Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yang Li | -- | 2832 | 2022-09-27 08:42:15 | | | |
| 2 | Sirius Huang | Meta information modification | 2832 | 2022-09-28 03:55:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, Y.; Li, X.; Liang, Z.; Chang, X.; Li, F.; Wang, X.; Lian, X. Microencapsulation Technology of Phycocyanin. Encyclopedia. Available online: https://encyclopedia.pub/entry/27636 (accessed on 07 March 2026).
Li Y, Li X, Liang Z, Chang X, Li F, Wang X, et al. Microencapsulation Technology of Phycocyanin. Encyclopedia. Available at: https://encyclopedia.pub/entry/27636. Accessed March 07, 2026.
Li, Yang, Xu Li, Zi-Peng Liang, Xin-Ying Chang, Fu-Tong Li, Xue-Qing Wang, Xi-Jun Lian. "Microencapsulation Technology of Phycocyanin" Encyclopedia, https://encyclopedia.pub/entry/27636 (accessed March 07, 2026).
Li, Y., Li, X., Liang, Z., Chang, X., Li, F., Wang, X., & Lian, X. (2022, September 27). Microencapsulation Technology of Phycocyanin. In Encyclopedia. https://encyclopedia.pub/entry/27636
Li, Yang, et al. "Microencapsulation Technology of Phycocyanin." Encyclopedia. Web. 27 September, 2022.
Copy Citation
Phycocyanin (PC) is a blue fluorescent protein with multi-bioactive functions. The multi-bioactivities and spectral stability of phycocyanin are susceptible to external environmental conditions, which limit its wide application. Microencapsulation is a micro-packaging technology that encapsulates trace substances with polymer films. Multifarious strategies have been successfully investigated for the microencapsulation of PC.
phycocyanin
microencapsulation
biologically active substance
1. Introduction
Phycocyanin (PC) separated from Spirulina platensis is a soluble pigment-protein which is formed from the thioether covalent bond between open-chain tetrapyrrole chromophores (called Phycocyanobilin (PCB) or Phycoerthrobilin (PEB)) and apoproteins (protein components which conjugate the chromophores). As a food additive, it is permitted by many countries in the world [1], and its global marketing value is expected to reach USD 245.5 million in 2027 [2]. PC has anti-inflammatory, -oxidant, -tumor, and -modulating immunity and other biological functions [3] and been commonly used as an additive in food or cosmetics. By folding the primary structure of apoprotein, the compact secondary, tertiary, or quaternary structures are formed; meanwhile, the linear chromophores are rearranged into a cyclic form, and the cyclic chromophores as well as the conjugated apoprotein form a protein–chromophore complex. Moreover, the complex increases the wavelength utilized by Spirulina platensis for photosynthesis, extends the photosynthesis’ wavelength range, and gives the blue color of PC [4]. Evidence has showed that the antioxidant ability of PC was higher than that of its apoproteins, which indicated that the antioxidant ability of PC was mainly provided by the chromophore [5]. Nicotinamide adenine dinucleotide phosphate (NAPDH) is a coenzyme, also called reduced coenzyme II, which is formed in the light reaction stage of photosynthesis. It enters the carbon reaction with ATP and participates in the fixation of CO2. The tetrapyrrole structure of PC chromophore could activate NADPH oxidase through hydrophobic interaction and inhibit the production of NADPH-dependent superoxide [6]. PC chromophore also played an anti-inflammatory role in inhibiting nuclear factor-kappa B (NF-κB) activation in various inflammatory models of animal and cell [7][8], thereby effectively improving renal injury [9] and intestinal inflammation [10]. PC scavenged external free radicals by radical addition reaction at conjugated double bonds of the tetrapyrrole structure’s chromophores, leading to the oxidative degradation of covalently linked chromophores to mesobiliviolin and biliviolins [11] and lost the color of the chromophore itself [12]. In addition, the reasons for PC chromophores easily losing color also include being susceptible to pH, light, temperature, and other environmental factors [13]. Therefore, the color stability and bioactivity of PC are closely related to the existence environmental conditions of chromophores. When PC was either processed or taken orally, the color changed, as did the biological activity, because the former was subjected to external hostile environments, while the latter was subjected to the internal gastrointestinal extremely environment, and then the application range of PC was limited. To overcome the abovementioned shortcomings, targeted delivery protective measures should be given to escape the harmful adversity and to improve the color and structural stability of PC.
Microencapsulation is a micro-packaging technology that encapsulates trace substances with polymer films. Protected substances, called core materials, which can exist in solid, liquid, and gas phase, are wrapped by polymer film-forming materials, known as wall materials [14]. At present, this technology has been successfully applied to encapsulate natural pigments such as anthocyanins [15], curcumin [16], and other plant extracts [17][18], for protection and utilization of such compounds.
Up to now, multifarious strategies have been successfully investigated for the microencapsulation of PC. Table 1 presents the summary of the advantages and disadvantages of the recent encapsulation strategies [15]. Encapsulation of PC by microcapsule technology can significantly enhance its stability and improve its bioavailability both in vivo and in vitro. The prepared microcapsules exhibit the features in the encapsulation efficiency, particle size, and controlled release ability, meeting the need of corresponding fields. Moreover, microcapsules can synergize with the wall material to exert biological activity.
