Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ramon Farre | -- | 2124 | 2022-09-27 10:58:36 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2124 | 2022-09-28 03:59:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Farré, R.; Gozal, D.; Nguyen, V.; Pearce, J.M.; Dinh-Xuan, A.T. Low-Cost, Open-Source Devices for Respiratory Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/27668 (accessed on 08 February 2026).
Farré R, Gozal D, Nguyen V, Pearce JM, Dinh-Xuan AT. Low-Cost, Open-Source Devices for Respiratory Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/27668. Accessed February 08, 2026.
Farré, Ramon, David Gozal, Viet-Nhung Nguyen, Joshua M. Pearce, Anh Tuan Dinh-Xuan. "Low-Cost, Open-Source Devices for Respiratory Diseases" Encyclopedia, https://encyclopedia.pub/entry/27668 (accessed February 08, 2026).
Farré, R., Gozal, D., Nguyen, V., Pearce, J.M., & Dinh-Xuan, A.T. (2022, September 27). Low-Cost, Open-Source Devices for Respiratory Diseases. In Encyclopedia. https://encyclopedia.pub/entry/27668
Farré, Ramon, et al. "Low-Cost, Open-Source Devices for Respiratory Diseases." Encyclopedia. Web. 27 September, 2022.
Copy Citation
Respiratory diseases pose an increasing socio-economic burden worldwide given their high prevalence and their elevated morbidity and mortality. Medical devices play an important role in managing acute and chronic respiratory failure, including diagnosis, monitoring, and providing artificial ventilation.
medical devices
low-cost
low- and middle-income countries
flowmeters
1. Device to Measure Maximal Inspiratory and Expiratory Pressures
Measurement of the maximal inspiratory (MIP) and expiratory (MEP) pressures that a patient can exert allows for assessing the functional performance of his/her respiratory muscles. MIP and MEP are altered in very prevalent diseases such as chronic obstructive pulmonary disease (COPD), neuromuscular diseases (e.g., multiple sclerosis, muscular dystrophies), or chronic heart failure. Since measuring MIP and MEP is non-invasive, this technique helps in diagnosing and characterizing the disease and in monitoring the evolution of the patient’s disease status and response to therapy. Given that commercial devices for measuring MIP/MEP are expensive, a low-cost, open-source device has been recently proposed [1]. The MIP/MEP measurement technique is straightforward from both conceptual and technical viewpoints. It is based on recording pressures at a mouthpiece, computing the average of the highest pressures generated over a 1 s period of stable inspiratory/expiratory effort and providing the variability across values in subsequent maneuvers to select the maximum value from several representative efforts. The device (Figure 1A) was designed using simple, inexpensive, and easy-to-find components (most of them purchased from e-commerce sources). The setting consisted of a development board with an Arduino microcontroller, an LCD screen, a pressure transducer, a rechargeable 9 V battery block, a switch, a power supply base, and a customized enclosure produced by using any plastic-based 3D printer (most of which are open-source or derived from RepRap 3D printers). A measuring session starts by asking the user to select a MIP or MEP measurement to run; then, data acquisition begins immediately. After 5 s of pressure signal sampling, the device screen shows the corresponding pressure–time curve and the MIP/MEP values are computed according to conventional rules [1]. The device asks the user whether the maneuver should be accepted or rejected and whether a new maneuver should be carried out (Figure 1B). After repeated maneuvers, the device shows all data from previously accepted tests and informs on whether the quality control criterion to select the final result has been achieved [1]. Figure 1A shows that the device has two independent blocks connected through a 1 m length (3 mm ID) silicone tube. One of them is the handheld framework, used by the health technician, containing the electronics and digital display of the measurement process and results. The second block is a handheld mouthpiece support for a disposable patient’s mouthpiece. The cost of all the components was ≈ 80 €. Figure 1C,D show the results obtained when the low-cost, open-source device was evaluated by simultaneous comparison with a laboratory reference setting (Bland-Altman and linear regression plots, respectively). The average difference in MIP/MEP values between the low-cost device and the lab reference setting was only 0.13 cmH2O (limits of agreement from −0.86 to 1.12 cmH2O), which corresponds to ±1% accuracy. Accordingly, the developed device is suitable for performing MIP and MEP measurements within clinical ranges. The device design was published using the open-source hardware approach. Therefore, it can facilitate measuring MIP/MEP by readily available point-of-care devices for patient monitoring. Most importantly, it can make this respiratory function measurement tool affordable to users in LMICs.
