Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hamizah Shahirah Hamezah | -- | 5640 | 2022-09-25 17:04:27 | | | |
| 2 | Beatrix Zheng | Meta information modification | 5640 | 2022-09-27 08:12:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Azlan, U.K.; Mediani, A.; Rohani, E.R.; Tong, X.; Han, R.; Misnan, N.M.; Jam, F.A.; Bunawan, H.; Sarian, M.N.; Hamezah, H.S. Pharmacological Aspects of Moringa oleifera. Encyclopedia. Available online: https://encyclopedia.pub/entry/27613 (accessed on 07 February 2026).
Azlan UK, Mediani A, Rohani ER, Tong X, Han R, Misnan NM, et al. Pharmacological Aspects of Moringa oleifera. Encyclopedia. Available at: https://encyclopedia.pub/entry/27613. Accessed February 07, 2026.
Azlan, Ummi Kalthum, Ahmed Mediani, Emelda Rosseleena Rohani, Xiaohui Tong, Rongchun Han, Norazlan Mohmad Misnan, Faidruz Azura Jam, Hamidun Bunawan, Murni Nazira Sarian, Hamizah Shahirah Hamezah. "Pharmacological Aspects of Moringa oleifera" Encyclopedia, https://encyclopedia.pub/entry/27613 (accessed February 07, 2026).
Azlan, U.K., Mediani, A., Rohani, E.R., Tong, X., Han, R., Misnan, N.M., Jam, F.A., Bunawan, H., Sarian, M.N., & Hamezah, H.S. (2022, September 27). Pharmacological Aspects of Moringa oleifera. In Encyclopedia. https://encyclopedia.pub/entry/27613
Azlan, Ummi Kalthum, et al. "Pharmacological Aspects of Moringa oleifera." Encyclopedia. Web. 27 September, 2022.
Copy Citation
Moringa oleifera is an ancient remedy plant, known as the miraculous plant due to its many prominent uses and significant health benefits. It is a nutrient-rich plant, with exceptional bioactive compounds, such as polyphenols that possess several medicinal properties. Many significant studies have been carried out to evaluate the ethnomedicinal and pharmacological properties of M. oleifera in various applications.
M. oleifera
bioactive compounds
polyphenols
pharmacological properties
1. Introduction
Plant-based products in medical research are currently one of the significant initiatives utilizing the potential properties carried by the bioactive compounds naturally found in plants. Many works have been carried out to incorporate plant products into safe drugs by synthetic strategies as well as to incorporate their potential effect into a regular diet. The Moringa oleifera plant, commonly known as ‘pokok kelor’ in Malaysia (natively known as drumstick tree or horseradish tree), is a plant that belongs to the Moringaceae family. It contains a great number of bioactive compounds, providing the pharmacological properties of the plant extract and contributing to the beneficial effects in humans [1][2][3].
Perpetually, this plant is a good source of naturally acquired medical benefits as most of them carry functional bioactive compounds, such as polyphenols and carotenoids [4]. Many studies have been carried out to investigate the medical significance of the bioactive compounds, possessing several biological activities such as antimicrobe, anti-inflammation and antioxidant. On top of that, the establishment of an optimized extraction method was also a game changer as the functional compounds can be extracted while keeping the original composition and structure. This has thus shown the authentic compounds to be better agents replacing synthetic compounds that are commonly toxic and have more carcinogenic effects.
Yet, M. oleifera have been practically and traditionally used for many purposes such as traditional remedies for many diseases, food consumption and cosmetic value preparation even long before its nutritional and potential medical properties were discovered [5][6]. Since the 1970s, many significant studies have been carried out with remarkable findings that show the M. oleifera plant to be a nutrient-rich plant, with an exceptional combination of nutrients, amino acids and many more properties that are valuable medically [6]. Thus, the use of M. oleifera has been applied extensively in many applications following its potential properties (Figure 1).
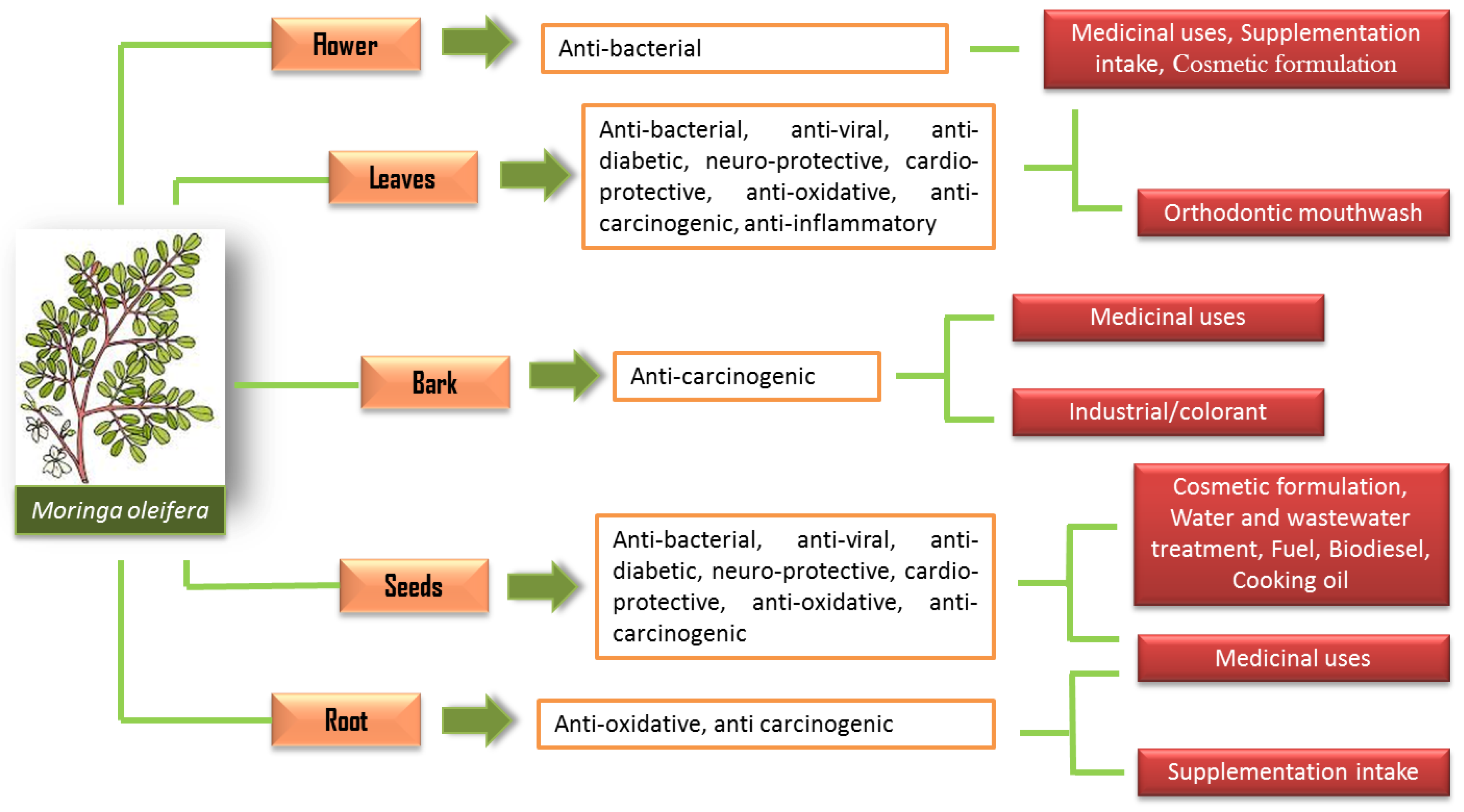
Figure 1. The uses of M. oleifera in various applications.