Table 1. Overview of the advantages and disadvantages of various methods for preparing PC microcapsules.
| Method | Benefits | Drawbacks | Reference |
|---|---|---|---|
| Spray drying | Rapid, cost-effective, easy to scale up. C-PC retention, and relatively good storage stability. | Production of nonuniform particles with a wide size distribution. C-PC degradation and loss of product. | [19][20][21][22][23][24] |
| Electrospinning | Suitable for heat-sensitive compounds. | Slow, produces low encapsulation yield, and limited scope of application. | [25][26][27][28][29] |
| Electrospraying | Produces particles with a high surface-to-volume ratio, controlled release, improved functionality, and physical properties. | Time-consuming and hardly repeatable, especially at the industrial level. | [30][31][32] |
| Liposome delivery | Increased adsorption bioavailability, and nontoxic. | Low encapsulation efficiency. Lipid oxidation, poor physicochemical stability, and wide particle size distribution. Postprocess step is required. | [33][34][35][36][37] |
| Sharp-hole coagulation bath | High loading capacity, low temperature operating requirements, reduced evaporation losses of volatile compounds, and thermal degradation. Tailored release of active compounds. | Special instruments are required and are not suitable for large-scale industrial production. | [38][39][40][41][42] |
| Ion Gelation | Low cost and does not require advanced equipment, high temperatures, and organic solvents. | Produced particles are very sensitive to pH and ionic strength. Agglomeration of particles and particle size control. | [43][44][45][46][47][48][49] |
2. Microencapsulation Technology of PC
2.1. Spray Drying
Spray drying is a technique involving water removal from the material through contact with hot air after the material is dispersed into micro-particles by the mechanical centrifugal force or a pressure difference. The microcapsule wall material along with the water is believed to wrap the thermo-sensitive PC in the core through electrostatic attraction and hydrogen bonding due to the dissociation of a large number of functional groups of wall materials and PC, such as -CH3, -NH2, -COOH, -CONH2, -OH, etc., [19][20]. In the spray drying process, the water of the wall material is evaporated as the heat releases and temperature increases slowly to achieve the wet-bulb temperature of the air. This process effectively protects the PC from damage during the heat treatment and demonstrates the purpose of the microencapsulation technology. The spray drying treatment can make the wall material of microcapsules form a dense barrier, which improves the encapsulation and the protection of core materials. This technology, being used for the PC microencapsulation, exhibits high yield and low processing cost. The major morphology of microcapsules produced by the spray drying is a multi-core type. Meanwhile, the microcapsules could also form a composite type if the produced microcapsules are re-encapsulated with other compounds during the spray drying. The inlet temperature and wall materials are significant factors that affect the types of microcapsules [50]. Iqbal reported that the optimum inlet temperature of dried PC microcapsules was 110 °C [21]. The technical schematic is shown in Figure 1.
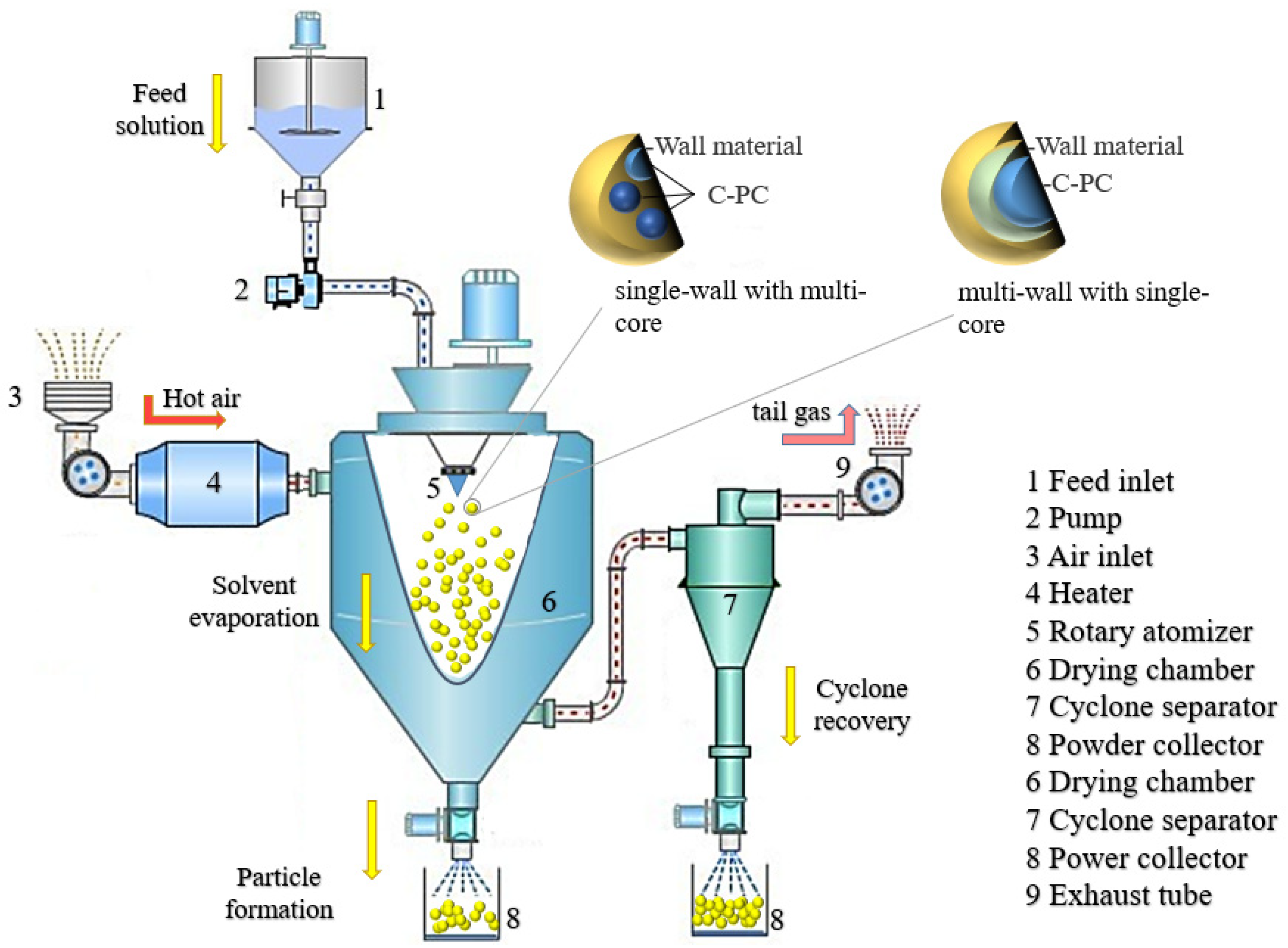
Figure 1. Schematic illustration of the process for preparing PC microcapsules through the spray drying technique.
