- Parkinson, L.; Cicerale, S. The health benefiting mechanisms of virgin olive oil phenolic compounds. Molecules 2016, 21, 1734.
- Soldevila-Domenech, N.; Boronat, A.; Mateus, J.; Diaz-Pellicer, P.; Matilla, I.; Pérez-Otero, M.; Aldea-Perona, A.; De La Torre, R. Generation of the antioxidant hydroxytyrosol from tyrosol present in beer and red wine in a randomized clinical trial. Nutrients 2019, 11, 2241.
- Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236.
- Castañer, O.; Covas, M.-I.; Khymenets, O.; Nyyssonen, K.; Konstantinidou, V.; Zunft, H.-F.; de la Torre, R.; Muñoz-Aguayo, D.; Vila, J.; Fitó, M. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am. J. Clin. Nutr. 2012, 95, 1238–1244.
- Moreno-Luna, R.; Muñoz-Hernandez, R.; Miranda, M.L.; Costa, A.F.; Jimenez-Jimenez, L.; Vallejo-Vaz, A.J.; Muriana, F.J.G.; Villar, J.; Stiefel, P. Olive Oil Polyphenols Decrease Blood Pressure and Improve Endothelial Function in Young Women with Mild Hypertension. Am. J. Hypertens. 2012, 25, 1299–1304.
- Liu, Z.; Wang, N.; Ma, Y.; Wen, D. Hydroxytyrosol improves obesity and insulin resistance by modulating gut microbiota in high-fat diet-induced obese mice. Front. Microbiol. 2019, 10, 390.
- Hernáez, Á.; Fernández-Castillejo, S.; Farràs, M.; Catalán, Ú.; Subirana, I.; Montes, R.; Solà, R.; Muñoz-Aguayo, D.; Gelabert-Gorgues, A.; Díaz-Gil, Ó.; et al. Olive oil polyphenols enhance high-density lipoprotein function in humans: A randomized controlled trial. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2115–2119.
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258.
- Violi, F.; Loffredo, L.; Pignatelli, P.; Angelico, F.; Bartimoccia, S.; Nocella, C.; Cangemi, R.; Petruccioli, A.; Monticolo, R.; Pastori, D.; et al. Extra virgin olive oil use is associated with improved post-prandial blood glucose and LDL cholesterol in healthy subjects. Nutr. Diabetes 2015, 5, e172.
- Bonura, A.; Vlah, S.; Longo, A.; Bulati, M.; Melis, M.R.; Cibella, F.; Colombo, P. Hydroxytyrosol modulates Par j 1-induced IL-10 production by PBMCs in healthy subjects. Immunobiology 2016, 221, 1374–1377.
- Del Río, L.F.; Gutiérrez-Casado, E.; Varela-López, A.; Villalba, J.M. Olive oil and the hallmarks of aging. Molecules 2016, 21, 163.
- Lockyer, S.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Olive leaf phenolics and cardiovascular risk reduction: Physiological effects and mechanisms of action. Nutr. Aging 2012, 1, 125–140.
- Markellos, C.; Ourailidou, M.-E.; Gavriatopoulou, M.; Halvatsiotis, P.; Sergentanis, T.N.; Psaltopoulou, T. Olive oil intake and cancer risk: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0261649.
- Sirangelo, I.; Borriello, M.; Liccardo, M.; Scafuro, M.; Russo, P.; Iannuzzi, C. Hydroxytyrosol Selectively Affects Non-Enzymatic Glycation in Human Insulin and Protects by AGEs Cytotoxicity. Antioxidants 2021, 10, 1127.
- de Bock, M.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) Leaf Polyphenols Improve Insulin Sensitivity in Middle-Aged Overweight Men: A Randomized, Placebo-Controlled, Crossover Trial. PLoS ONE 2013, 8, e57622.
- Fytili, C.; Nikou, T.; Tentolouris, N.; Tseti, I.K.; Dimosthenopoulos, C.; Sfikakis, P.P.; Simos, D.; Kokkinos, A.; Skaltsounis, A.L.; Katsilambros, N.; et al. Effect of Long-Term Hydroxytyrosol Administration on Body Weight, Fat Mass and Urine Metabolomics: A Randomized Double-Blind Prospective Human Study. Nutrients 2022, 14, 1525.
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257.
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135.
- Serreli, G.; Deiana, M. Biological relevance of extra virgin olive oil polyphenols metabolites. Antioxidants 2018, 7, 170.
- De La Torre, R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology 2008, 16, 245–247.
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588.
- Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. ADMET-score—A comprehensive scoring function for evaluation of chemical drug-likeness. Medchemcomm 2019, 10, 148–157.
- Borah, P.; Hazarika, S.; Deka, S.; Venugopala, K.N.; Nair, A.B.; Attimarad, M.; Sreeharsha, N.; Mailavaram, R.P. Application of Advanced Technologies in Natural Product Research: A Review with Special Emphasis on ADMET Profiling. Curr. Drug Metab. 2020, 21, 751–767.
- Paine, M.F. Natural Products: Experimental Approaches to Elucidate Disposition Mechanisms and Predict Pharmacokinetic Drug Interactions. Drug Metab. Dispos. 2020, 48, 956–962.
- Galmés, S.; Reynés, B.; Palou, M.; Palou-March, A.; Palou, A. Absorption, Distribution, Metabolism, and Excretion of the Main Olive Tree Phenols and Polyphenols: A Literature Review. J. Agric. Food Chem. 2021, 69, 5281–5296.
- Ooka, M.; Lynch, C.; Xia, M. Application of In Vitro Metabolism Activation in High-Throughput Screening. Int. J. Mol. Sci. 2020, 21, 8182.
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22.
- Koppel, N.; Rekdal, V.M.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, 1246–1257.
- Cheng, F.; Li, W.; Liu, G.; Tang, Y. In Silico ADMET Prediction: Recent Advances, Current Challenges and Future Trends. Curr. Top. Med. Chem. 2013, 13, 1273–1289.
- Reichel, A.; Lienau, P. Pharmacokinetics in Drug Discovery: An Exposure-Centred Approach to Optimising and Predicting Drug Efficacy and Safety. Handb. Exp. Pharmacol. 2016, 232, 235–260.
- Derendorf, H. Pharmacokinetics of Natural Compounds. Planta Med. 2012, 78, IL41.
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249.
- González-Santiago, M.; Fonollá, J.; Lopez-Huertas, E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol. Res. 2010, 61, 364–370.
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M.; et al. Nutrigenomics of extra-virgin olive oil: A review. BioFactors 2017, 43, 17–41.
- Landberg, R.; Manach, C.; Kerckhof, F.-M.; Minihane, A.-M.; Saleh, R.N.M.; De Roos, B.; Tomas-Barberan, F.; Morand, C.; Van de Wiele, T. Future prospects for dissecting inter-individual variability in the absorption, distribution and elimination of plant bioactives of relevance for cardiometabolic endpoints. Eur. J. Nutr. 2019, 58, 21–36.
- Gallardo, E.; Sarria, B.; Espartero, J.L.; Correa, J.A.G.; Bravo-Clemente, L.; Mateos, R. Evaluation of the Bioavailability and Metabolism of Nitroderivatives of Hydroxytyrosol Using Caco-2 and HepG2 Human Cell Models. J. Agric. Food Chem. 2016, 64, 2289–2297.
- Suárez, M.; Romero, M.P.; Macià, A.; Valls, R.M.; Fernández, S.; Solà, R.; Motilva, M.J. Improved method for identifying and quantifying olive oil phenolic compounds and their metabolites in human plasma by microelution solid-phase extraction plate and liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 4097–4106.
- Corona, G.; Spencer, J.; Dessì, M. Extra virgin olive oil phenolics: Absorption, metabolism, and biological activities in the GI tract. Toxicol. Ind. Health 2009, 25, 285–293.
- de la Torre-Carbot, K.; Chávez-Servín, J.L.; Jaúregui, O.; Castellote, A.I.; Lamuela-Raventós, R.M.; Fitó, M.; Covas, M.I.; Muñoz-Aguayo, D.; López-Sabater, M.C. Presence of virgin olive oil phenolic metabolites in human low density lipoprotein fraction: Determination by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Anal. Chim. Acta 2007, 583, 402–410.
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644.
- Visioli, F.; Galli, C.; Galli, G.; Caruso, D. Biological activities and metabolic fate of olive oil phenols. Eur. J. Lipid Sci. Technol. 2002, 104, 677–684.
- Vissers, M.N.; Zock, P.L.; Katan, M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur. J. Clin. Nutr. 2004, 58, 955–965.
- Mosele, J.I.; Martín-Peláez, S.; Macià, A.; Farràs, M.; Valls, R.M.; Catalán, Ú.; Motilva, M.J. Faecal microbial metabolism of olive oil phenolic compounds: In vitro and in vivo approaches. Mol. Nutr. Food Res. 2014, 58, 1809–1819.
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
771
Revisions:
2 times
(View History)
Update Date:
28 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