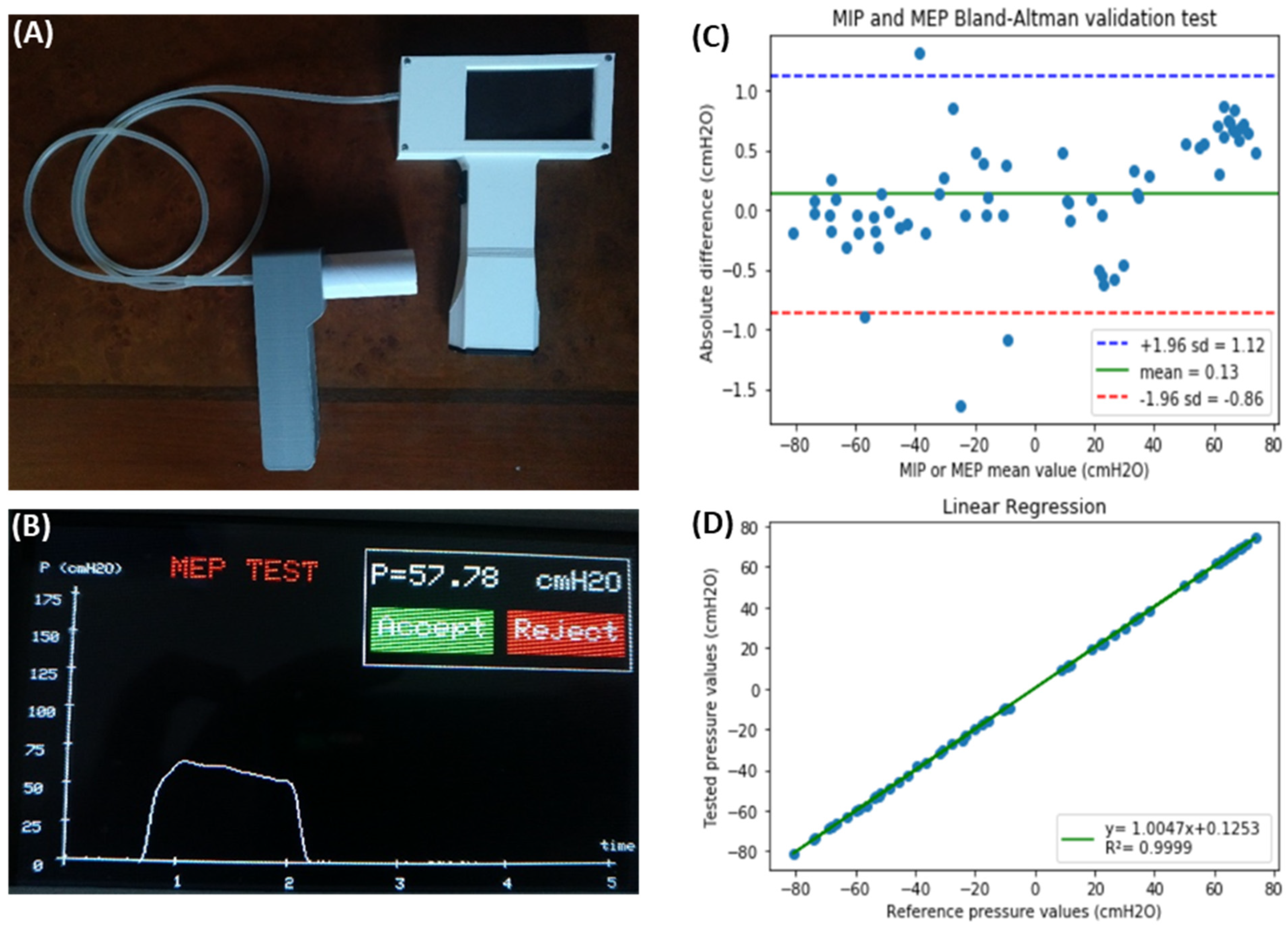
Figure 1. (A) Complete external view of the device showing the operator’s handheld block and the patient’s mouthpiece block, connected through a flexible silicone tube. (B) Example of one of the screens during device operation, showing the result of an MEP maneuver test (time course of expiratory pressure, MEP result, and the option to allow the user to accept or reject this specific maneuver). (C) Bland–Altman plot showing the difference between values measured by the prototype and by laboratory reference equipment, as a function of the measured values for both MIP (negative values) and MEP (positive values). The green line is the prototype bias, and the blue–red lines indicate the limits of agreement. (D) Linear regression of the values obtained with the developed device and the laboratory reference. Reprinted with permission from Ref. [1]. Creative Commons CC-BY license.