The encapsulation wall materials applied in the spray drying technology are commonly soluble in water and have good thermal stability. Maltodextrin is a polyhydroxy compound, which can form a protective layer by secondary bonds such as hydrogen bonds to encapsulate proteins, reduce the collision between protein molecules, inhibit protein hydrolysis, and endow it with good thermal stability. Faieta et al. [22] used maltodextrin and trehalose to construct PC microcapsules with high carrying capacity. After the encapsulation of the microcapsules, the residual amount of PC was more than 89%, and the concentration of maltodextrin was positively correlated to the thermal degradation resistance and color protection ability of PC. Da Silva et al. [23] prepared the alginate extract microcapsules encapsulated by citric acid-crosslinked maltodextrin using the spray drying technique. The encapsulation efficiency of the microcapsules was 75%, presenting higher thermal stability than the base materials and better anti-inflammatory activity.
In the early stage of constant rate drying, the temperature around PC is not too high and does not exceed the wet-bulb temperature of the air. Along with the microcapsule powders’ drop in the bottom of the drying tower, the drying process occurs in the late stage of reduced rate drying, and the temperature in the out-layer of microcapsule the can exceed the wet-blub temperature because of a lack of excess water that is evaporated for absorbing heat. Therefore, the microcapsule contacts with the hot air again and results in the PC color change. In order to overcome this limitation, some researchers constructed a two-phase system of polyethylene glycol (PEG) and dextran to encapsulate PC. By protecting the dispersed phase of enriched PC from the continuous phase, through spray drying with continuous mixing (to prevent phase separation), double-layer enclosed microcapsules were formed. The microcapsules prepared in this system could be stored for 6 months without a change in the color of PC, showing that its stability was higher than any other malt dextrin single encapsulation systems [24]. Microcapsules of PC prepared by spray drying with maltodextrin or other water-soluble wall materials are easily soluble in water and can be used to improve the storage stability without sustained-release ability.
2.2. Electrospinning Technology
Electrospinning is a technique normally used for the manufacturing of polymer fibers by applying an electrostatic field force. Typically, a solution composited of core and wall material forms a polymer when exposed to the electric field through the nozzle. The produced polymer is then transferred onto the electrode and, due to the charge repulsion, is extended into fiber morphology and coated on the core material. Compared to the traditional spay drying technique, which always causes a partial loss of biological activity PC under the processing environmental conditions, the electrospinning treatment with strong electric field extrusion is usually carried out at room temperature to avoid heat denaturation and PC inactivation. The technical schematic is shown in Figure 2.
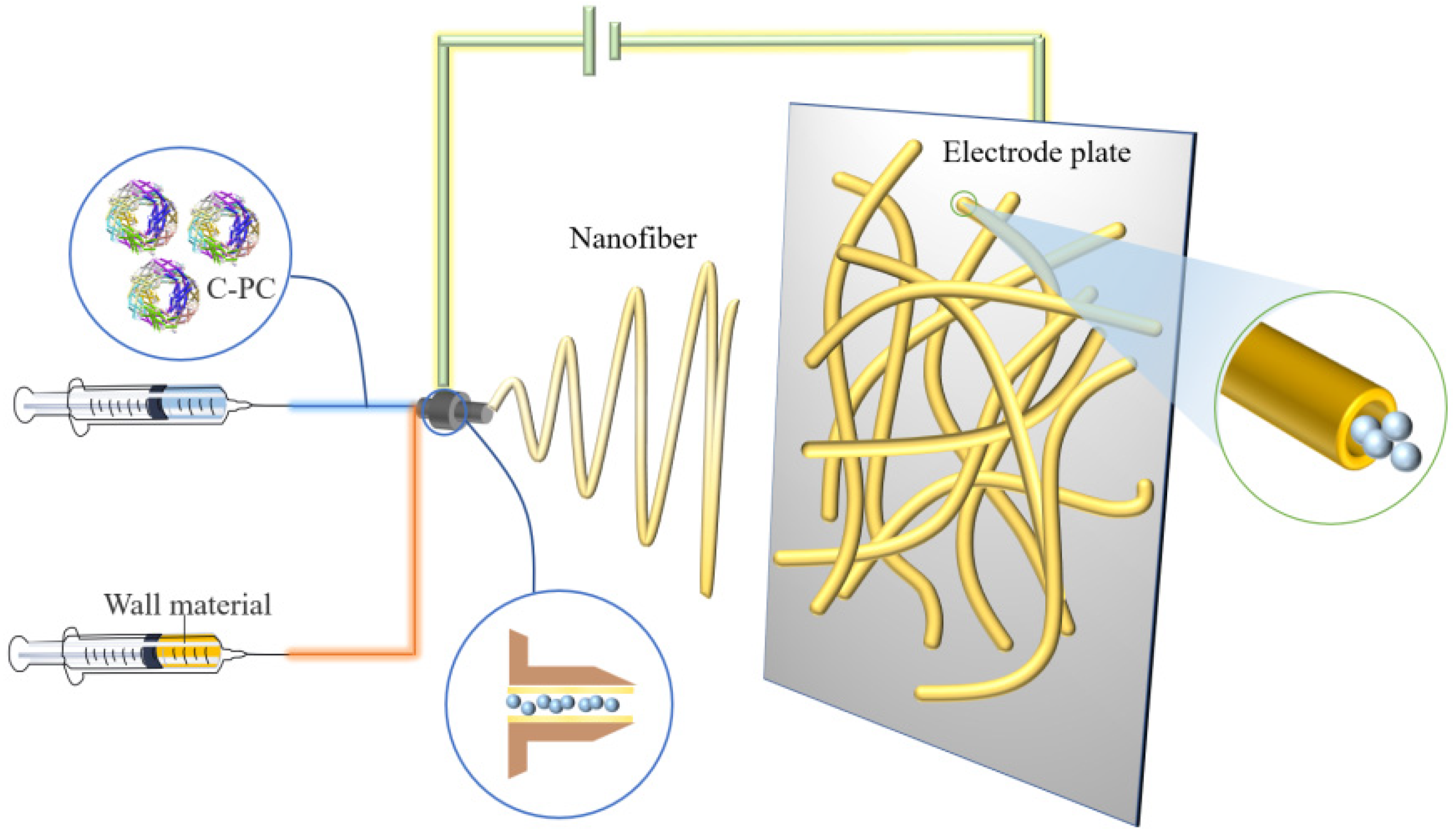
Figure 2. Schematic illustration of preparing PC microcapsules using the electrospinning method.