Each part of the M. oleifera carries its own benefits, and the most widely studied parts are the leaves and seeds. The high polyphenolic contents in M. oleifera have been suggested to be one of the significant contributory factors to its beneficial effects on health. For instance, a study has reported that the ethanolic extract of M. oleifera leaves has been characterized by a high content of flavonoid constituents, such as isoquercetin, quercetin and kaempferol [7]. These compounds contribute to many of its pharmacological properties [8]. The bioactive compounds of M. oleifera have presented many remarkable medicinal properties with various potential biological activities.
2. Pharmacological Properties of M. oleifera
The pharmacological properties of M. oleifera have been studied for various potential biological properties, such as cardio-protective, anti-oxidative, antiviral, antibacterial, anti-diabetic and anti-carcinogenic effects (Figure 2).
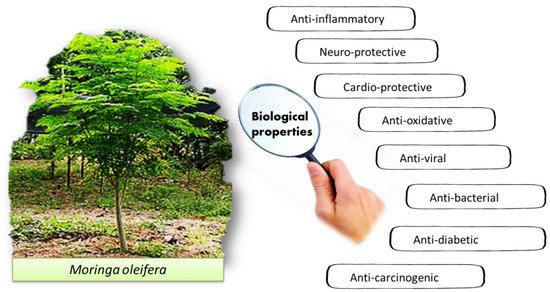
Figure 2. The reported biological properties of M. oleifera.
2.1. Anti-Oxidative Effects of M. oleifera
The high antioxidant activities of M. oleifera are often associated with its high content of polyphenol compounds. Antioxidant activities can be beneficial in many applications, and much evidence has shown that dietary polyphenols help to alleviate the complications of many critical illnesses, such as cancer, cardiac diseases and chronic inflammation that are commonly related to oxidative stress. Mechanistically, polyphenol compounds are secondary metabolites in plants known to be potent antioxidant agents that complement and add value to the activities of antioxidant vitamins and enzymes against oxidative stress [9]. They can neutralize free radicals by donating an electron or hydrogen atom dubbed as the main contributor to overall antioxidants in fruits, followed by vitamins [10]. However, studies found that chlorophyll has higher radical scavenger and reducing agent potential than phenolic compounds and flavonoids [11][12].
The nephroprotective and antioxidant effects of M. oleifera were appraised in paracetamol-induced nephrotoxic albino rabbits [13]. The research used the M. oleifera seed powder for oral administration in the treatment group at different doses (200, 400 and 600 mg/kg). That research found that, at an optimum dose of 600 mg/kg, the seed-powder-treated group demonstrated an alleviated damaging effect of paracetamol-induced renal damage in the rabbit. The authors stipulated that the alleviated damage was due to an altered lipid peroxidation process, and this may suggest the promising potential of M. oleifera in the treatment of renal failure or as an alternative to enhance the therapeutic value of the nephrotoxic agent [13].
The antioxidant effect of Japanese M. oleifera products, which consist of the herbal leaf tea and stem, have been investigated via free radical assays that target superoxide anion (O2−) radical generation systems [14]. This research used Trolox as the control standard for the determination of free radical scavenging capacity in the sample as it is an analog of α-tocopherol which is water soluble. Results show that the hot extracts of Moringa teas have lower scavenging activities than the Trolox standard in the tested synthetic free radical models [14]. However, the extracts also demonstrated an elevated O2− radical scavenging activity than Trolox in the phenazine methosulfate–NADH–nitroblue tetrazolium and xanthine oxidase assay systems. Other than that, the tea extracts potently suppressed the cellular O2− radical generation in incubated human neutrophils as compared to the Trolox standard. It was stipulated that, among the polyphenol contents of M. oleifera, caffeic acid and chlorogenic acid are the two compounds that are crucial for O2− specific radical scavenging capacity that is stronger than Trolox. Thus, it was suggested that the tea extracts consisting of leaves and stem parts of Moringa are a good alternative for natural antioxidants that help prevent O2− radical mediated conditions.
The antioxidant activity of the M. oleifera leaf has been investigated across the age of the leaf (30, 45 and 60 days) and extraction solvent (methanol, ethanol and aqueous) by using radical scavenging assays such as DPPH, 2, 2′-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic (ABTS) and anti-peroxide activity (APA) [15]. The TPC, TFC and chlorophyll content of the leaves was determined as part of the correlation in the research. The research found that total phenolic (TPC) and flavonoid (TFC) content was increased with age as the highest readings were observed at 60 days of leaf maturation in ethanol and methanol solvents but peaked at 45 days for the aqueous extract. The highest TPC was observed for the methanolic extract while the highest TFC was observed in the ethanolic extract. On the other hand, the ethanol and methanol extracts were observed to have similar chlorophyll contents that were significantly higher than in the aqueous extract. However, the chlorophyll content remained constant or reduced after it peaked at 30 days for all three solvents. Ethanolic leaf extracts showed the highest DPPH activity, while both ethanolic and methanolic extracts demonstrated similar ABTS+ activity. However, the authors also proposed that chlorophyll is the main contributor to antioxidant activities as there is evidence of a positive correlation between chlorophyll content and DPPH, ABTS and APA. The research concluded that ethanolic and methanolic extracts showed higher antioxidant activity than the leaf aqueous extract and 45 days of age is the optimum condition for extraction with the highest antioxidant potential.
Aside from that, the phytochemical content and antioxidant activity for different types of extraction of M. oleifera seed kernels (methanol, acetone and water) originating from Bangladesh were also appraised in a previous study [16]. In addition to the TPC, TFC and total tannin content evaluation, the in vitro antioxidant activities were determined by performing DPPH, ABTS, NO (nitric oxide) free radical scavenging and FRAP assays [16]. It was observed that the aqueous extracts showed the highest activities for scavenging DPPH, ABTS and NO free radicals as well as significant free radical scavenging activities and reducing power, higher TPC and TFC than the methanol and acetone extracts. This is in contrast with the previous finding as the aqueous extract was dubbed the most potent natural antioxidant agent [15]. However, the author also suggested that as future research, the study of the isolation of active compounds from the extracts may elucidate more rationale on the current finding.
In another locality, a study has been conducted to systematically evaluate the anti-inflammatory and antioxidant activities of ethanolic extracts of the leaves, seeds and roots of M. oleifera harvested in Kenya [17]. It was demonstrated that the leaf extracts showed the highest DPPH and FRAP activities while the leaf and root extracts displayed potential ABTS radical scavenging activities [17]. In addition, the leaf and seed extracts exhibited anti-inflammatory activities by the suppression of NO production. Phytochemical analysis via HPLC-UV/ESI-MS/MS found that the leaves of M. oleifera contain substantial amounts of flavonoid and phenolic acids as compared to the seed and root parts. As the positive correlation analysis found that flavonoid content is directly associated with antioxidant and anti-inflammatory activities, the high TPC and TFC of the leaves thus suggest it is a more potent source of anti-inflammatory and antioxidant activities as compared to other parts of the plant.
