
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kevin Honeychurch | -- | 4113 | 2022-09-27 09:35:10 | | | |
| 2 | Beatrix Zheng | Meta information modification | 4113 | 2022-09-28 03:08:51 | | |
Video Upload Options
The detection of analytes is optically difficult in planta due to tissue thickness and the presence of photosynthetic pigments in plant tissues. Nanosensors are well-suited for the detection of analytes as they are easily embedded in plant tissues. They are thus well-suited for in vivo studies of cellular signalling and metabolism.
1. Detection of Molecular Oxygen
2. Water and Humidity Nanosensors
3. Detection of Adenosine Triphosphate
4. Detection of Calcium Ions
5. Detection of Reactive Oxygen Species
6. Detection of Nitric Oxide
7. Detection of Plant Hormones
8. Determination of Fruit Ripening
9. Plant Pathogen Detection
10. Fertiliser and Pesticide Management
11. Summary
The application of nanosensors has been shown to meet some of the greatest challenges presently facing us, allowing for insights that can be developed to support plant growth and food security. The ability to monitor and determine plant characteristics is essential for plant breeding programmes and incorporation of desirable traits in plants. The application of nanosensors in the plant science offers opportunities to investigate distribution and transport of analytes, nutrients and pathogens in vivo, as well as plant signalling, and plant responses to environmental conditions.
References
- Ast, C.; Schmälzlin, E.; Löhmannsröben, H.-G.; van Dongen, J.T. Optical Oxygen Micro- and Nanosensors for Plant Applications. Sensors 2012, 12, 7015–7032.
- Considine, M.J.; Diaz-Vivancos, P.; Kerchev, P.; Signorelli, S.; Agudelo-Romero, P.; Gibbs, D.J.; Foyer, C.H. Learning to Breathe: Developmental Phase Transitions in Oxygen Status. Trends Plant Sci. 2017, 22, 140–153.
- Clark, L.C.; Wolf, R.; Granger, D.; Taylor, Z. Continuous Recording of Blood Oxygen Tensions by Polarography. J. Appl. Physiol. 1953, 6, 189–193.
- Bykova, N.V.; Keerberg, O.; Pärnik, T.; Bauwe, H.; Gardeström, P. Interaction between Photorespiration and Respiration in Transgenic Potato Plants with Antisense Reduction in Glycine Decarboxylase. Planta 2005, 222, 130–140.
- Day, D.; Neuburger, M.; Douce, R. Biochemical Characterization of Chlorophyll-Free Mitochondria from Pea Leaves. Funct. Plant Biol. 1985, 12, 219.
- Shaw, D.S.; Meitha, K.; Considine, M.J.; Foyer, C.H. Mitochondrial Respiration and Oxygen Tension. Methods Mol. Biol. 2017, 1670, 97–113.
- Kearns, A.; Whelan, J.; Young, S.; Elthon, T.E.; Day, D.A. Tissue-Specific Expression of the Alternative Oxidase in Soybean and Siratro. Plant Physiol. 1992, 99, 712–717.
- Alova, A.; Erofeev, A.; Gorelkin, P.; Bibikova, T.; Korchev, Y.; Majouga, A.; Bulychev, A. Prolonged Oxygen Depletion in Microwounded Cells of Chara Corallina Detected with Novel Oxygen Nanosensors. J. Exp. Bot. 2020, 71, 386–398.
- Buck, S.M.; Xu, H.; Brasuel, M.; Philbert, M.A.; Kopelman, R. Nanoscale Probes Encapsulated by Biologically Localized Embedding (PEBBLEs) for Ion Sensing and Imaging in Live Cells. Talanta 2004, 63, 41–59.
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; ISBN 0387312781.
- Schmälzlin, E.; Van Dongen, J.T.; Klimant, I.; Marmodée, B.; Steup, M.; Fisahn, J.; Geigenberger, P.; Löhmannsröben, H.G. An Optical Multifrequency Phase-Modulation Method Using Microbeads for Measuring Intracellular Oxygen Concentrations in Plants. Biophys. J. 2005, 89, 1339–1345.
- Chen, Y.; Tian, Y.; Wang, X.; Dong, L. Miniaturized Soil Sensor for Continuous, In-Situ Monitoring of Soil Water Potential. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (Transducers & Eurosensors XXXIII), Berlin, Germany, 23–27 June 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 2025–2028.