Poly(ethylene oxide) (PEO) is a water-soluble polymer material and suitable for the electrospinning nanofibers production. By mixing the PEO with PC, the PEO nanofibers produced through the electrospinning technique could expand to an average diameter of 295 nm and protected the inside PC to perform good thermal stability [25]. Moreira et al. [26] reported that an antioxidant ultrafine composite fiber with PC and PEO was made by free surface electrospinning technology. The composite fiber had a PC encapsulation rate of 5–10% (w/w) and was 269–542 nm in diameter, exhibiting a promising application in the food preservation industry. Wen et al. [27] constructed a safe, nontoxic, and probiotic-loaded PC nanofiber membrane by ion-coagulation combined with coaxial electrospinning technology. The diameter and release rate of the composite was 590 nm and 82%, respectively, and the release amount of PC in simulated colon fluids followed in a dose-time-dependent manner due to the existence of prebiotics and polysaccharides. Furthermore, the composite inhibited the growth of human colon cancer cells by blocking the cell cycle at the G0/G1 phase and inducing cell apoptosis. The mechanism involved a decrease in B-cell lymphoma-2 (Bcl-2)/ Bcl-2-associated x protein (Bax), the activation of Caspase 3, and the release of cytochrome c [28].
At the same time, it promoted the proliferation of intestinal probiotics, maintained the biological activity of PC, and realized its colon targeting. In the food quality detection field, PC-based composite nanofibers prepared by electrospinning could be used as a pH indicator to detect perishable products [29].
2.3. Electrospraying Technology
Electrospraying is a method that, when used under a high voltage electric field, the wall material mixture solution through the nozzle can be cracked into a charged spray, which is sprayed onto the core material surface to form a core-shell composite.
The difference between electrospraying and electrospinning is that, under the applied voltage, the electrospraying method makes polymer into ultrafine particles, while the electrospinning technology produces polymer fibers. Electrospraying technology is employed for the encapsulation of small molecular substances or proteins exhibiting good efficiency of encapsulation and high efficiency of producing monodisperse particles. The rapid encapsulation of compounds can be realized by electrical spraying technology to prepare bioactive microspheres with smooth surfaces and small particle sizes [30]. Several researchers used polyvinyl alcohol (PVA) ultrafine particles to spray onto PC, showing 75% encapsulation efficiency and producing the compound with 395 nm as the average diameter. The microcapsules could tolerate the temperature up to 216 °C and still maintained the antioxidant activity of PC [31]. Figure 3 shows the preparation process of PC microcapsules by electrospraying.
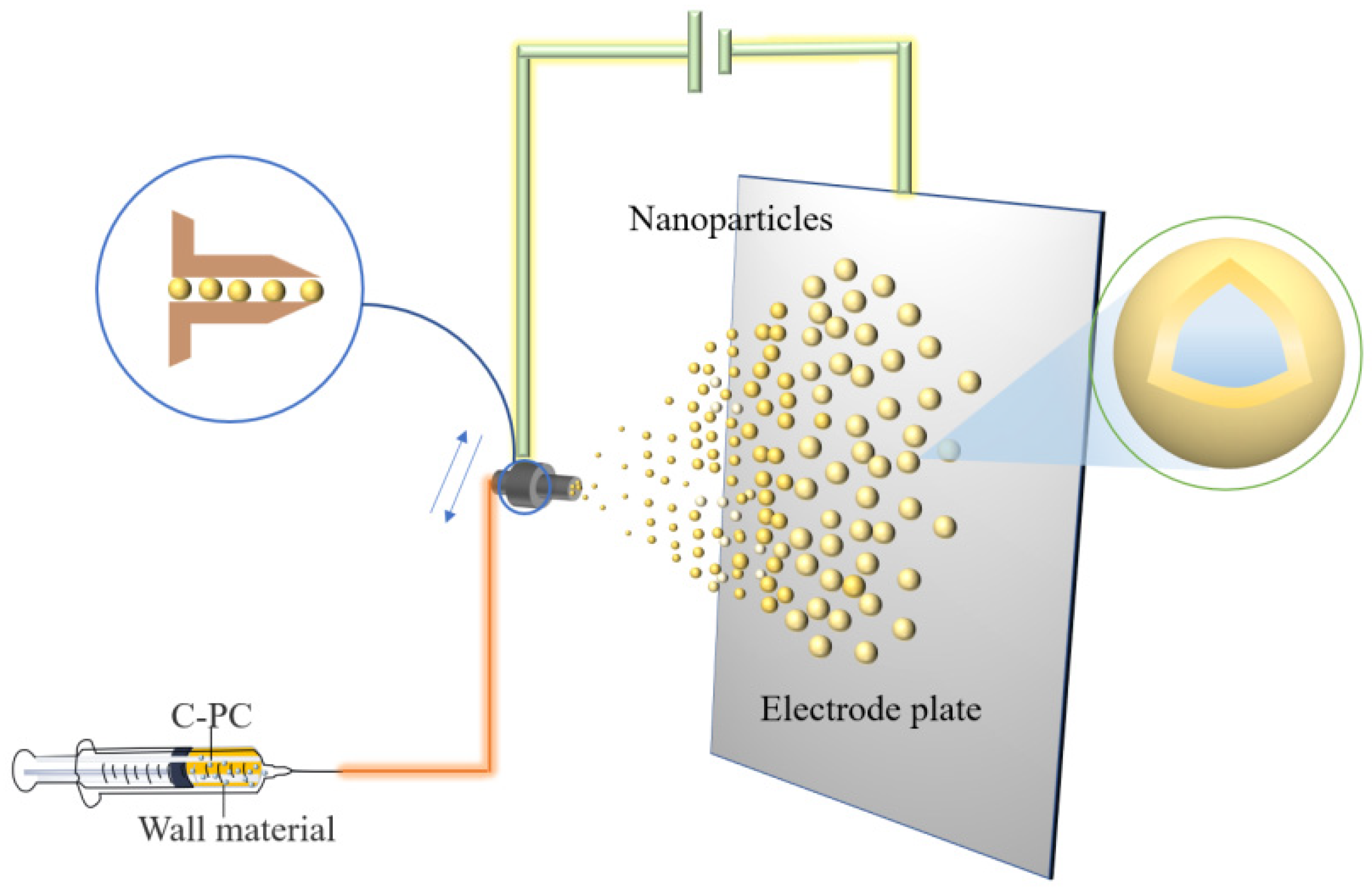
Figure 3. Schematic illustration showing the preparation of PC microcapsules using the electrospraying method.