2.2. Antiviral Effects of M. oleifera
M. oleifera has been vastly studied as a potent antiviral agent. Years before the establishment of vaccine development and advancement, Moringa plants were traditionally used to treat many viral infections such as smallpox and chickenpox. Even though it has never been scientifically proven that M. oleifera plants are effective against viral infection due to the inability to conduct extensive research as the World Health Organization (WHO) has declared the world free of smallpox infection since May 1980, the potential of the plants as promising antiviral agents against other viral infection has continually been studied [18][19]. Following the reports of many authors, M. oleifera extracts exhibited potent inhibitory activities against many viral infections such as the influenza A virus (IAV) [20], Herpes simplex virus type 1 (HSV-1) [21][22][23], foot and mouth disease virus (FMDV) [24][25], hepatitis B virus (HBV) [26][27], human immunodeficiency virus (HIV) [28][29], Epstein–Barr virus (EBV) [30] and Newcastle disease virus (NDV) [31][32].
A study has demonstrated that Moringa A, the new compound from the M. oleifera seed, is effective against IAVs as it impedes the replication of the virus and protects the host cells from the cytopathic effect [20]. It was also found in the in vitro study that the compound can disrupt the cellular protein transcription factor EB (TFEB) and in turn decelerate autophagy in infected cells [20]. The M. oleifera leaf extract has also been found to be effective in an in vitro study against FMDV at 100 µg/mL, and it is toxic to the cells at a concentration of 200 µg/mL and higher [24][25]. It has been proposed that one of the thiocarbamate compounds, namely the niaziminin found in M. oleifera leaves, exhibits antiviral activities against the FMDV. This niaziminin compound has been discovered before against EBV, where the reaction of the compound and 4-[(4′-O-acetyl-alpha-L-rhamnose loxy) benzyl] isothiocyanate inhibited the activation of EBV [30].
From the survey, it was found that the extracts of M. oleifera are often consumed as part of a supplementation diet as part of an alternative to consuming conventional medicine. Even though research on the risk of the herb–drugs interaction is still scarce, no adverse effect has ever been reported. As there are many claims suggesting the ability of Moringa to improve the quality of life of people living with HIV/AIDS (PLWHA), a study has been carried out to investigate the in vitro inhibitory activities of M. oleifera extracts on lentiviral vector infectivity [28]. Results show that all ethanolic, methanolic and water extracts of M. oleifera were active against the HIV-1 lentiviral vector, and the early event of viral replication was inhibited. The potential of M. oleifera extracts in the selective inhibition of viral replication has suggested that they could serve as potent antiretroviral lead molecules.
In addition, considering the recent COVID-19 outbreak that has now become a pandemic, there has also been an attempt to investigate the potential of M. oleifera as a supplementary diet in enhancing the immune system. A review has suggested that M. oleifera can be effective against COVID-19 in a comprehensive way such that the plant acts as an immune booster and may increase the survival rate of people with SARS-CoV-2 infection [33]. There are many bioactive compounds of M. oleifera that show promising potential against COVID-19 infection such as kaempferol, quercetin, morphine, pterygospermin and apigenin-7-O-rutinoside [33][34][35]. Among all, the apigenin compound showed the highest activity against SARS-CoV-2- MPro, one of the main proteases of COVID-19, further concluding the potential of the Moringa plant as an immune booster against SARS- CoV-2 (COVID-19).
2.3. Antibacterial Effects of M. oleifera
The bacterial species that have been tested against the potent M. oleifera include water-borne pathogens, diarrhea-causing bacteria, drug-resistant bacteria and many more. A study has observed that the hexane and methanol seed extract of the plants exhibited inhibition of water-borne pathogens such as Salmonella typhii, Vibrio cholera and Escherichia coli [36]. Therefore, it was proposed that the antibacterial effect of M. oleifera could serve as a natural antibacterial agent in managing bacteria-caused water-borne diseases. Another study has also been carried out to investigate the antibacterial properties of different parts of M. oleifera in an approach to create natural dental care from the plant. Among the many attempts to formulate the right extracts into an experimental toothpaste and mouthwash, an ethanol extract of the leaves showed the highest antibacterial activities against S. aureus and Streptococcus mutant growth, with the experimental toothpaste exhibiting higher activities than the mouthwash [37]. The vast application of antibacterial agents derived from natural products has been crucial as it is more environmentally friendly, less toxic and a cheap and sustainable method for disease management and to improve the quality of life, especially in rural and developing countries.
In one study, both ethanol and methanol extracts of M. oleifera leaves showed a significantly higher (p < 0.05) inhibitory effect at a higher concentration of 120 mg/mL as compared to an aqueous extract against E. coli, S. aureus and Pseudomonas aeruginosa [38]. The finding suggests that the antibacterial activity of Moringa leaves is effective against both Gram-positive bacteria (S. aureus) and Gram-negative bacteria (E. coli and P. aeruginosa). In another study, an M. oleifera leaf extract was also tested against isolated multidrug-resistant (MDR) E. coli, S. aureus and P. aeruginosa by using the agar disc diffusion method. The results show that the chloroform extract had the highest antibacterial activity (9.32 ± 1.45 mm), while the aqueous extract had the lowest activity (0.27 ± 0.27 mm) [39]. The antibacterial activity observed against MDR bacteria added value to M. oleifera as a promising treatment alternative for infections caused by MDR bacteria.
The antibacterial effect of M. oleifera is the most anticipated property due to the massive application of antibacterial agents in various settings. M. oleifera is astonishing as a plant because every part of it, which includes the seed, root, bark, stem and leaf, has been described to harbor its own potential, coupled with the best extraction method and solvents that established its potency. Table 1 describes more studies from different authors that have investigated the antibacterial properties of M. oleifera against various species in many applications.
Table 1. Extracts of M. oleifera and the extensive findings on antibacterial properties.