- Leone, M.; Consales, M.; Passeggio, G.; Buontempo, S.; Zaraket, H.; Youssef, A.; Persiano, G.V.; Cutolo, A.; Cusano, A. Fiber Optic Soil Water Content Sensor for Precision Farming. Opt. Laser Technol. 2022, 149, 107816.
- Lan, L.; Le, X.; Dong, H.; Xie, J.; Ying, Y.; Ping, J. One-Step and Large-Scale Fabrication of Flexible and Wearable Humidity Sensor Based on Laser-Induced Graphene for Real-Time Tracking of Plant Transpiration at Bio-Interface. Biosens. Bioelectron. 2020, 165, 112360.
- Saito, K.; Chang, Y.F.; Horikawa, K.; Hatsugai, N.; Higuchi, Y.; Hashida, M.; Yoshida, Y.; Matsuda, T.; Arai, Y.; Nagai, T. Luminescent Proteins for High-Speed Single-Cell and Whole-Body Imaging. Nat. Commun. 2012, 3, 1262.
- Krebs, M.; Held, K.; Binder, A.; Hashimoto, K.; den Herder, G.; Parniske, M.; Kudla, J.; Schumacher, K. FRET-Based Genetically Encoded Sensors Allow High-Resolution Live Cell Imaging of Ca2+ Dynamics. Plant J. 2012, 69, 181–192.
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399.
- Foyer, C.H. Reactive Oxygen Species, Oxidative Signaling and the Regulation of Photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142.
- Mattila, H.; Khorobrykh, S.; Havurinne, V.; Tyystjärvi, E. Reactive Oxygen Species: Reactions and Detection from Photosynthetic Tissues. J. Photochem. Photobiol. B Biol. 2015, 152, 176–214.
- Neill, S.J.; Desikan, R.; Clarke, A.; Hurst, R.D.; Hancock, J.T. Hydrogen Peroxide and Nitric Oxide as Signalling Molecules in Plants. J. Exp. Bot. 2002, 53, 1237–1247.
- Quan, L.-J.; Zhang, B.; Shi, W.-W.; Li, H.-Y. Hydrogen Peroxide in Plants: A Versatile Molecule of the Reactive Oxygen Species Network. J. Integr. Plant Biol. 2008, 50, 2–18.
- Hanson, G.T.; Aggeler, R.; Oglesbee, D.; Cannon, M.; Capaldi, R.A.; Tsien, R.Y.; Remington, S.J. Investigating Mitochondrial Redox Potential with Redox-Sensitive Green Fluorescent Protein Indicators. J. Biol. Chem. 2004, 279, 13044–13053.
- Wierer, S.; Peter, S.; Elgass, K.; Mack, H.-G.; Bieker, S.; Meixner, A.J.; Zentgraf, U.; Schleifenbaum, F. Determination of the in vivo Redox Potential by One-Wavelength Spectro-Microscopy of RoGFP. Anal. Bioanal. Chem. 2012, 403, 737–744.
- Schnaubelt, D.; Queval, G.; Dong, Y.; Diaz-Vivancos, P.; Makgopa, M.E.; Howell, G.; de Simone, A.; Bai, J.; Hannah, M.A.; Foyer, C.H. Low Glutathione Regulates Gene Expression and the Redox Potentials of the Nucleus and Cytosol in Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 266–279.
- García-Quirós, E.; Alché, J.d.D.; Karpinska, B.; Foyer, C.H. Glutathione Redox State Plays a Key Role in Flower Development and Pollen Vigour. J. Exp. Bot. 2020, 71, 730–741.
- Jiang, K.; Schwarzer, C.; Lally, E.; Zhang, S.; Ruzin, S.; Machen, T.; Remington, S.J.; Feldman, L. Expression and Characterization of a Redox-Sensing Green Fluorescent Protein (Reduction-Oxidation-Sensitive Green Fluorescent Protein) in Arabidopsis. Plant Physiol. 2006, 141, 397–403.
- Rosenwasser, S.; Rot, I.; Meyer, A.J.; Feldman, L.; Jiang, K.; Friedman, H. A Fluorometer-Based Method for Monitoring Oxidation of Redox-Sensitive GFP (RoGFP) during Development and Extended Dark Stress. Physiol. Plant. 2010, 138, 493–502.
- Schwarzländer, M.; Fricker, M.D.; Müller, C.; Marty, L.; Brach, T.; Novak, J.; Sweetlove, L.J.; Hell, R.; Meyer, A.J. Confocal Imaging of Glutathione Redox Potential in Living Plant Cells. J. Microsc. 2008, 231, 299–316.