Microcapsules encapsulated by electrospraying have specific particle states and dispersion properties and keep the biological activity unchanged [32]. However, higher requirements are needed regarding the viscosity, fluidity, and constituent and solvent volatility of the solution system to meet the electrospraying technique. The irregular movement of charged particles during spraying results in a loss of wall material and an uneven distribution of the coating.
2.4. Liposome Delivery
Liposomes are small vesicles formed by lipids or a liposoluble substance, such as phospholipids or cholesterol, which is dissolved in organic solvents and then added to water. Liposomes have a bimolecular layer structure similar to the cell membrane and can encapsulate hydrophilic substances in a water cavity acting as the controlled-release substances [33]. The microcapsule formed from liposomes encapsulating PC is shown in Figure 4. This microcapsule can effectively improve the stability and protect the protein compounds’ oral bioavailability; therefore, they can smoothly pass through the acidic environment of the stomach without any damage, thereby maintaining their anti-inflammatory activity [34].
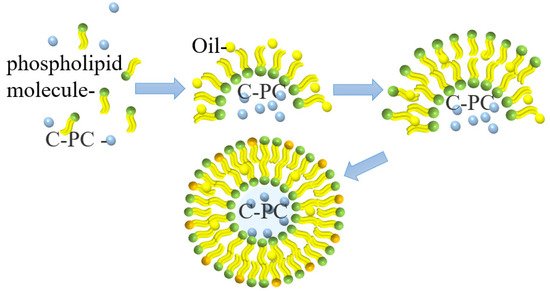
Figure 4. Schematic illustration of PC liposome microcapsule formation.
Yagoubi et al. [35] used an ultrasound-assisted method to prepare solid lipid-nanocarriers for microencapsulated PC producing a uniform spherical particle with a particle size of 50–80 nm. The encapsulation efficiency of this process is 37–69%. These lipid nanocarriers can control the release of PC.
Hardiningtyas et al. [36] prepared composite particles with the particle size of 220–270 nm by forming water in oil (W/O) emulsion with more than 99% encapsulation efficiency. The microencapsulated PC effectively maintained the active structure of PC and released it. Castangia et al. [37] used sodium hyaluronate to immobilize liposomes prepared by concentrated soybean phosphatidylcholine, and the obtained multilayer liposomes had a unique hyaluronic acid–phospholipid structure, which was conducive to the maintenance of PC activity and deep accumulation. Liposomes can be used as osmotic carriers to encapsulate PC for targeted delivery; however, the yield of water-soluble compounds encapsulated by liposomes is low, and the microcapsules produced are easily oxidized in the air, which greatly limits their applications.
2.5. Sharp-Hole Coagulation Bath
The sharp-hole coagulation bath method is a technique that involves dispersing the core material in a soluble polymer solution and adding spherical droplets into the coagulation bath with the help of the sharp-hole instrument for rapid precipitation or crosslinking solidification to form the cystic structure complex. Sodium alginate presents good biological activity and biocompatibility [38], and it is commonly used as condensation material. With the negative charge, sodium alginate can form gel by complexing with calcium ions, which was further employed to encapsulate PC to form calcium alginate/PC microcapsules through extrusion [39], as shown in Figure 5. Moreover, the encapsulation efficiency of microcapsules prepared by this method can reach 98%.
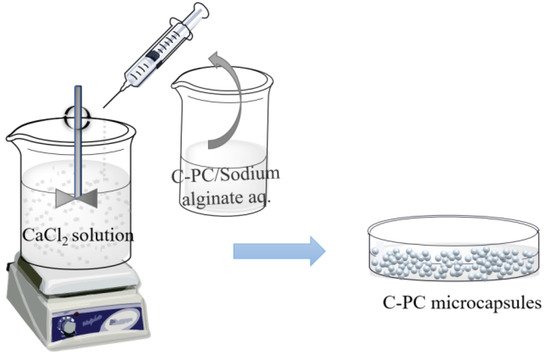
Figure 5. Schematic illustration showing the preparation of PC microcapsules using the sharp-hole coagulation bath method.
The calcium alginate/PC microcapsules treated by the sharp-hole coagulation bath were spherical, with an average size of about 1.2 mm. The addition of calcium alginate did not destroy the structure of PC. Moreover, the produced compound could tolerate an acidic environment with a pH of 4.5 and demonstrates a degradation rate that is slower than unencapsulated PC [40][41]. The sharp-hole coagulation bath method is also suitable for the complex system of alginate and other substances. Yan et al. [42] used alginate and chitosan as coating materials to prepare PC microcapsules by the extrusion method, forming a dense spherical complex with an average diameter of 1.03 mm. The encapsulation of alginate and chitosan can reduce the temperature sensitivity of PC during storage and can enhance the sustained-release effect of single alginate-encapsulated microcapsules due to the addition of chitosan.