| Extracts | Application | Finding | Citation |
|---|---|---|---|
| 70% Ethanol, 80% methanol, petroleum ether and aqueous extracts of M. oleifera leaves, flower, pulp and seed |
Method: Agar-well diffusion method Bacteria species: E. coli and S. aureus |
Maximum zone of inhibition: Leaves: 80% methanol extract against E. coli (28 mm) and S. aureus (26 mm). Flower: 70% ethanol extract against E. coli (23 mm) and S. aureus (17 mm). Pulp: 80% methanol extract against E. coli (15.33 mm). Aqueous extracts against S. aureus (18.33 mm). Seed: 80% methanol extract against E. coli (18.33 mm). 70% ethanol extracts against S. aereus (15.66 mm). |
[40] |
| Aqueous, petroleum ether and methanolic (20, 40, 60%) extracts of M. oleifera leaf | Method: In vitro, cup–plate method and disc diffusion method Bacteria species: S. aureus, E. coli, Klebsiella pneumonia, P. aeruginosa and Proteus vulgaris |
Methanolic extracts (20, 40, 60%) had high inhibitory effects on S. aureus, K. pneumoniae standard strains and S. aureus, S. saprophyticus and E.coli isolated from urinary tract infection. Aqueous extract only showed effects on P. vulgaris standard strain. Petroleum ether extracts showed no inhibitory activity at all. |
[41] |
| Methanol (99.9%), n-hexane (98.9%) and aqueous extracts of M. oleifera and M. ovalifolia seeds and bark | Method: Paper-disc diffusion method Bacteria species: E. coli, Enterococcus faecalis and Bacillus cereus |
M. oleifera extracts showed higher inhibitory activities than M. ovolifolia. Seed extracts of M. oleifera exhibit a wider range of antibacterial activity than M. ovalifolia. M. oleifera bark extracts showed higher antibacterial activity than M. ovalifolia against all tested species. n-hexane extracts for both M. ovalifolia and M. oleifera showed similar inhibitory activities but were generally lower than other solvents. |
[42] |
| Aqueous and ethanolic M. oleifera leaf | Method: Agar diffusion and microbroth dilution methods Bacteria species: S. aureus, Streptococcus pyogenes, Bacillus cereus, E. coli, P. aeruginosa, Shigella sonnei, Shigella dysenteriae, Shigella flexneri, Shigella boydii and Proteus mirabilis |
All tested bacterial isolates were observed to be susceptible to both extracts at 100 µg concentration, but susceptibility decreased as the extract concentration reduced. M.oleifera leaf extract showed broad spectrum of antibacterial activities as it works on both Gram-positive and -negative bacteria. Ethanol extract exhibited higher inhibition and minimal inhibitory concentrations. |
[43] |
| Chloroform, ethyl acetate, butanol and aqueous extracts M. oleifera leaf | Method: In vitro, agar-well diffusion method Bacteria species: E. coli, P. vulgaris, K. pneumoniae, Salmonella enterica, P. aeruginosa, S. aureus, Staphylococcus epidermidis and B. cereus |
Ethyl acetate extract observed the highest antibacterial activity against S. epidermidis, S. aureus, P. aeruginosa and B. cereus. Butanol extract reacted against S. epidermidis and S. aureus. Aqueous and chloroform extract only showed activity against S. epidermidis and S. aureus, respectively. |
[44] |
| Methanolic M. oleifera leaf | Method: In vitro, agar-well diffusion method Bacteria species: E. coli and Klebsiella |
The extracts showed activities against both bacteria in dose-dependent manner, as such highest activities were observed at dose of 200 mg/L. Methanol extract of M. oleifera was shown to have minimum inhibitory concentration (MIC) value against Kleibsilla at 45 mg %. |
[45] |
| Aqueous (hot and cold), ethanolic and methanolic extracts of M. oleifera seeds | Method: In vitro, disc diffusion method and broth dilution method Bacteria species: S. aureus, E. coli and P. aeruginosa |
The MIC for different extracts was observed as follows: aqueous (cold) extract showed no MIC, but aqueous (hot) extract was 100 mg/mL. The MIC for ethanolic and methanolic extracts is also capped at 100 mg/mL, except for E. coli and S. aureus with MIC of 50 mg/mL. The minimum bactericidal concentration (MBC) of aqueous (hot), ethanolic and methanolic extracts on tested bacteria was 200 mg/mL, but methanolic extract showed MBC of 100 mg/mL on E. coli. |
[46] |
| Methanol, acetone and aqueous extracts of M. oleifera seeds | Method: In vitro, agar-well diffusion technique and MIC and MBC Bacteria species: E. coli, Shigella typhii and Shigella dysenteriae |
Acetone extracts showed highest antibacterial activity against S. typhii and least sensitivity with the aqueous extract. The most observed MIC value was 6.25 mg/mL, then 12.5 mg/mL. Acetone extract is the most potent in exhibiting inhibitory activities at very low concentration for Shigella typhii |
[47] |
| 80% methanolic extracts of M. oleifera leaf and seeds | Method: In vitro, agar-well diffusion method Bacteria species: E. coli, s. typhi, salmonella paratyphi- A, salmonella paratyphi-B, shigella dysentriae, s. aureus, streptococcus feacalis, p. aeruginosa, Proteus mirabilis and k. Pneumoniae |
Both leaf and seed extracts exhibit antibacterial activity against all bacterial ATCC strains, but for leaf extract, highest activity was observed on S. typhi (ATCC19430) while the seed extracts showed on E. coli (ATCC25922). Both extracts also showed activities in clinically isolated bacterial strains, but for leaf extract, highest activity was observed against S. aureus, and for seed extract, highest activities was observed against K. pneumoniae, P. mirabilis and S. typhi. Overall, the results show higher antibacterial activity in leaf extract as compared to seed extract. |
[48] |
| Ethanolic extracts of M. oleifera leaf | Method: Bacteria inhibition microplate assay Bacteria species: Agrobacterium tumefeciens (At), Clavibacter michiganensis subsp. michiganensis (Cmm), Pseudomonas syringae pv. tomato (Pst), Ralstonia solanacearum (Rs) and Xanthomonas axonopodis (Xa) |
Results show that the extract exhibits inhibitory effects on the growth of phytopathogenic bacteria At, Cmm, Pst, Rs and Xa in dose-dependent manner. Higher inhibition was observed at higher concentration of extract. At was found to be most susceptible to the exact treatment while Rs was more resistant. Ethanolic extracts of M. oleifera leaf showed prominent bio-bactericide potential. |
[49] |
2.4. Anti-Diabetic Effects of M. oleifera
Diabetes mellitus (DM) is a metabolic disease that causes high blood glucose due to the body’s inability to produce sufficient or functional hormone insulin to regulate blood glucose. Many studies have demonstrated the potential of M. oleifera as anti-diabetic agents for the treatment of this metabolic disease due to the high presence of polyphenols that help to reduce blood glucose [50] and improve sexual dysfunction [51]. The leaf powder of M. oleifera was found to have quercetin-3-glucoside and fibers that give mitigating effects on glucose intolerance [52]. In addition, the leaves are also rich in unique Moringa isothiocyanate (MIC) compounds that possess high biological activities and evidence of therapeutically active constituents [53]. It is also evidently suggested that a potential wound dressing formulation containing extracts of M. oleifera may help with wound management that potentially aggravates diabetic conditions [54].
The oral administration of ethanolic extract of M. oleifera leaves has been investigated for the anti-diabetic and liver function indices in Alloxan-induced diabetic rats via glucometer and spectrophotometric methods [55]. The results show that there is a significant decline in the glucose level of the treated rats and elevated levels of liver indices ALT, AST and ALP in a dose-dependent manner. It was also demonstrated that the levels of albumin and bilirubin changed according to doses; for example, a 200 mg/kg dose showed an increase in the albumin level, but at higher doses, the albumin levels were reduced. It can be stipulated that aside from providing anti-diabetic potential, the extracts also help to protect from liver damage, and 400 mg/kg was observed to be the safest dose.
In another study, the aqueous ethanolic extract (95, 75, 50, 25% v/v and 100% water) of M. oleifera leaves was fed orally to experimental rats to investigate the hypoglycemic activities and contribution to intraperitoneal glucose tolerance test (IPGTT) data [56]. As a 95% (v/v) ethanolic extract (at 1000 mg/kg) showed the highest activities, it was submitted for liquid-to-liquid fractionation into hexane, chloroform, ethyl acetate, butanol and water for more screening of potent anti-diabetic activities [56]. Among all extracts and fractions, the 95% ethanolic extract and only butanol fraction showed an effect by alleviating blood glucose concentration after administration to diabetic rats. However, no hyperglycemic effect was observed in normal rats. The TLC and HPLC analysis determined the presence of quercetin 3-β-D-glucoside, kaempferol-3-O-glucoside and crypto chlorogenic acid in the extracts that stipulated antihyperglycemic potential. Thus, the authors suggested the potential of the extract and fraction as alternative treatments for diabetes and recommended further investigation for drug discovery.