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically Encoded Fluorescent Indicator for Intracellular Hydrogen Peroxide. Nat. Methods 2006, 3, 281–286.
- Costa, A.; Drago, I.; Behera, S.; Zottini, M.; Pizzo, P.; Schroeder, J.I.; Pozzan, T.; Schiavo, F. lo H2O2 in Plant Peroxisomes: An in Vivo Analysis Uncovers a Ca2+-Dependent Scavenging System. Plant J. 2010, 62, 760–772.
- Hernández-Barrera, A.; Velarde-Buendía, A.; Zepeda, I.; Sanchez, F.; Quinto, C.; Sánchez-Lopez, R.; Cheung, A.Y.; Wu, H.M.; Cardenas, L. Hyper, a Hydrogen Peroxide Sensor, Indicates the Sensitivity of the Arabidopsis Root Elongation Zone to Aluminum Treatment. Sensors 2015, 15, 855–867.
- Ai, F.; Chen, H.; Zhang, S.-H.; Liu, S.-Y.; Wei, F.; Dong, X.-Y.; Cheng, J.-K.; Huang, W.-H. Real-Time Monitoring of Oxidative Burst from Single Plant Protoplasts Using Microelectrochemical Sensors Modified by Platinum Nanoparticles. Anal. Chem. 2009, 81, 8453–8458.
- Kolbert, Z.; Barroso, J.B.; Brouquisse, R.; Corpas, F.J.; Gupta, K.J.; Lindermayr, C.; Loake, G.J.; Palma, J.M.; Petřivalský, M.; Wendehenne, D.; et al. A Forty Year Journey: The Generation and Roles of NO in Plants. Nitric Oxide 2019, 93, 53–70.
- Hu, J.; Yang, H.; Mu, J.; Lu, T.; Peng, J.; Deng, X.; Kong, Z.; Bao, S.; Cao, X.; Zuo, J. Nitric Oxide Regulates Protein Methylation during Stress Responses in Plants. Mol. Cell 2017, 67, 702–710.e4.
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant Nanobionics Approach to Augment Photosynthesis and Biochemical Sensing. Nat. Mater. 2014, 13, 400–408.
- Tsuchiya, Y.; Yoshimura, M.; Sato, Y.; Kuwata, K.; Toh, S.; Holbrook-Smith, D.; Zhang, H.; McCourt, P.; Itami, K.; Kinoshita, T.; et al. Probing Strigolactone Receptors in Striga Hermonthica with Fluorescence. Science 2015, 349, 864–868.
- Janssen, S.; Schmitt, K.; Blanke, M.; Bauersfeld, M.L.; Wöllenstein, J.; Lang, W. Ethylene Detection in Fruit Supply Chains. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130311.
- Cristescu, S.M.; Mandon, J.; Arslanov, D.; De Pessemier, J.; Hermans, C.; Harren, F.J.M. Current Methods for Detecting Ethylene in Plants. Ann. Bot. 2013, 111, 347–360.
- McLamore, E.S.; Diggs, A.; Calvo Marzal, P.; Shi, J.; Blakeslee, J.J.; Peer, W.A.; Murphy, A.S.; Porterfield, D.M. Non-Invasive Quantification of Endogenous Root Auxin Transport Using an Integrated Flux Microsensor Technique. Plant J. 2010, 63, 1004–1016.
- Liu, J.T.; Hu, L.S.; Liu, Y.L.; Chen, R.S.; Cheng, Z.; Chen, S.J.; Amatore, C.; Huang, W.H.; Huo, K.F. Real-Time Monitoring of Auxin Vesicular Exocytotic Efflux from Single Plant Protoplasts by Amperometry at Microelectrodes Decorated with Nanowires. Angew. Chem.-Int. Ed. 2014, 53, 2643–2647.
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-la-Peña, C.; Loyola-Vargas, V.M. Signaling Overview of Plant Somatic Embryogenesis. Front. Plant Sci. 2019, 10, 77.
- Pierre-Jerome, E.; Drapek, C.; Benfey, P.N. Regulation of Division and Differentiation of Plant Stem Cells. Annu. Rev. Cell Dev. Biol. 2018, 34, 289–310.