2.6. Ion Gelation
Ion gelation is a method used to encapsulate bioactive substances by which electrolyte materials are normally electrolyzed into anions and cations during the polymer molecular chains’ crosslink to form a spatial network structure through electrostatic attraction or forming hydrogen bonds. The ion gelation method is a new preparation method for drug-loaded microcapsules with mild preparation conditions. In the field of healthcare products and medicine, ion gelation can use PC composite photosensitizers to assist in antibacterial photodynamic therapy [43].
Mohan et al. [44] prepared chitosan nanoparticles with an average particle size of 20 nm by using the ion gelation method, exhibiting 36.45% encapsulation efficiency of PC and maintaining the antibacterial activity of PC. The negative-charged sodium tripolyphosphate could combine with the positive-charged chitosan by ion gelation method to encapsulate the anti-tumor polypeptide Y2 from hydrolyzed PC to form microcapsules. The maximum encapsulation efficiency was 49% with 15% polypeptide content [45]. In addition, natural food additives, carrageenan, and PC can also form porous network materials with hydrophilic and mechanical strength through ion crosslinking [46]. To realize the oral administration of PC, Yang et al. [47] used carboxymethyl chitosan to prepare PC complexes by ion gel method. The particles were spherical, with a diameter of about 200 nm, and with 65% encapsulation efficiency. The nanoparticles were pH sensitive to the release of PC and had sustained-release effect in vitro. Manconi et al. [48], utilizing chitosan and xanthan gum as polyelectrolyte complexes to encapsulate PC (shown in Figure 6), found that, when the mass ratio of chitosan and xanthan gum was 0.5/8.0 (w/w), the surface of the microcapsules, after drying, was regular. The microcapsules satisfy the Fick diffusion characteristics of drug release and presented good in vitro adhesion, which could be useful in colon administration.
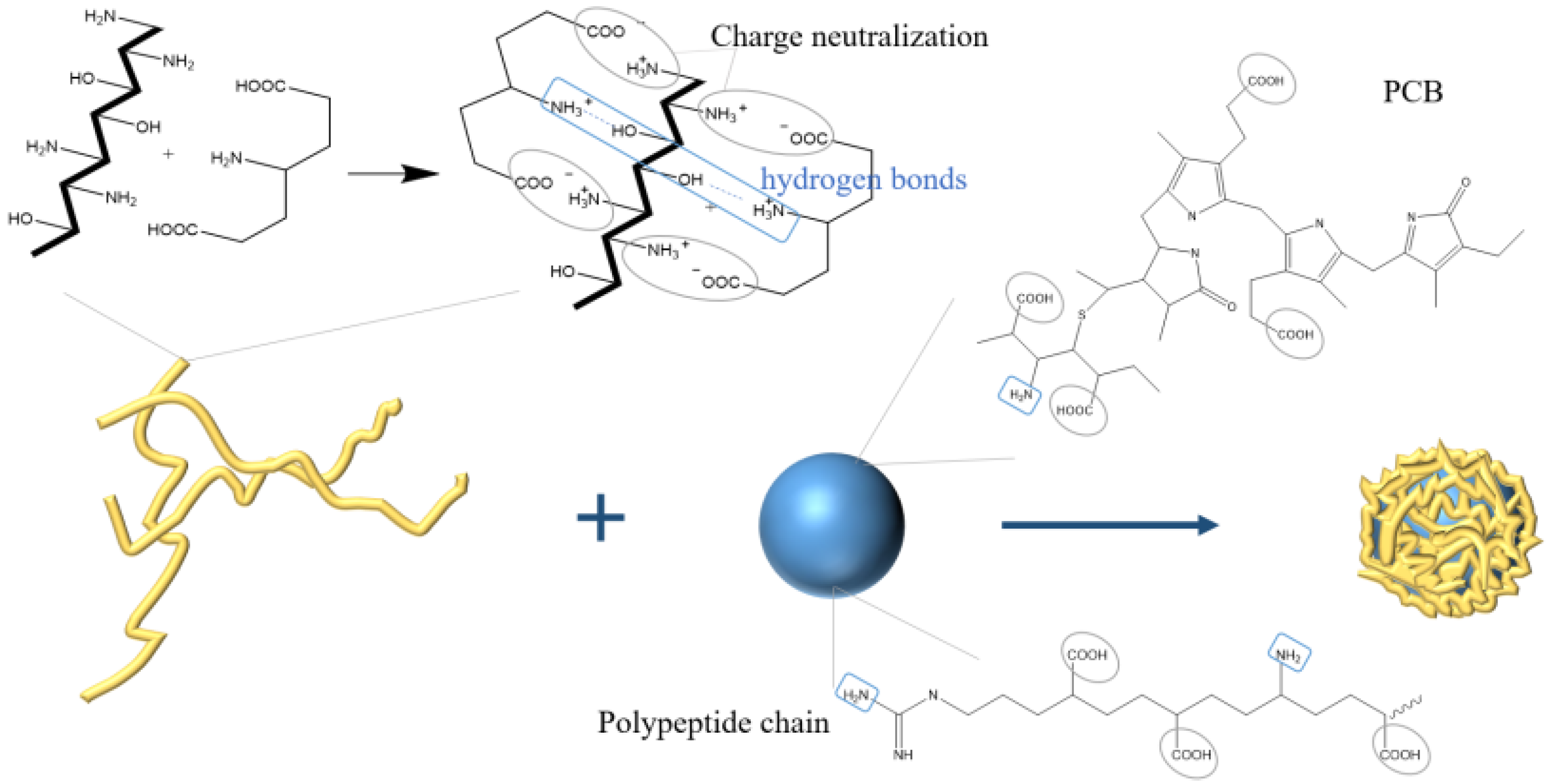
Figure 6. Schematic illustration showing the preparation of PC microcapsules using ion gelation method.
References
- El-Baky, H.H.A.; El-Baroty, G.S. Characterization and bioactivity of phycocyanin isolated from Spirulina maxima grown under salt stress. Food Funct. 2012, 3, 381–388.
- Meticulous Research (2020). January 30, 2021. Phycocyanin Market Worth $245.5 Million by 2027. Available online: https://www.meticulousresearch.com/pressrelease/30/phycocyanin-market-2027 (accessed on 30 January 2021).