Aside from the leaves, the seed extracts of M. oleifera have also been studied for their potential as anti-diabetics. There was a potential of the aqueous extract and oil of M. oleifera seeds against several biochemical markers in streptozotocin-induced diabetes mellitus albino rats [57]. The serum was collected for the determination of blood glucose, body weight, albumin, urea, creatinine, electrolytes (Na+, K+ and Cl−)- and the levels of enzyme markers for liver damage (AST and ALT). The results show that at 100 mg/kg and 200 mg/kg doses of aqueous extract treatment of diabetic rats, a significant reduction in serum glucose was observed [57]. Other than that, there was also a decrease in urea and creatinine levels that were significant as compared to the diabetic untreated group. In addition to that, the extract was also observed to ameliorate the hepatic function as low levels of enzyme markers were recorded in the treated group. Thus, this proposed the potential of the M. oleifera seed extract as an anti-diabetic with remarkable nephron-protective activity.
In a more recent study, the effect of the ethanolic leaf extract at two different doses (250 and 500 mg/kg) on the metabolic glucose, melatonin and lipid profile and liver and kidney function in Alloxan-induced diabetes was investigated [58]. After 60 days of oral treatment of the extracts, results showed that there was a significant decline in blood glucose, total cholesterol, triglycerides and the levels of low-density lipoprotein (LDL), ALP, ALT and AST. Elevated levels of serum melatonin, lactate dehydrogenase (LDH) and high-density lipoprotein (HDL) were also recorded in the diabetic group as compared to the control group. The authors thus stipulated that the ethanolic leaf extract treatment of diabetic rats helps to reinstitute the metabolic changes to normal levels.
2.5. Anti-Carcinogenic Effects of M. oleifera
The anti-carcinogenic potential of M. oleifera is one of its medical benefits that is worth investigating due to the high content of various phytochemicals, supported by much evidence of low toxicity that ensures the safe application of the plant [59][60][61]. M. oleifera is rich in phenolic acids and flavonoid compounds that are known for their potential as antioxidants and anti-cancer agents. As oxidative stress is one of the causative agents of cancer development, the presence of compounds harboring antioxidant properties may interfere with the floating free radicals and reduce oxidative stress. This will consequently help to prevent cancer.
The anti-carcinogenic effect of different parts of M. oleifera (leaf, bark and seed extracts) against the MDA-MB-231 and HCT-8 cancer cell lines has been studied [62]. The reesarch found that the leaf and bark extract showed significant anti-cancer effects as compared to the seed extract. The leaf- and bark-extract-treated cell lines showed low cell survival with a remarkable reduction in cell growth as well as cell motility. In addition, the apoptosis assays showed significant increments of apoptotic cells for the two extract groups. The GC-MS analyses demonstrated the presence of many targeted anti-cancer compounds such as eugenol, isopropyl isothiocyanate, D-allose and hexadeconoic acid ethyl ester that indicated the anti-cancer properties of M. oleifera. The authors claimed that the research was the first to report the anti-cancer potential of the bark. It was suggested that leaf and bark extracts of M. oleifera exhibited anti-cancer activity in both cell lines, and thus new potent agents can be proposed in the treatment of breast and colorectal cancers [62].
The potential of M. oleifera leaves has been further investigated in another study, against different cell lines of human hepatocellular carcinoma HepG2 cells [63]. Following the analysis of apoptotic signals, results show that the leaf extract triggers the apoptosis reaction in HepG2 cells. Moreover, the hollow fiber assay (HFA), using immunodeficient nude mice, demonstrated a notable reduction in both HepG2 cells and A549 non-small cell lung cancer cell proliferation after the oral administration of the leaf extract. It was proposed that the remarkable tumor inhibition activities may have resulted from the high bioactive compound content in the extracts, thus suggesting its potential as a promising anti-carcinogenic agent [63].
The ethanolic extract of M. oleifera has also been evaluated for its regulatory activity in leukemic Wistar rats via a tumor necrosis factor-α (TNF-α) assay [64]. The ethanolic extract was orally administered to the rats pre-, during and post-leukemia induction, in a compelling 8 weeks of total duration. The plasma sample was collected for the TNF-α analysis by using an enzyme-linked immunosorbent assay (ELISA). The level of TNF-α was the highest in the non-treated group, followed by the M. oleifera-treated group, and the lowest was observed in the control group. TNF-α is a known pro-inflammatory cytokine that is released upon the activation of macrophages or monocytes to mediate various cellular events such as the stimulation of other functional cytokines, cell proliferation, differentiation and apoptosis. One previous study has found that the reduction in the TNF-α level signifies the response against treatment while elevated TNF-α levels are indicative of the active disease progress [65]. Thus, the authors proposed that the TNF-α level may be a suitable indicator for the clinical efficacy of anti-cancer therapy.
The extract of M. oleifera seeds, roots, stems and leaves in different ethanol concentrations (50, 70 and 90%) was appraised for the antioxidant and anti-proliferative properties in different cell lines from a previously reviewed study, the head and neck cancer (HNC) cell lines, CNE-1 and CAL27 [66]. Prior to the investigation of the cell line, the TPC, TFC and antioxidant levels were determined for all the different extracts. The results of this research suggest that the aqueous leaf extract showed the highest antioxidant activities, but the 70% ethanolic extract recorded high antioxidant activity for the other parts of the plants (seeds, roots and stems). In addition to that, all the extracts showed notable anti-cancer activities in the tested cell line where the proliferations of HNC cells were impeded by the suspected apoptosis inducement. Interestingly, the stem extracts exhibited the strongest apoptotic induction, followed by the leaf extracts. This thus concluded that the M. oleifera extract possesses remarkable antioxidants and anti-proliferative potentials that may be helpful in the management and treatment of head and neck cancer.
2.6. Cardio-Protective Effects of M. oleifera
Cardiovascular abnormalities are one of the most concerning conditions ever existing medically, and all related complications have contributed to the high mortality throughout the world. The use of phytochemicals from natural medicinal plants has been extended to various applications including as a cardio-protective agent as more evidence from scientific research has suggested its potential. Bioactive compounds such as diosgenin, isoflavones, sulforaphane, carotinized, catechin and quercetin have been determined to contribute to cardio-protection and alleviating cardiac-related complications [67]. M. oleifera has been studied as potent medicinal plants for cardio-protection due to their abundant phytochemicals such as polyphenols that perform cardio-protection by impeding hepatic cholesterol and lipoprotein metabolism and mitigating the inflammatory response [68]. The ethanol and aqueous extracts were found to contribute significantly to reduced systolic and diastolic blood pressure in spontaneously hypertensive rats [69].
A study has evaluated the cardio-protective effect of the aqueous extract of M. oleifera leaves on Wistar albino rats via investigations of the lipid profile as well as the cardio-toxicity effect [70]. In this research, the rats were administered with potassium bromate to induce toxicity on the cardiac tissue, and then Moringa extracts were applied to investigate the detoxifying effect. Potassium bromate is a potent cardio-toxin that increases lipid peroxidation and reduces heart antioxidant activities. In the potassium bromate-induced rats only, cardiac dysfunction was indicated by the elevated cardiac biomarker enzymes AST, ALT, ALP and other tested components on cardiac tissues. Results show that the extract of M. oleifera demonstrated cardio-protection potential on the potassium bromate- induced cardiac oxidative damage in rats as the antioxidant loss was alleviated and the cardiac dysfunction was restored [70].