- Shigenaga, A.M.; Argueso, C.T. No Hormone to Rule Them All: Interactions of Plant Hormones during the Responses of Plants to Pathogens. Semin. Cell Dev. Biol. 2016, 56, 174–189.
- Bürger, M.; Chory, J. Stressed Out About Hormones: How Plants Orchestrate Immunity. Cell Host Microbe 2019, 26, 163–172.
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206.
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones Enhanced Drought Tolerance in Plants: A Coping Strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118.
- Umehara, M.; Cao, M.; Akiyama, K.; Akatsu, T.; Seto, Y.; Hanada, A.; Li, W.; Takeda-Kamiya, N.; Morimoto, Y.; Yamaguchi, S. Structural Requirements of Strigolactones for Shoot Branching Inhibition in Rice and Arabidopsis. Plant Cell Physiol. 2015, 56, 1059–1072.
- Aliche, E.B.; Screpanti, C.; De Mesmaeker, A.; Munnik, T.; Bouwmeester, H.J. Science and Application of Strigolactones. New Phytol. 2020, 227, 1001–1011.
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga Lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190.
- Cook, C.E.; Whichard, L.P.; Wall, M.; Egley, G.H.; Coggon, P.; Luhan, P.A.; McPhail, A.T. Germination Stimulants. II. Structure of Strigol, a Potent Seed Germination Stimulant for Witchweed (Striga Lutea). J. Am. Chem. Soc. 1972, 94, 6198–6199.
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant Sesquiterpenes Induce Hyphal Branching in Arbuscular Mycorrhizal Fungi. Nature 2005, 435, 824–827.
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C.; et al. Strigolactone Inhibition of Shoot Branching. Nature 2008, 455, 189–194.
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of Shoot Branching by New Terpenoid Plant Hormones. Nature 2008, 455, 195–200.
- Lin, Z.; Zhong, S.; Grierson, D. Recent Advances in Ethylene Research. J. Exp. Bot. 2009, 60, 3311–3336.
- Wang, K.L.C.; Li, H.; Ecker, J.R. Ethylene Biosynthesis and Signaling Networks. Plant Cell 2002, 14, S131–S151.
- Esser, B.; Schnorr, J.M.; Swager, T.M. Selective Detection of Ethylene Gas Using Carbon Nanotube-Based Devices: Utility in Determination of Fruit Ripeness. Angew. Chem. Int. Ed. 2012, 51, 5752–5756.
- Chauhan, R.; Moreno, M.; Banda, D.M.; Zamborini, F.P.; Grapperhaus, C.A. Chemiresistive Metal-Stabilized Thiyl Radical Films as Highly Selective Ethylene Sensors. RSC Adv. 2014, 4, 46787–46790.
- Krivec, M.; Mc Gunnigle, G.; Abram, A.; Maier, D.; Waldner, R.; Gostner, J.; Überall, F.; Leitner, R. Quantitative Ethylene Measurements with MOx Chemiresistive Sensors at Different Relative Air Humidities. Sensors 2015, 15, 28088–28098.
- Mirica, K.A.; Azzarelli, J.M.; Weis, J.G.; Schnorr, J.M.; Swager, T.M. Rapid Prototyping of Carbon-Based Chemiresistive Gas Sensors on Paper. Proc. Natl. Acad. Sci. USA 2013, 110, E3265–E3270.
- Su, Z.; Xu, X.; Cheng, Y.; Tan, Y.; Xiao, L.; Tang, D.; Jiang, H.; Qin, X.; Wang, H. Chemical Pre-Reduction and Electro-Reduction Guided Preparation of a Porous Graphene Bionanocomposite for Indole-3-Acetic Acid Detection. Nanoscale 2019, 11, 962–967.
- Sun, T. Gibberellin Metabolism, Perception and Signaling Pathways in Arabidopsis. Arab. Book 2008, 6, e0103.
- Rizza, A.; Walia, A.; Lanquar, V.; Frommer, W.B.; Jones, A.M. In Vivo Gibberellin Gradients Visualized in Rapidly Elongating Tissues. Nat. Plants 2017, 3, 803–813.
- Ueguchi-Tanaka, M.; Nakajima, M.; Katoh, E.; Ohmiya, H.; Asano, K.; Saji, S.; Hongyu, X.; Ashikari, M.; Kitano, H.; Yamaguchi, I.; et al. Molecular Interactions of a Soluble Gibberellin Receptor, GID1, with a Rice DELLA Protein, SLR1, and Gibberellin. Plant Cell 2007, 19, 2140–2155.