- Deng, W.; Wang, X.Q. Progress of anti-tumor activity of phycocyanin and its enzymolied products. Mar. Sci. Bull. 2010, 29, 357–360.
- Böcker, L.; Ortmann, S.; Surber, J.; Leeb, E. Biphasic short time heat degradation of the blue microalgae protein phycocyanin from Arthrospira platensis. Innov. Food Sci. Emerg. Technol. 2018, 52, 116–121.
- Pleonsil, P.; Soogarun, S.; Suwanwong, Y. Anti-oxidant activity of holo- and apo-C-phycocyanin and their protective effects on human erythrocytes. Int. J. Biol. Macromol. 2013, 60, 393–398.
- Hirata, T.; Tanaka, M.; Ooike, M.; Sakaguchi, T.T.M. Antioxidant activities of phycocyanobilin prepared from Spirulina platensis. J. Appl. Phycol. 2000, 12, 435–439.
- Liu, Q.; Li, W.; Lu, L.; Liu, B.; Du, Z.N.; Qin, S. Phycocyanin attenuates X-ray-induced pulmonary inflammation via the TLR2-MyD88-NF-kappa B signaling pathway. J. Oceanol. Limnol. 2019, 37, 1678–1685.
- Alzokaky, A.A.; Abdelkader, E.M.; El-Dessouki, A.M.; Khaleel, S.A.; Raslan, N.A. C-phycocyanin protects against ethanol-induced gastric ulcers in rats: Role of HMGB1/NLRP3/NF-kappa B pathway. Basic Clin. Pharmacol. Toxicol. 2020, 127, 265–277.
- Pliego, E.G.; Franco-Colin, M.; Franco, P.R.; Blas-Valdivia, V. Phycocyanobilin is the molecule responsible for the nephroprotective action of phycocyanin in acute kidney injury caused by mercury. Food Funct. 2021, 12, 2985–2994.
- Guo, W.; Zeng, M.Y.; Zhu, S.Q.; Li, S.Y.; Qian, Y.L.; Wu, H.H. Phycocyanin ameliorates mouse colitis via phycocyanobilin-dependent antioxidant and anti-inflammatory protection of intestinal epithelial barrier. Food Funct. 2022, 13, 3294–3307.
- Bhat, V.B.; Madyastha, K.M. Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: Protection against oxidative damage to DNA. Biochem. Biophys. Res. Commun. 2001, 285, 262–266.
- Abeliovich, A.; Shilo, M. Photooxidative reactions of c-phycocyanin. Biochim Biophys Acta. 1972, 283, 483–491.
- Liang, X.; Wang, C.H.; Lu, X.; Pang, Y.Y.; Jiang, H.R.; Liu, X.L. Stability and fluorescence characteristics of C-phycocyanin in Spirulina platensis. Mod. Food Sci. Technol. 2020, 36, 89–96.
- Kaya-Celiker, H.; Mallikarjunan, K. Better nutrients and therapeutics delivery in food through nanotechnology. Food Eng. Rev. 2012, 4, 114–123.
- Mohammadalinejhad, S.; Kurek, M.A. Microencapsulation of anthocyanins—Critical review of techniques and wall materials. Appl. Sci. 2021, 11, 3936.
- Jiang, T.; Wei, L.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035.
- Chu, C.C.; Chew, S.C.; Nyam, K.L. Recent advances in encapsulation technologies of kenaf (Hibiscus cannabinus) leaves and seeds for cosmeceutical application. Food Bioprod. Process. 2021, 127, 99–113.
- Siddiqui, S.A.; Redha, A.A.; Esmaeili, Y.; Mehdizadeh, M. Novel insights on extraction and encapsulation techniques of elderberry bioactive compounds. Crit. Rev. Food Sci. Nutr. 2022, 12, 1–16.
- İlter, I.; Koç, M.; Demirel, Z.; Dalay, M.C. Improving the Stability of Phycocyanin by Spray Dried Microencapsulation. J. Food Processing Preserv. 2021, 45, 15646.
- Ilter, I.; Akyil, S.; Koc, M.; Demirel, Z.; Dalay, M.C.; Ertekin, F.K. Microencapsulation of phycocyanin by spray drying technique. J. Biotechnol. 2017, 256, S26.
- Iqbal, M.N.; Hadiyanto, H. Experimental investigation of phycocyanin microencapsulation using maltodextrin as a coating material with spray drying method. AIP Conf. Proc. 2020, 2197, 100002.
- Faieta, M.; Corradini, M.G.; Michele, A.D.; Ludescher, R.D. Effect of encapsulation process on technological functionality and stability of Spirulina platensis extract. Food Biophys. 2020, 15, 50–63.
- Silva, S.C.; Fernandes, I.P.; Barros, L.; Fernandes, A.; Alves, M.J.; Calhelha, R.C.; Pereira, C.; Barreira, J.C.M.; Manrique, Y.; Colla, E.; et al. Spray-dried Spirulina platensis as an effective ingredient to improve yogurt formulations: Testing different encapsulating solutions. J. Funct. Foods 2019, 60, 103427.
- Ayekpam, C.; Hs, P.P.; Tavanandi, H.A.; Raghavarao, K. A novel method for double encapsulation of C-phycocyanin using aqueous two phase systems for extension of shelf life. J. Food Sci. Technol. 2020, 57, 3545–3555.
- Braga, A.R.C.; Figueira, F.D.S.; Silveira, J.T.D.; Morais, M.G.D.; Costa, J.A.V.; Kalil, S.J. Improvement of thermal stability of C-phycocyanin by nanofiber and preservative agents. J. Food Process. Preserv. 2016, 40, 1264–1269.
- Moreira, J.B.; Lim, L.T.; Zavareze, E.D.R.; Dias, A.R.G.; Costa, J.A.V.; Morais, M.G.D. Antioxidant ultrafine fibers developed with microalga compounds using a free surface electrospinning. Food Hydrocoll. 2019, 93, 131–136.