2. Device to Measure the Tidal Volume Delivered by Mechanical Ventilators
Tidal volume (VT), the volume of air achieved during inspiration, is one of the main parameters required during mechanical ventilation in patients with respiratory failure. Therefore, precise measurement of VT is of great importance for both controlling blood gases and avoiding ventilator-induced lung injury. However, this measurement, usually carried out by the ventilator, is complex since it requires a series of corrections for oxygen concentration in the air, dead space of the ventilator circuit, and temperature and humidity conditions. As each of these three corrections may induce up to 10% variance in measured VT, periodic quality control testing is required, particularly in clinical settings where medical device maintenance could be suboptimal, for instance, in LMICs. To avoid the need for reference devices measuring VT, which are based on expensive gold standard sensors, a simple procedure has been recently described [2]. This procedure can be readily followed by clinical staff who are not experts in instrumentation techniques. Figure 2A shows a diagram of the rationale, which is based on measuring the volume of inspired air (VT) directly from water displacement. Interestingly, for users in LMICs, Figure 2B shows a low-cost implementation made using 15 cm diameter PVC tubing components that are widely available in hardware stores. Assuming the 1 mm resolution in the common ruler for assessing h in the setting in Figure 2B, the resolution in VT measurement corresponds to 0.43% and 0.86% for maximum and typical VT values of 1000 and 500 mL, respectively, being by far sufficient to detect any potential real-life errors when tidal volume is measured by mechanical ventilators.
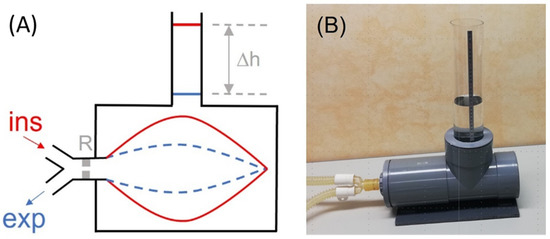
Figure 2. (A) Diagram of the method described for directly measuring the tidal volume (VT) delivered by a mechanical ventilator. A lung test, consisting of an orifice-type resistor (R) and a compliant bag enclosed in a water chamber open to the atmosphere through a vertical tube, is connected to the inspiratory and expiratory lines of the mechanical ventilator. The VT introduced into the bag induces an increase in the height (∆h) of the water level in the tube, from end-expiration (blue) to end-inspiration (red). (B) Example of low-cost implementation of the measuring setting. The chamber was made with 15 cm diameter PVC drainpipe fittings. One of the cylinder bases was a screw cap to allow replacing the bag. The transparent vertical tube has an internal diameter of 7.4 cm (section: 43.01 cm2); hence, VT (in mL) = 43.01 · h (in cm). Reprinted with permission from Ref. [2]. Creative Commons CC-BY license.
3. Construction and Calibration of Accurate Pneumotachographs
Pneumotachographs, which are the sensors measuring ventilation airflow (and volume by flow integration), are basic integral components required in mechanical ventilators. Given that standard pneumotachographs are expensive and require careful cleaning and maintenance, a recent publication has provided full details for low-cost and simple construction and calibration of robust pneumotachographs made by manual perforation of a plate with a domestic drill [3]. Their pressure–volume relationship is characterized by a quadratic equation with parameters that can be tailored by the number and diameter of the perforations (Figure 3A–E). The calibration parameters of the pneumotachographs can be measured through two maneuvers with a conventional resuscitation bag and by assessing the maneuver volumes with an inexpensive and straightforward water displacement setting. The performance of these simple, inexpensive pneumotachographs was assessed by comparison with a reference gold standard pneumotachograph in a bench test where conventional mechanical ventilation was applied to a simulated patient. As shown in Figure 3F,G, under realistic mechanical ventilation settings (pressure- and volume-control; 200−600 mL), the simple pneumotachographs were able to accurately measure inspiratory flow and tidal volume (VT errors of 2.1% on average and <4% in the worst case). Therefore, these easy-to-reproduce pneumotachographs and the calibration method facilitate the low-cost and simple availability of pneumotachographs for accurately controlling mechanical ventilation in low-resource settings, either by incorporating them into the ventilators or as external measuring devices for quality control.