- Sun, T. Gibberellin-GID1-DELLA: A Pivotal Regulatory Module for Plant Growth and Development. Plant Physiol. 2010, 154, 567–570.
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338.
- Chen, C.; Feng, S.; Zhou, M.; Ji, C.; Que, L.; Wang, W. Development of a Structure-Switching Aptamer-Based Nanosensor for Salicylic Acid Detection. Biosens. Bioelectron. 2019, 140, 111342.
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and Vegetable Waste Management and the Challenge of Fresh-Cut Salad. Trends Food Sci. Technol. 2017, 63, 51–59.
- Dubois, M.; van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323.
- Light, K.M.; Wisniewski, J.A.; Vinyard, W.A.; Kieber-Emmons, M.T. Perception of the Plant Hormone Ethylene: Known-Knowns and Known-Unknowns. JBIC J. Biol. Inorg. Chem. 2016, 21, 715–728.
- Saltveit, M.E. Effect of Ethylene on Quality of Fresh Fruits and Vegetables. Postharvest Biol. Technol. 1999, 15, 279–292.
- Pranamornkith, T.; East, A.; Heyes, J. Influence of Exogenous Ethylene during Refrigerated Storage on Storability and Quality of Actinidia Chinensis (Cv. Hort16A). Postharvest Biol. Technol. 2012, 64, 1–8.
- Li, Y.; Golding, J.B.; Arcot, J.; Wills, R.B.H. Continuous Exposure to Ethylene in the Storage Environment Adversely Affects ‘Afourer’ Mandarin Fruit Quality. Food Chem. 2018, 242, 585–590.
- Sun, M.; Yang, X.; Zhang, Y.; Wang, S.; Wong, M.W.; Ni, R.; Huang, D. Rapid and Visual Detection and Quantitation of Ethylene Released from Ripening Fruits: The New Use of Grubbs Catalyst. J. Agric. Food Chem. 2019, 67, 507–513.
- Zhang, J.; Cheng, D.; Wang, B.; Khan, I.; Ni, Y. Ethylene Control Technologies in Extending Postharvest Shelf Life of Climacteric Fruit. J. Agric. Food Chem. 2017, 65, 7308–7319.
- Jedermann, R.; Praeger, U.; Geyer, M.; Lang, W. Remote Quality Monitoring in the Banana Chain. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130303.
- Spaniolas, S.; Bazakos, C.; Awad, M.; Kalaitzis, P. Exploitation of the Chloroplast Trn L (UAA) Intron Polymorphisms for the Authentication of Plant Oils by Means of a Lab-on-a-Chip Capillary Electrophoresis System. J. Agric. Food Chem. 2008, 56, 6886–6891.
- Bilodeau, G.J.; Lévesque, C.A.; De Cock, A.W.A.M.; Duchaine, C.; Brière, S.; Uribe, P.; Martin, F.N.; Hamelin, R.C. Molecular Detection of Phytophthora Ramorum by Real-Time Polymerase Chain Reaction Using TaqMan, SYBR Green, and Molecular Beacons. Phytopathology 2007, 97, 632–642.
- Sanzani, S.M.; Li Destri Nicosia, M.G.; Faedda, R.; Cacciola, S.O.; Schena, L. Use of Quantitative PCR Detection Methods to Study Biocontrol Agents and Phytopathogenic Fungi and Oomycetes in Environmental Samples. J. Phytopathol. 2014, 162, 1–13.
- Weller, S.A.; Elphinstone, J.G.; Smith, N.C.; Boonham, N.; Stead, D.E. Detection of Ralstonia Solanacearum Strains with a Quantitative, Multiplex, Real-Time, Fluorogenic PCR (TaqMan) Assay. Appl. Environ. Microbiol. 2000, 66, 2853–2858.
- Jongman, M.; Carmichael, P.C.; Bill, M. Technological Advances in Phytopathogen Detection and Metagenome Profiling Techniques. Curr. Microbiol. 2020, 77, 675–681.
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The New Perspective in Precision Agriculture. Biotechnol. Rep. 2017, 15, 11–23.
- Hong, S.; Lee, C. The Current Status and Future Outlook of Quantum Dot-Based Biosensors for Plant Virus Detection. Plant Pathol. J. 2018, 34, 85–92.