- Wen, Y.; Wen, P.; Hu, T.G.; Linhardt, R.J.; Zong, M.H.; Wu, H.; Chen, Z.Y. Encapsulation of phycocyanin by prebiotics and polysaccharides-based electrospun fibers and improved colon cancer prevention effects. Int. J. Biol. Macromol. 2020, 149, 672–681.
- Wen, P.; Hu, T.G.; Wen, Y.; Linhardt, R.J. Targeted delivery of phycocyanin for the prevention of colon cancer using electrospun fibers. Food Funct. 2019, 10, 1816–1825.
- Terra, A.; Moreira, J.B.; Costa, J.; Morais, M.G.D. Development of time-pH indicator nanofibers from natural pigments: An emerging processing technology to monitor the quality of foods. LWT Food Sci. Technol. 2021, 142, 111020.
- González, Y.V.; Prieto, C.; Filizoglu, M.F.; Ragazzo-Sanchez, J.A.; Calderon-Santoyo, M.; Furtado, R.F.; Chen, H.N.; Biswas, A.; Lagaron, J.M. Electrosprayed cashew gum microparticles for the encapsulation of highly sensitive bioactive materials. Carbohydr. Polym. 2021, 264, 118060.
- Schmatz, D.; Mastrantonio, D.J.D.S.; Costa, J.A.V.; Morais, M.G.D. Encapsulation of phycocyanin by electrospraying: A promising approach for the protection of sensitive compounds. Food Bioprod. Process. 2020, 119, 206–215.
- Rostamabadi, H.; Falsafi, S.R.; Rostamabadi, M.M.; Assadpour, E.; Jafari, S.M. Electrospraying as a novel process for the synthesis of particles/nanoparticles loaded with poorly water-soluble bioactive molecules. Adv. Colloid Interface Sci. 2021, 290, 102384.
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306.
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnurch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 6, 114097.
- Yagoubi, A.S.; Shahidi, F.; Mohebbi, M.; Varidi, M.; Golmohammadzadeh, S. Preparation, characterization and evaluation of physicochemical properties of phycocyanin-loaded solid lipid nanoparticles and nanostructured lipid carriers. J. Food Meas. Charact. 2018, 12, 378–385.
- Hardiningtyas, S.D.; Wakabayashi, R.; Kitaoka, M.; Tahara, Y.; Minamihata, K.; Goto, M.; Kamiya, N. Mechanistic investigation of transcutaneous protein delivery using solid-in-oil nanodispersion: A case study with phycocyanin. Eur. J. Pharm. Biopharm. 2018, 127, 44–50.
- Castangia, I.; Manca, L.M.; Latorre, A.C.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Phycocyanin-encapsulating hyalurosomes as carrier for skin delivery and protection from oxidative stress damage. J. Mater. Sci. Mater. Med. 2016, 27, 75.
- Zhu, Y.L.; Ma, Z.J.; Kong, L.Z.; He, Y.H.; Chan, H.F.; Li, H.Y. Modulation of macrophages by bioactive glass/sodium alginate hydrogel is crucial in skin regeneration enhancement. Biomaterials 2020, 256, 120216.
- Pan-Utai, W.; Iamtham, S. Physical extraction and extrusion entrapment of C-phycocyanin from Arthrospira platensis. J. King Saud Univ.-Sci. 2018, 31, 1535–1542.
- Pradeep, H.; Nayak, C. Enhanced stability of C-phycocyanin colorant by extrusion encapsulation. J. Food Sci. Technol. 2019, 56, 4526–4534.
- Pan-Utai, W.; Kahapana, W.; Iamtham, S. Extraction of C-phycocyanin from Arthrospira (Spirulina) and its thermal stability with citric acid. J. Appl. Phycol. 2017, 30, 231–242.
- Yan, M.Y.; Liu, B.; Jiao, X.D.; Qin, S. Preparation of phycocyanin microcapsules and its properties. Food Bioprod. Process. 2014, 92, 89–97.
- Hashemikamangar, S.S.; Alsaedi, R.J.F.; Chiniforush, N.; Motevaselian, F. Effect of antimicrobial photodynamic therapy with different photosensitizers and adhesion protocol on the bond strength of resin composite to sound dentin. Clin. Oral Investig. 2022, 26, 4011–4019.
- Mohan, P. Chitosan nanoparticle-mediated delivery of curcumin and phycocyanin for photodynamic therapy against biofilm forming bacteria. Mater. Express 2020, 10, 1854–1870.
- Zhang, B.C.; Zhang, X.W. Separation and nanoencapsulation of antitumor polypeptide from Spirulina platensis. Biotechnol. Prog. 2013, 29, 1230–1238.
- Dev, A.; Mohanbhai, S.J.; Kushwaha, A.C.; Sood, A.; Sardoiwala, M.N.; Choudhury, S.R.; Karmakar, S. κ-carrageenan-C-phycocyanin based smart injectable hydrogels for accelerated wound recovery and real-time monitoring. Acta Biomater. 2020, 109, 121–131.
- Yang, P.; Li, B.; Yin, Q.F.; Wang, Y.J. Carboxymethyl chitosan nanoparticles coupled with CD59-specific ligand peptide for targeted delivery of C-phycocyanin to HeLa cells. Tumor Biol. 2017, 39, 87–108.
- Manconi, M.; Mura, S.; Manca, M.L.; Fadda, A.M.; Dolz, M.; Hernandez, M.J.; Casanovas, A.; Diez-Sales, O. Chitosomes as drug delivery systems for C-phycocyanin: Preparation and characterization. Int. J. Pharm. 2010, 392, 92–100.
- Wang, X.Q.; Li, L.N.; Chang, W.R.; Zhang, J.P.; Gui, L.L.; Guo, B.J.; Liang, D.C. Structure of C-phycocyanin from Spirulina platensisat 2.2 Å resolution: A novel monoclinic crystal form for phycobiliproteins in phycobilisomes. Acta Crystallogr. 2001, 57, 784–792.
- Shishir, M.R.I.; Chen, W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends Food Sci. Technol. 2017, 65, 49–67.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
28 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





