Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mati Ullah | -- | 2403 | 2022-09-26 16:20:30 | | | |
| 2 | Rita Xu | Meta information modification | 2403 | 2022-09-27 04:07:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ullah, M.; Liu, P.; Xie, S.; Sun, S. Challenges in Lignin Valorization. Encyclopedia. Available online: https://encyclopedia.pub/entry/27606 (accessed on 01 March 2026).
Ullah M, Liu P, Xie S, Sun S. Challenges in Lignin Valorization. Encyclopedia. Available at: https://encyclopedia.pub/entry/27606. Accessed March 01, 2026.
Ullah, Mati, Pengyang Liu, Shangxian Xie, Su Sun. "Challenges in Lignin Valorization" Encyclopedia, https://encyclopedia.pub/entry/27606 (accessed March 01, 2026).
Ullah, M., Liu, P., Xie, S., & Sun, S. (2022, September 26). Challenges in Lignin Valorization. In Encyclopedia. https://encyclopedia.pub/entry/27606
Ullah, Mati, et al. "Challenges in Lignin Valorization." Encyclopedia. Web. 26 September, 2022.
Copy Citation
The aromatic hetero-polymer lignin is industrially processed in the paper/pulp and lignocellulose biorefinery, acting as a major energy source. It has been proven to be a natural resource for useful bioproducts; its depolymerization and conversion into high-value-added chemicals is the major challenge due to the complicated structure and heterogeneity.
lignin
biocatalysis
valorization
green synthesis
1. Introduction
The most profuse and renewable energy source on the planet is lignocellulosic biomass derived from plants; it comprises the two carbohydrates polymers, cellulose and hemicellulose, with the phenolic polymer lignin. The lignocellulosic biomass provides an alternative basis to fossil fuels for second-generation biofuels and other biobased chemicals production; however, the lignin biomass delivers excessive recalcitrance [1][2]. Lignin is a non-carbohydrate aromatic hetero-polymer with rich content and a complex structure in nature, present in the walls of the vascular tissue of plants [3][4]. This aromatic polymer is believed to be the second-most-abundant renewable resource, accounting for up to 25% of the total land-based biomass. Lignin does not have a fixed composition in all plant species, as its composition varies in different plant species; variation even exists in different tissues of the same plant. Cork lignin is mostly acetylated on the γ-OH of the side-chain (forty-eight percent acetylation) over the G units, whereas the lignin from the phloem and the xylem are barely acetylated, and this occurs mainly on the S units [5][6][7].
Lignin was considered a waste product in biorefinery during the production of biofuel from lignocellulosic biomass. After extraction, this polymer received attention and was found to have much potential as a natural reservoir of different chemicals and valuable products [8]. Thus, the release of lignin from conventional biomass refineries along with the pulp and paper industry has resulted in urgent requirements and also created the opportunity to increase the transformation of lignin into value-added products dramatically [9][10]. Many chemical and physical methods were explored to acquire the desired compounds from the lignin, but they all have certain limitations. Hence, for obtaining access to polysaccharides, the microbes have to break down or modify the lignin molecular structure [11][12]. The polysaccharide in the cell wall is hydrophilic, while the lignin is hydrophobic (Figure 1A); this combination of hydrophobic lignin and hydrophilic polysaccharides gives plants a significant advantage. The need for energy consumption for the non-specific breaking of these rich energy bonds is highly challenging; thus, the microbial degradation of lignin diverted the researchers’ attention and interest. For the better bioconversion of residual lignin-enriched refinery waste from upstream pretreatment, the most strategic process is to depolymerize macromolecular lignin into small molecular aromatic compounds for further conversion through microorganisms [13]. This conversion is similar to saccharification in cellulose; however, unlike the β-1,4-glucosidic linkage of cellulose, lignin contains diverse aromatic monomers and various types of chemical bonds or interunit linkages such as β-O-4, α-O-4/β-5, β–β, 4-O-5, and 5–5 [10].