- Yao, K.S.; Li, S.J.; Tzeng, K.C.; Cheng, T.C.; Chang, C.Y.; Chiu, C.Y.; Liao, C.Y.; Hsu, J.J.; Lin, Z.P. Fluorescence Silica Nanoprobe as a Biomarker for Rapid Detection of Plant Pathogens. Adv. Mater. Res. 2009, 79–82, 513–516.
- Firrao, G.; Moretti, M.; Ruiz Rosquete, M.; Gobbi, E.; Locci, R. Nanobiotransducer for Detecting Flavescence Dorée Phytoplasma. J. Plant Pathol. 2005, 87, 101–107.
- Lau, H.Y.; Wu, H.; Wee, E.J.H.; Trau, M.; Wang, Y.; Botella, J.R. Specific and Sensitive Isothermal Electrochemical Biosensor for Plant Pathogen DNA Detection with Colloidal Gold Nanoparticles as Probes. Sci. Rep. 2017, 7, 38896.
- Lattanzio, V.M.T.; Nivarlet, N. Multiplex Dipstick Immunoassay for Semiquantitative Determination of Fusarium Mycotoxins in Oat. In Methods in Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 137–142.
- Lattanzio, V.M.; Nivarlet, N.; Lippolis, V.; Della Gatta, S.; Huet, A.C.; Delahaut, P.; Granier, B.; Visconti, A. Multiplex Dipstick Immunoassay for Semi-Quantitative Determination of Fusarium Mycotoxins in Cereals. Anal. Chim. Acta 2012, 718, 99–108.
- Medintz, I.L.; Sapsford, K.E.; Konnert, J.H.; Chatterji, A.; Lin, T.; Johnson, J.E.; Mattoussi, H. Decoration of Discretely Immobilized Cowpea Mosaic Virus with Luminescent Quantum Dots. Langmuir 2005, 21, 5501–5510.
- Sun, W.; Zhong, J.; Qin, P.; Jiao, K. Electrochemical Biosensor for the Detection of Cauliflower Mosaic Virus 35 S Gene Sequences Using Lead Sulfide Nanoparticles as Oligonucleotide Labels. Anal. Biochem. 2008, 377, 115–119.
- Shojaei, T.R.; Salleh, M.A.M.; Sijam, K.; Rahim, R.A.; Mohsenifar, A.; Safarnejad, R.; Tabatabaei, M. Fluorometric Immunoassay for Detecting the Plant Virus Citrus Tristeza Using Carbon Nanoparticles Acting as Quenchers and Antibodies Labeled with CdTe Quantum Dots. Microchim. Acta 2016, 183, 2277–2287.
- Moreau, A.L.D.; Janissen, R.; Santos, C.A.; Peroni, L.A.; Stach-Machado, D.R.; de Souza, A.A.; de Souza, A.P.; Cotta, M.A. Highly-Sensitive and Label-Free Indium Phosphide Biosensor for Early Phytopathogen Diagnosis. Biosens. Bioelectron. 2012, 36, 62–68.
- Shojaei, T.R.; Salleh, M.A.M.; Sijam, K.; Rahim, R.A.; Mohsenifar, A.; Safarnejad, R.; Tabatabaei, M. Detection of Citrus Tristeza Virus by Using Fluorescence Resonance Energy Transfer-Based Biosensor. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2016, 169, 216–222.
- Tereshchenko, A.; Fedorenko, V.; Smyntyna, V.; Konup, I.; Konup, A.; Eriksson, M.; Yakimova, R.; Ramanavicius, A.; Balme, S.; Bechelany, M. ZnO Films Formed by Atomic Layer Deposition as an Optical Biosensor Platform for the Detection of Grapevine Virus A-Type Proteins. Biosens. Bioelectron. 2017, 92, 763–769.
- Zhang, M.; Chen, W.; Chen, X.; Zhang, Y.; Lin, X.; Wu, Z.; Li, M. Multiplex Immunoassays of Plant Viruses Based on Functionalized Upconversion Nanoparticles Coupled with Immunomagnetic Separation. J. Nanomater. 2013, 1–8.
- Yazgan, I.; Osonga, F.J.; Miller, R.M.; Kariuki, V.M.; Zhang, J.; Feng, J.; Skeete, Z.; Crapo, H.; Schulte, J.; Sadik, O.A. Greener One-Pot Synthesis of Gold Nanoparticle Glycoconjugates Using Functionalized Sugars. ACS Agric. Sci. Technol. 2021, 1, 379–389.