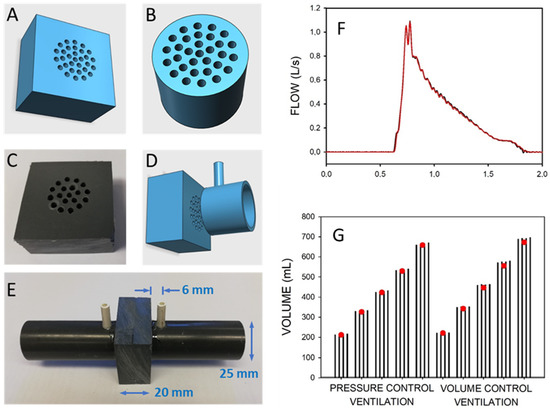
Figure 3. Low-cost pneumotachograph. (A,B) Diagrams of resistors for the pneumotachographs. (B,C) Photograph of the manually drilled resistor. (C,D) Diagram of resistor and (one side) standard PVC tube piece to assemble the pneumotach. (E) Photograph of the assembled pneumotachograph. (F) Example of the flow signals during pressure-controlled mechanical ventilation simultaneously measured by a reference pneumotachograph (red) and by the pneumotachograph constructed and calibrated by the low-cost procedures (black). (G) Volume measured during different magnitudes of pressure-control and volume-control mechanical ventilation of a patient model. Volume was simultaneously measured with the low-cost and the reference pneumotachograph (open circles). Each set of four bars corresponds to the volumes measured by the low-cost pneumotachograph using independent calibrations. Reprinted with permission from Ref. [3]. Creative Commons CC-BY license.
4. Pediatric Continuous Positive Airway Pressure (CPAP)
The provision of therapy with continuous positive airway pressure (CPAP) has proven efficacy in the treatment of pneumonia in children, the leading cause of death in under-5-year-old patients in LMICs. Given the high cost of conventionally marketed CPAP devices, which are unaffordable for the majority of healthcare systems in low-resource regions, expanding access to potential CPAP treatment relies on the provision of simple and cheap devices for the application of this respiratory support therapy. Figure 4 shows in detail the example of a recent open-source proposal to facilitate the local construction of pediatric CPAP devices [4]. The setting (Figure 4A) is based on off-the-shelf, easy-to-purchase components (a pump and a heater/controller domestic aquarium, and two rotameters), which are assembled into a 3D printed enclosure. The total cost of the CPAP device was <100 €. When tested on the bench on a simulated patient with realistic breathing [4], the device was shown to provide CPAP support with precision and stability similar to commercial devices (Figure 4B).
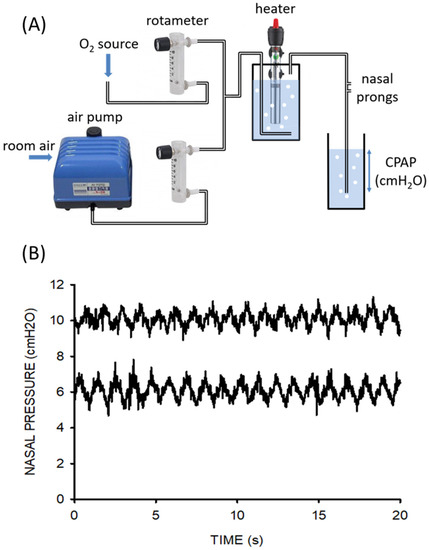
Figure 4. (A) Diagram of the CPAP setting, including its essential components: domestic aquarium air pump, rotameters, and domestic aquarium water heater/controller. (B) Nasal pressures actually applied at nasal prongs by the novel device for CPAP settings of 6 and 10 cm H2O. The simulated newborn infant was breathing with a tidal volume of 20 mL and frequency of 55 breaths/min while 8 L/min of humidified heated airflow circulated through the circuit. Reprinted with permission from Ref. [4]. Copyright by the American Thoracic Society.
5. Non-Invasive Pressure Support Ventilator
Current pricing of commercial mechanical ventilators in LMICs markedly restricts their availability, and consequently, a considerable number of patients with severe respiratory diseases cannot be adequately treated. To reduce the serious shortage of ventilators in low-resource regions, a recent simple, open-source, non-invasive bilevel pressure ventilator has been designed and evaluated in human subjects [5]. The ventilator (Figure 5A) was built using off-the-shelf materials available via e-commerce and was based on a high-pressure blower, two pressure transducers, and an Arduino Nano controller with a digital display (total retail cost < 75 USD$). The ventilator was first evaluated and compared with a commercially available device (Lumis-150, Resmed) on the bench using an actively breathing patient simulator mimicking a range of obstructive/restrictive diseases. The low-cost ventilator was able to provide inspiratory/expiratory pressures of up to 20/10 cmH2O, respectively, with no faulty triggering or cycling. The ventilator was also tested in 12 healthy volunteers wearing a high airway resistance and thoracic/abdominal bands to mimic obstructive/restrictive patients. When applied under conditions mimicking patients, the ventilator was able to support the subjects’ breathing with highly demanding inspiratory and expiratory pressures with no artifacts in cycling and triggering. Figure 5B shows an example of the pressure signal (provided by the ventilator sensor) when a volunteer mimicking an obstructive–restrictive patient was supported with strenuous inspiratory and expiratory pressures of 22 and 10 cm H2O, respectively. Interestingly, Figure 6 shows that the ventilator function was comfortable for subjects. Indeed, the breathing difficulty score rated (1–10 scale) by the loaded breathing subjects was significantly (p < 0.005) decreased from 5.45 ± 1.68 without support to 2.83 ± 1.66 when using the prototype ventilator, which showed no difference compared with the commercial device (2.80 ± 1.48; p = 1.000). Consequently, this open-source proposal for a low-cost, easy-to-build, non-invasive ventilator performs similarly to a high-quality commercial device, thereby allowing for free replication and use in LMICs, and thus, facilitating the application of this life-saving therapy to patients who otherwise could not be treated.