Other than that, the potential of M. oleifera seed powder has been evaluated in spontaneously hypertensive rats (SHRs) where the SHRs were given oral administration of food containing the seed powder, and the cardiac effects were determined [71]. Hypertension is a condition of perpetuated high blood pressure that may result in cardiac complications with an escalated risk of heart attack/heart failure. Upon oral treatment of Moringa seed powder, no changes were observed in the rats’ blood pressure, except for a decrease in nocturnal heart rate with ameliorated cardiac diastolic function. The authors also suggested that the seed powder treatment may have an effect on the signaling pathways associated with pressure-overload-induced left ventricular hypertrophy such as the calcium-regulated mechanism. However, an in-depth study is needed to elucidate the exact mechanism involved in the Moringa cardio-protective potential.
A study has been conducted to investigate the effect of M. oleifera seed powder on the oxidative and nitrosative vascular stresses in SHRs [72]. Reduced vascular stresses were observed in the Moringa-treated SHR aortas, associated with a decline in the free 8-isoprostane circulating level, vascular p22phox and p47phox expressions and the upregulation of SOD2. After the treatment, it was found that there were decreased iNOS and NF-κB protein expressions, which resulted in reduced circulating nitrites and C-reactive proteins that are often elevated in normal SHRs. The research also found that the treated-SHR group showed an enhanced resistance of the arteries against the endothelium-dependent carbachol-induced relaxation functional test. This research presented an overall vascular antioxidant, anti-inflammatory and endothelial protective potential of M. oleifera seed powder in a supplementary diet against cardiovascular complications indicated by oxidative stress and inflammation [72].
In addition, the cardio-protective effect of the methanolic M. oleifera seed extract has been studied in isoproterenol-induced myocardial infarction (MI) in Wistar albino rats [73]. The treatment lasted for 32 days, the fasting blood samples were collected for the determination of serum cardiac biomarker enzymes and the lipid profile, while the heart tissue was collected for the evaluation of myocardial marker enzymes (LDH, CPK, AST, ALT and CK-MB) and antioxidant enzymes (GSH and LPO). The research found that the rats treated with the methanolic seed extracts showed a positive effect that reversed all the altered regulation of the tested biomarkers as compared to isoproterenol-induced rats. The positive reversed effect of Moringa was observed as follows: the isoproterenol caused a significant increment in serum myocardial enzymes (LDH, CPK, AST, ALT and CK-MB) and lipid profiling parameters, but the Moringa-treated group showed a decrease in the levels. The isoproterenol reduced the myocardial enzymes in the heart tissue significantly, but the Moringa pre-treated rats presented elevated biomarker enzyme levels in a dose-dependent manner.
The M. oleifera seed has also been evaluated for its potential in ischemic heart diseases. The M. oleifera seed powder was orally administered to wild-type C57/BL6 male mice by feeding the mice a diet containing the seed powder. The research found that the M. oleifera treated group had a reduced MI-induced mortality and alleviated cardiac dysfunctions in MI mice [74]. In addition, it was observed that post 28 days of MI inducement in mice, there was a significant increment in ejection fraction and fractional shortening, with more data suggesting that the Moringa treatment attenuates MI-induced infarction size and cardiac remodeling. The research elucidated that the mechanistic role of M. oleifera seeds in ischemic heart diseases is indicated by the inhibition of MI-induced apoptosis and subdued cardiac fibrosis. The authors concluded that oral administration of M. oleifera seed powder potentially exhibits anti-apoptosis and antioxidant effects which are critical for mitigating MI damage to cardiac function in MI-induced mice model.
References
- Luetragoon, T.; Sranujit, R.P.; Noysang, C.; Thongsri, Y.; Potup, P.; Suphrom, N.; Nuengchamnong, N.; Usuwanthim, K. Bioactive Compounds in Moringa oleifera Lam. Leaves Inhibit the Pro-Inflammatory Mediators in Lipopolysaccharide-Induced Human Monocyte-Derived Macrophages. Molecules 2020, 25, 191.
- Padayachee, B.; Baijnath, H. An overview of the medicinal importance of Moringaceae. J. Med. Plants Res. 2012, 6, 5831–5839.
- Padayachee, B.; Baijnath, H. An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. S. Afr. J. Bot. 2020, 129, 304–316.
- Proestos, C.; Varzakas, T. Aromatic Plants: Antioxidant Capacity and Polyphenol Characterisation. Foods 2017, 6, 28.
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835.
- Mahmood, K.T.; Mugal, T.; Ul Haq, I. Moringa oleifera: A natural gift—A review. J. Pharm. Sci. Res. 2010, 2, 775–781.
- Zhu, Y.; Yin, Q.; Yang, Y. Comprehensive investigation of Moringa oleifera from different regions by simultaneous determination of 11 polyphenols using UPLC-ESI-MS/MS. Molecules 2020, 25, 676.
- Jaja-Chimedza, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, e0182658.
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246.
- Wang, H.; Cao, G.; Prior, R.L. Total Antioxidant Capacity of Fruits. J. Agric. Food Chem. 1996, 44, 701–705.
- Hsu, C.-Y.; Chao, P.-Y.; Hu, S.-P.; Yang, C.-M. The Antioxidant and Free Radical Scavenging Activities of Chlorophylls and Pheophytins. Food Nutr. Sci. 2013, 4, 35234.
- Khattab, R.; Goldberg, E.; Lin, L.; Thiyam, U. Quantitative analysis and free-radical-scavenging activity of chlorophyll, phytic acid, and condensed tannins in canola. Food Chem. 2010, 122, 1266–1272.
- Ijaz, A.; Javed, I.; Aslam, B.; Khan, J.A.; Khaliq, T.; Khan, M.Z.; Iqbal, Z.; Naeem, M.A.; Ashraf, M.M. Nephroprotective and antioxidant effects of Moringa oleifera (Sohanjna) in paracetamol induced nephrotoxic Albino rabbits. Pak. Vet. J. 2016, 36, 292–296.
- Sugahara, S.; Chiyo, A.; Fukuoka, K.; Ueda, Y.; Tokunaga, Y.; Nishida, Y.; Kinoshita, H.; Matsuda, Y.; Igoshi, K.; Ono, M.; et al. Unique antioxidant effects of herbal leaf tea and stem tea from Moringa oleifera L. especially on superoxide anion radical generation systems. Biosci. Biotechnol. Biochem. 2018, 82, 1973–1984.
- Nobossé, P.; Fombang, E.N.; Mbofung, C.M.F. Effects of age and extraction solvent on phytochemical content and antioxidant activity of fresh Moringa oleifera L. leaves. Food Sci. Nutr. 2018, 6, 2188–2198.
- Jahan, I.A.; Hossain, M.H.; Ahmed, K.S.; Sultana, Z.; Biswas, P.K.; Nada, K. Antioxidant activity of Moringa oleifera seed extracts. Orient. Pharm. Exp. Med. 2018, 18, 299–307.
- Xu, Y.; Chen, G.; Guo, M. Antioxidant and anti-inflammatory activities of the crude extracts of Moringa oleifera from Kenya and their correlations with flavonoids. Antioxidants 2019, 8, 296.
- Biswas, D.; Nandy, S.; Mukherjee, A.; Pandey, D.; Dey, A. Moringa oleifera Lam. and derived phytochemicals as promising antiviral agents: A review. S. Afr. J. Bot. 2020, 129, 272–282.
- WHO. Small Pox Is Dead; WHO Report; World Health Organization: Geneva, Switzerland, 1980; Volume 23, pp. 1–40.