- Yazgan, I.; Zhang, J.; Kariuki, V.; Akgul, A.; Cronmiller, L.E.; Akgul, A.; Osonga, F.; McMahon, A.; Gao, Y.; Eshun, G.; et al. Selective Sensing and Imaging of Penicillium Italicum Spores and Hyphae Using Carbohydrate–Lectin Interactions. ACS Sens. 2018, 3, 648–654.
- Yazgan, I.; Gümüş, A.; Gökkuş, K.; Demir, M.A.; Evecen, S.; Sönmez, H.A.; Miller, R.M.; Bakar, F.; Oral, A.; Popov, S.; et al. On the Effect of Modified Carbohydrates on the Size and Shape of Gold and Silver Nanostructures. Nanomaterials 2020, 10, 1417.
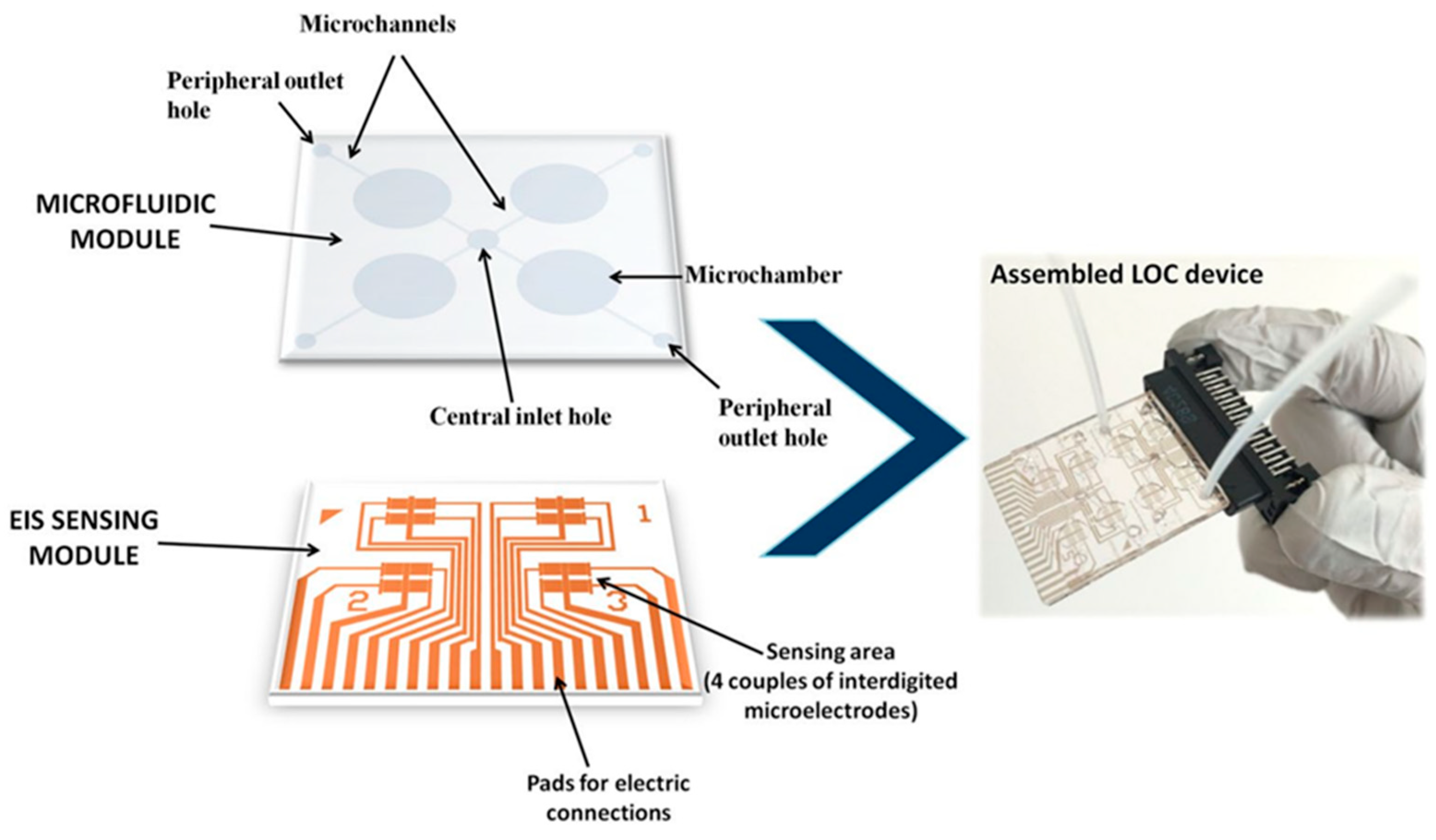
- Chiriacò, M.S.; Luvisi, A.; Primiceri, E.; Sabella, E.; de Bellis, L.; Maruccio, G. Development of a Lab-on-a-Chip Method for Rapid Assay of Xylella Fastidiosa Subsp. Pauca Strain CoDiRO. Sci. Rep. 2018, 8, 7376.