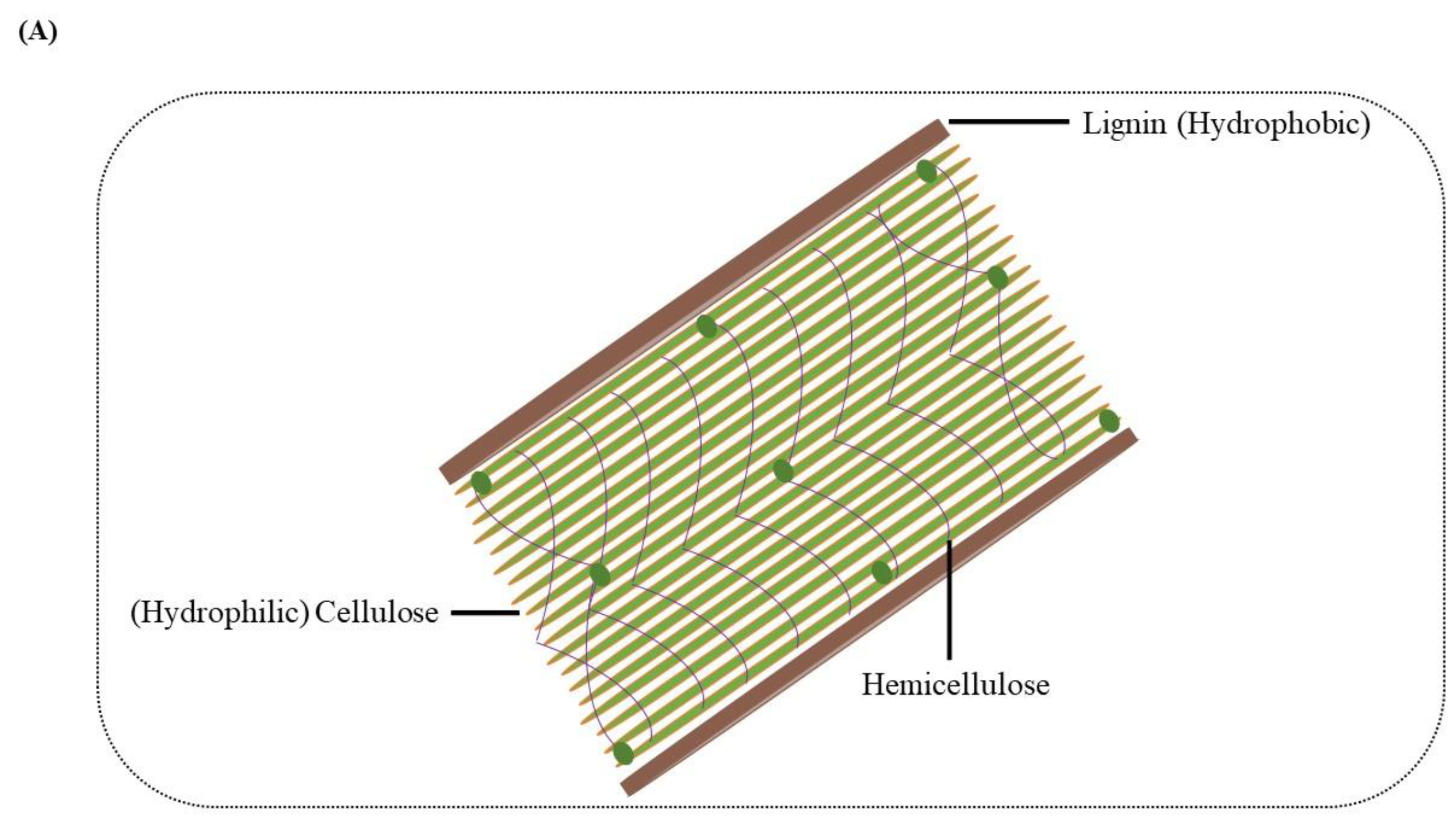