- Xiong, Y.; Rajoka, M.S.R.; Mehwish, H.M.; Zhang, M.; Liang, N.; Li, C.; He, Z. Virucidal activity of Moringa A from Moringa oleifera seeds against Influenza A Viruses by regulating TFEB. Int. Immunopharmacol. 2021, 95, 107561.
- Goswami, D.; Mukherjee, P.K.; Kar, A.; Ojha, D.; Roy, S.; Chattopadhyay, D. Screening of ethnomedicinal plants of diverse culture for antiviral potentials. Indian J. Tradit. Knowl. 2016, 15, 474–481.
- Kurokawa, M.; Wadhwani, A.; Kai, H.; Hidaka, M.; Yoshida, H.; Sugita, C.; Watanabe, W.; Matsuno, K.; Hagiwara, A. Activation of Cellular Immunity in Herpes Simplex Virus Type 1-Infected Mice by the Oral Administration of Aqueous Extract of Moringa oleifera Lam. Leaves. Phytother. Res. 2016, 30, 797–804.
- Lipipun, V.; Kurokawa, M.; Suttisri, R.; Taweechotipatr, P.; Pramyothin, P.; Hattori, M.; Shiraki, K. Efficacy of Thai medicinal plant extracts against herpes simplex virus type 1 infection in vitro and in vivo. Antivir. Res. 2003, 60, 175–180.
- Imran, I.; Altaf, I.; Ashraf, M.; Javeed, A.; Munir, N.; Bashir, R. In vitro evaluation of antiviral activity of leaf extracts of Azadirachta indica, Moringa oleifera, and Morus alba against the foot and mouth disease virus on BHK-21 cell line. Sci. Asia 2016, 42, 392.
- Younus, I.; Siddiq, A.; Assad, T.; Badar, S.; Jameel, S.; Ashraf, M. Screening antiviral activity of Moringa oliefera L. leaves against foot and mouth disease virus. Glob. Vet. 2015, 15, 409–413.
- Feustel, S.; Ayón-Pérez, F.; Sandoval-Rodriguez, A.; Rodríguez-Echevarría, R.; Contreras-Salinas, H.; Armendáriz-Borunda, J.; Sánchez-Orozco, L.V. Protective Effects of Moringa oleifera on HBV Genotypes C and H Transiently Transfected Huh7 Cells. J. Immunol. Res. 2017, 2017, 6063850.
- Waiyaput, W.; Payungporn, S.; Issara-Amphorn, J.; Panjaworayan, N.T.-T. Inhibitory effects of crude extracts from some edible Thai plants against replication of hepatitis B virus and human liver cancer cells. Complement. Altern. Med. 2012, 12, 246.
- Nworu, S.C.; Okoye, L.E.; Ezeifeka, O.G. Esimone Extracts of Moringa oleifera Lam. showing inhibitory activity against early steps in the infectivity of HIV-1 lentiviral particles in a viral vector-based screening. Afr. J. Biotechnol. 2013, 12, 4866–4873.
- Monera, T.; Maponga, C. Moringa oleifera supplementation by patients on antiretroviral therapy. J. Int. AIDS Soc. 2010, 13, P188.
- Murakami, A.; Kitazono, Y.; Jiwajinda, S.; Koshimizu, K.; Ohigashi, H. Niaziminin, a thiocarbamate from the leaves of Moringa oleifera, holds a strict structural requirement for inhibition of tumor- promoter-induced epstein-barr virus activa-tion. Planta Med. 1998, 64, 319–323.
- Eze, D.C.; Okwor, E.C.; Okoye, J.O.A.; Onah, D.N. Immunologic effects of Moringa oleifera methanolic leaf extract in chickens infected with Newcastle disease virus (kudu 113) strain. Afr. J. Pharm. Pharmacol. 2013, 7, 2231–2237.
- Tolba, H.M.N.; Elmaaty, A.A.; Farag, G.K.; Mansour, D.A.; Elakkad, H.A. Immunological effect of Moringa Oleifera leaf extract on vaccinated and non-vaccinated Hubbard chickens experimentally infected with Newcastle virus. Saudi J. Biol. Sci. 2022, 29, 420–426.
- Fajri, M. The potential of Moringa oleifera as immune booster against COVID 19. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 022008.
- Nair, D.A.; James, T. Computational screening of phytocompounds from Moringa oleifera leaf as potential inhibitors of SARS-CoV-2 Mpro. Res. Square. 2002, 1–14.
- Shaji, D. Computational identification of drug lead compounds for COVID-19. ChemRxiv 2020, 1–15.
- Walter, A.; Samuel, W.; Peter, A.; Joseph, O. Antibacterial activity of Moringa oleifera and Moringa stenopetala methanol and n-hexane seed extracts on bacteria implicated in water borne diseases. Afric. J. Microb. Res. 2011, 5, 153–157.
- Elgamily, H.; Moussa, A.; Elboraey, A.; El-Sayed, H.; Al-Moghazy, M.; Abdalla, A. Microbiological Assessment of Moringa Oleifera Extracts and Its Incorporation in Novel Dental Remedies against Some Oral Pathogens. Maced. J. Med. Sci. 2016, 4, 585–590.
- Singh, K.; Tafida, G. Antibacterial activity of Moringa oleifera (lam) leaves extracts against some selected bacteria. Int. J. Pharm. Pharmaceu. Sci. 2014, 6, 52–54.
- Eremwanarue, O.; Shittu, H. Antimicrobial activity of Moringa oleifera leaf extracts on multiple drug resistant bacterial isolates from urine samples in Benin City. Niger. J. Biotechnol. 2018, 35, 16–26.
- Dodiya, B.; Amin, B. Antibacterial activity and phytochemical screening of different parts of Moringa oleifera against selected gram positive and gram negative bacteria. Res. J. Pharm. Biol. Chem. Sci. 2015, 3, 421–425.
- Abdalla, A.M.; Alwasilah, H.Y.; Mahjoub, R.A.H.; Mohammed, H.I.; Yagoub, M. Evaluation of antimicrobial activ-ity of Moringa oleifera leaf extracts against pathogenic bacteria isolated from urinary tract infected patients. J. Adv. Lab. Res. 2016, 7, 47–51.
- Shailemo, D.H.P.; Kwaambwa, H.M.; Kandawa-Schulz, M.; Msagati, T.A.M. Antibacterial Activity of Moringa ovalifolia and Moringa oleifera Methanol, N-Hexane and Water Seeds and Bark Extracts against Pathogens That Are Implicated in Water Borne Diseases. Green Sustain. Chem. 2016, 6, 71–77.
- Isitua, C.; Ibeh, I.; Olayinka, J. Antibacterial activity of Moringa Oleifera Lam leaves on enteric human pathogens. Indian J. Appl. Res. 2016, 6, 553–557.
- Abdallah, E.M. Antibacterial Properties of Leaf Extracts of Moringa oleifera Lam. Growing in Sudan. J. Adv. Med. Pharm. Sci. 2016, 5, 1–5.
- FadiaTaufik, M.; Rashed, A.; Oshkondali, S.T.; Alacrouk, S.A.; Sleman, K. Antibacterial activities of Moringa oleifera leaf extract on some human pathogenic bacteria. Saudi J. Med. Pharm. Sci. 2021, 7, 426–431.
- Bello, S.; Jamiu, A. Antibacterial activity of Moringa oleifera seed extracts on Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Niger. J. Microb. 2017, 31, 3873–3881.