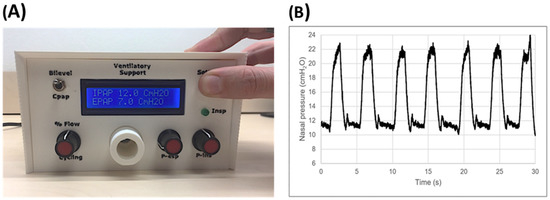
Figure 5. (A) Front view of the low-cost ventilator prototype. (B) Example of pressure signal recorded when the prototype ventilator supported a resistive–restrictive loaded breathing volunteer’s breathing. These are unfiltered raw data from the sensor within the ventilator. Reprinted with permission from Ref. [5]. Creative Commons CC-BY license.
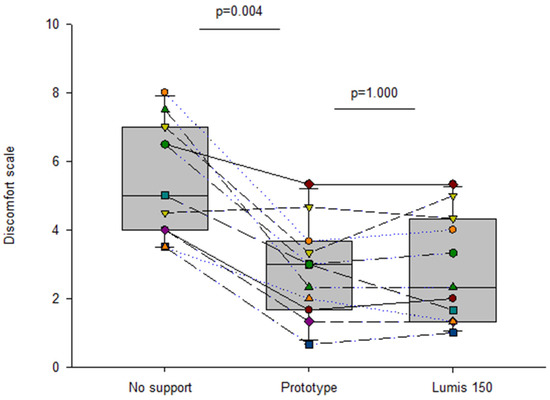
Figure 6. Discomfort scoring (Visual Analog Scale) in healthy volunteers subjected to obstructive–restrictive loaded breathing when unsupported and when supported by the prototype and Lumis 150 ventilators. Reprinted with permission from Ref. [5]. Creative Commons CC-BY license.
References
- Aymerich, C.; Rodríguez-Lázaro, M.; Solana, G.; Farré, R.; Otero, J. Low-Cost Open-Source Device to Measure Maximal Inspiratory and Expiratory Pressures. Front. Physiol. 2021, 12, 719372.
- Farré, R.; Artigas, A.; Torres, A.; Albaiceta, G.M.; Dinh-Xuan, A.T.; Gozal, D. A Simple Procedure to Measure the Tidal Volume Delivered by Mechanical Ventilators: A Tool for Bedside Verification and Quality Control. Arch. Bronconeumol. 2022; in press.
- Farré, R.; Rodríguez-Lázaro, M.A.; Gozal, D.; Trias, G.; Solana, G.; Navajas, D.; Otero, J. Simple low-cost construction and calibration of accurate pneumotachographs for monitoring mechanical ventilation in low-resource settings. Fron. Med. 2022, 9, 938949.
- Farré, R.; Trias, G.; Solana, G.; Ginovart, G.; Gozal, D.; Navajas, D. Novel Approach for Providing Pediatric Continuous Positive Airway Pressure Devices in Low-Income, Underresourced Regions. Am. J. Respir. Crit. Care Med. 2019, 199, 118–120.
- Garmendia, O.; Rodríguez-Lazaro, M.A.; Otero, J.; Phan, P.; Stoyanova, A.; Dinh-Xuan, A.T.; Gozal, D.; Navajas, D.; Montserrat, J.M.; Farré, R. Low-cost, easy-to-build noninvasive pressure support ventilator for under-resourced regions: Open source hardware description, performance and feasibility testing. Eur. Respir. J. 2020, 55, 2000846.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
965
Revisions:
2 times
(View History)
Update Date:
28 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