- Jiang, H.; Xu, Z.; Aluru, M.R.; Dong, L. Plant Chip for High-Throughput Phenotyping of Arabidopsis. Lab Chip 2014, 14, 1281–1293.
- Julich, S.; Riedel, M.; Kielpinski, M.; Urban, M.; Kretschmer, R.; Wagner, S.; Fritzsche, W.; Henkel, T.; Möller, R.; Werres, S. Development of a Lab-on-a-Chip Device for Diagnosis of Plant Pathogens. Biosens. Bioelectron. 2011, 26, 4070–4075.
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558.
- Vitosh, M.L.; Silva, G.H. A Rapid Petiole Sap Nitrate-Nitrogen Test for Potatoes. Commun. Soil Sci. Plant Anal. 1994, 25, 183–190.
- Errebhi, M.; Rosen, C.J.; Birong, D.E. Calibration of a Petiole Sap Nitrate Test for Irrigated “Russet Burbank” Potato. Commun. Soil Sci. Plant Anal. 1998, 29, 23–35.
- Kubota, A.; Thompson, T.L.; Doerge, T.A.; Godin, R.E. A Petiole Sap Nitrate Test for Broccoli. J. Plant Nutr. 1997, 20, 669–682.
- Zhang, H.; Zhang, G.; Xu, J.; Wen, Y.; Lu, B.; Zhang, J.; Ding, W. Novel Highly Selective Fluorescent Sensor Based on Electrosynthesized Poly(9-Fluorenecarboxylic Acid) for Efficient and Practical Detection of Iron(III) and Its Agricultural Application. Sens. Actuators B Chem. 2016, 230, 123–129.
- Roy, E.; Patra, S.; Madhuri, R.; Sharma, P.K. Simultaneous Determination of Heavy Metals in Biological Samples by a Multiple-Template Imprinting Technique: An Electrochemical Study. RSC Adv. 2014, 4, 56690–56700.
- Li, S.; Simonian, A.; Chin, B.A. Sensors for Agriculture and the Food Industry. Electrochem. Soc. Interface 2010, 19, 41–46.
- Gieling, T.H.; Van Straten, G.; Janssen, H.J.J.; Wouters, H. ISE and Chemfet Sensors in Greenhouse Cultivation. Sens. Actuators B Chem. 2005, 105, 74–80.
- Gutiérrez, M.; Alegret, S.; Cáceres, R.; Casadesús, J.; Marfà, O.; del Valle, M. Application of a Potentiometric Electronic Tongue to Fertigation Strategy in Greenhouse Cultivation. Comput. Electron. Agric. 2007, 57, 12–22.
- Sabzevari, S.; Hofman, J. A Worldwide Review of Currently Used Pesticides’ Monitoring in Agricultural Soils. Sci. Total Environ. 2022, 812, 152344.
- Yang, T.; Doherty, J.; Guo, H.; Zhao, B.; Clark, J.M.; Xing, B.; Hou, R.; He, L. Real-Time Monitoring of Pesticide Translocation in Tomato Plants by Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2019, 91, 2093–2099.
- Li, H.; Merkl, P.; Sommertune, J.; Thersleff, T.; Sotiriou, G.A. SERS Hotspot Engineering by Aerosol Self-Assembly of Plasmonic Ag Nanoaggregates with Tunable Interparticle Distance. Adv. Sci. 2022, 2201133.
- Strobel, R.; Pratsinis, S.E. Flame Aerosol Synthesis of Smart Nanostructured Materials. J. Mater. Chem. 2007, 17, 4743.
- Martinazzo, J.; Ballen, S.C.; Steffens, J.; Steffens, C. Long Term Stability of Cantilever Gas Nanosensors to Detect Euschistus Heros (F.) Pheromone Release by Rubber Septa. Sens. Actuators B Chem. 2022, 359, 131566.