- Delelegn, A.; Sahile, S.; Husen, A. Water purification and antibacterial efficacy of Moringa oleifera Lam. Agric. Food Secur. 2018, 7, 25.
- Kwami, W.S.; Saeed, H.A.; Mohammed Hamad, M.N. Screening the Antibacterial Activity of Moringa oleifera Leaves and Seeds Extract against Selected Members of Bacteria. Saudi J. Pathol. Microbiol. 2020, 5, 370–373.
- Arredondo-Valdés, R.; Hernández-Castillo, F.D.; Rocandio-Rodríguez, M.; Anguiano-Cabello, J.C.; Rosas-Mejía, M.; Vanoye-Eligio, V.; Ordaz-Silva, S.; López-Sánchez, I.V.; Carrazco-Peña, L.D.; Chacon-Hernandez, J.C. In vitro antibacterial activity of Moringa oleifera ethanolic extract against tomato phytopathogenic bacteria. J. Exp. Bot. 2021, 90, 895–906.
- Jacques, A.S.; Arnaud, S.S.S.; Fréjus, O.O.H.; Jacques, D.T. Review on biological and immunomodulatory properties of Moringa oleifera in animal and human nutrition. J. Pharmacogn. Phytother. 2020, 12, 1–9.
- Ain, N.; Bashah, K.; Noor, M.M.A.T. Antihyperglycemic and androgenic properties of Moringa oleifera leaves aqueous extract attenuate sexual dysfunction in diabetes-induced male rats. Malays. Appl. Biol. 2021, 50, 99–105.
- Ndong, M.; Uehara, M.; Katsumata, S.; Suzuki, K. Effects of oral administration of Moringa oleifera Lam on glucose tol-erance in Goto-Kakizaki and Wistar rats. J. Clin. Biochem. Nutri. 2007, 40, 229–233.
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 2014, 59, 1013–1024.
- Chin, C.-Y.; Jalil, J.; Ng, P.Y.; Ng, S.-F. Development and formulation of Moringa oleifera standardised leaf extract film dressing for wound healing application. J. Ethnopharmacol. 2018, 212, 188–199.
- Aja, P.M.; Igwenyi, I.; Orji, O. Evaluation of anti-diabetic effect and liver function indices of ethanol extracts of Moringa oleifera and Cajanus cajan leaves in alloxan induced diabetic Albino rats. Glob. Vet. 2015, 14, 439–447.
- Irfan, H.M.; Asmawi, M.Z.; Khan, N.A.K.; Sadikun, A.; Mordi, M.N. Anti-diabetic activity-guided screening of aqueous-ethanol Moringa oleifera extracts and fractions: Identification of marker compounds. Trop. J. Pharm. Res. 2017, 16, 543–552.
- Nadro, M.; Audu, A.; Glen, E. Anti-diabetic effects of aqueous extract and oil of Moringa oleifera seed on liver and kid-ney functions in streptozotocin-induced diabetes in rat. Am. J. Biochem. 2018, 8, 69–74.
- Saleh, S.S.; Sarhat, E.R. Effects of Ethanolic Moringa Oleifera Extract on Melatonin, Liver and Kidney Function Tests in Alloxan-Induced Diabetic Rats. Indian J. Forensic Med. Toxicol. 2019, 13, 1015–1019.
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of Drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155.
- Awodele, O.; Oreagba, I.A.; Odoma, S.; da Silva, J.A.T.; Osunkalu, V.O. Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae). J. Ethnopharmacol. 2012, 139, 330–336.
- Khor, K.Z.; Lim, V.; Moses, E.J.; Samad, N.A. The In Vitro and In Vivo Anticancer Properties of Moringa oleifera. Evid.-Based Complement. Altern. Med. 2018, 2018, 1071243.
- Al-Asmari, A.K.; AlBalawi, S.M.; Athar, T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS ONE 2015, 10, e0135814.
- Jung, I.L.; Lee, J.H.; Kang, S.C. A potential oral anticancer drug candidate, Moringa oleifera leaf extract, induces the apoptosis of human hepatocellular carcinoma cells. Oncol. Lett. 2015, 10, 1597–1604.
- Abutu, P.; Amuda, O.; Osinaya, O.; Babatunde, B. Regulatory activity of ethanol leaves extract of Moringa Oleifera on benzene induced leukemia in Wister Rat using TNF-α analysis. Am. J. Biomed. Res. 2020, 7, 6–11.
- Basu, S.; Pathak, S.K.; Banerjee, A.; Pathak, S.; Bhattacharyya, A.; Yang, Z.; Talarico, S.; Kundu, M.; Basu, J. Execution of Macrophage Apoptosis by PE_PGRS33 of Mycobacterium tuberculosis Is Mediated by Toll-like Receptor 2-dependent Release of Tumor Necrosis Factor-α. J. Biol. Chem. 2007, 282, 1039–1050.
- Wang, F.; Long, S.; Zhang, J.; Yu, J.; Xiong, Y.; Zhou, W.; Qiu, J.; Jiang, H. Antioxidant activities and anti-proliferative effects of Moringa oleifera L. extracts with head and neck cancer. Food Biosci. 2020, 37, 100691.
- Shah, S.M.A.; Akram, M.; Riaz, M.; Munir, N.; Rasool, G. Cardioprotective Potential of Plant-Derived Molecules: A Scientific and Medicinal Approach. Dose Response 2019, 17, 1559325819852243.
- Zern, T.L.; Fernandez, M.L. Cardioprotective Effects of Dietary Polyphenols. J. Nutr. 2005, 135, 2291–2294.
- Kumolosasi, E.; Wei, C.C.; Abdullah, A.Z.; Abd Manap, N.S.; Lee, W.L.; Yousuf, M.H.; Ying, L.S.; Buang, F.; Said, M.M.; Mohamad, H.F.; et al. Antihyperten-sive activities of standardised Moringa oleifera Lam. (Merunggai) extracts in spontaneously hypertensive rats. Sains Malays 2021, 50, 769–778.
- Oseni, O.; Ogunmoyole, T.; Idowu, K. Lipid profile and cardio-protective effects of aqueous extract of Moringa oleifera (lam) leaf on bromate- induced cardiotoxicity on Wistar albino rats. Eur. J. Adv. Res. Biol. Life Sci. 2015, 3, 52–66.
- Randriamboavonjy, J.I.; Loirand, G.; Vaillant, N.; Lauzier, B.; Derbré, S.; Michalet, S.; Pacaud, P.; Tesse, A. Cardiac Protective Effects of Moringa oleifera Seeds in Spontaneous Hypertensive Rats. Am. J. Hypertens. 2016, 29, 873–881.
- Randriamboavonjy Joseph Iharinjaka Rio, M.; Pacaud, P.; Loirand, G.; Tesse, A. Moringa oleifera seeds attenuate vas-cular oxidative and nitrosative stresses in spontaneously hypertensive rats. Oxid. Med. Cell. Longev. 2017, 2017, 4129459.
- Hugar, S.; Shivapraksha, S.; Biradar, S.; Shivakumar, B. Evaluation of Moringa oleifera seeds for the cardio protective efficacy. World J. Pharm. Res. 2018, 7, 1461–1473.
- Li, Y.-J.; Ji, Q.-Q.; Wang, Z.; Shen, L.-H.; He, B. Moringa oleifera seeds mitigate myocardial injury and prevent ventricular failure induced by myocardial infarction. Am. J. Transl. Res. 2020, 12, 4511–4521.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
27 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