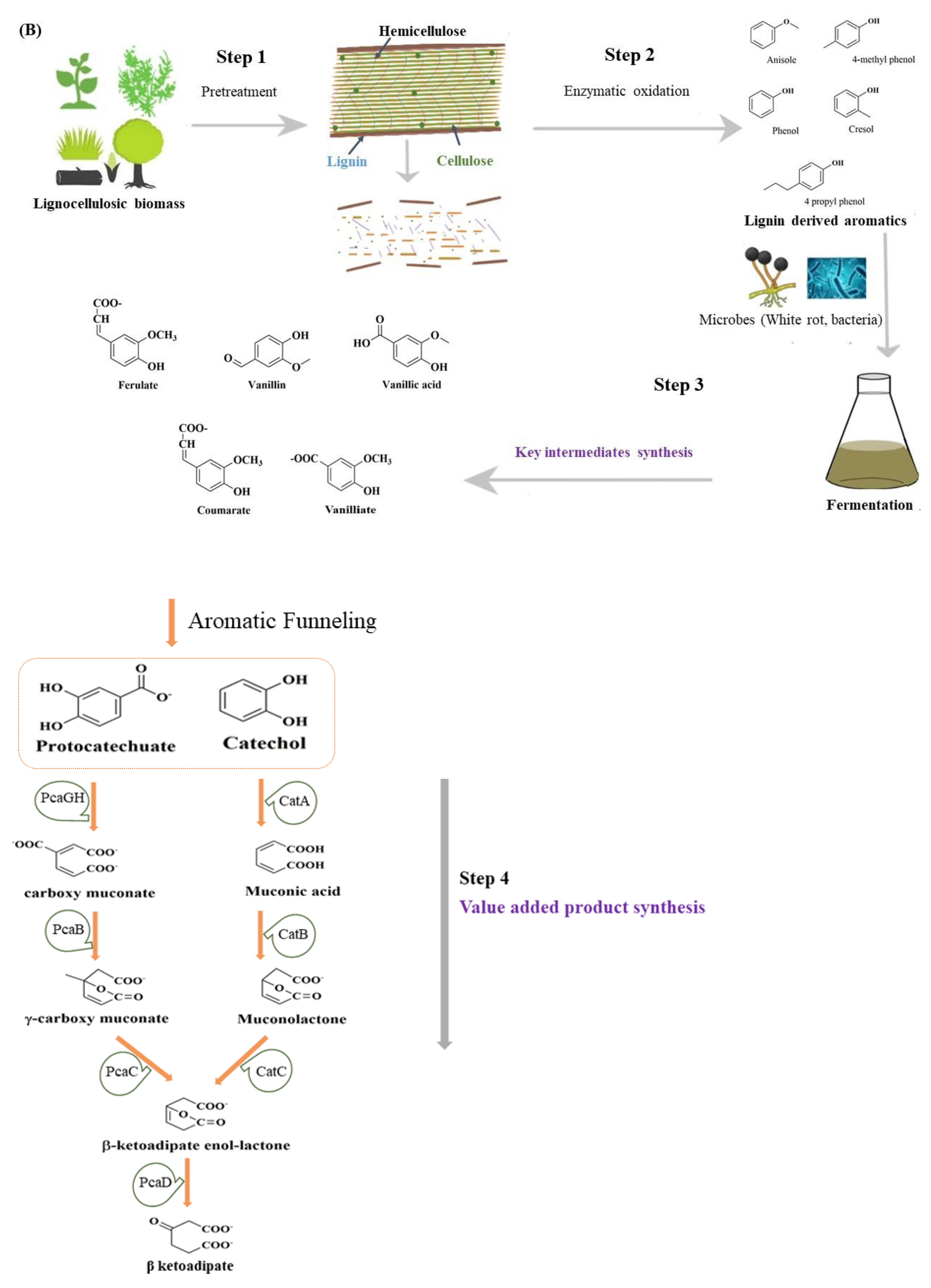
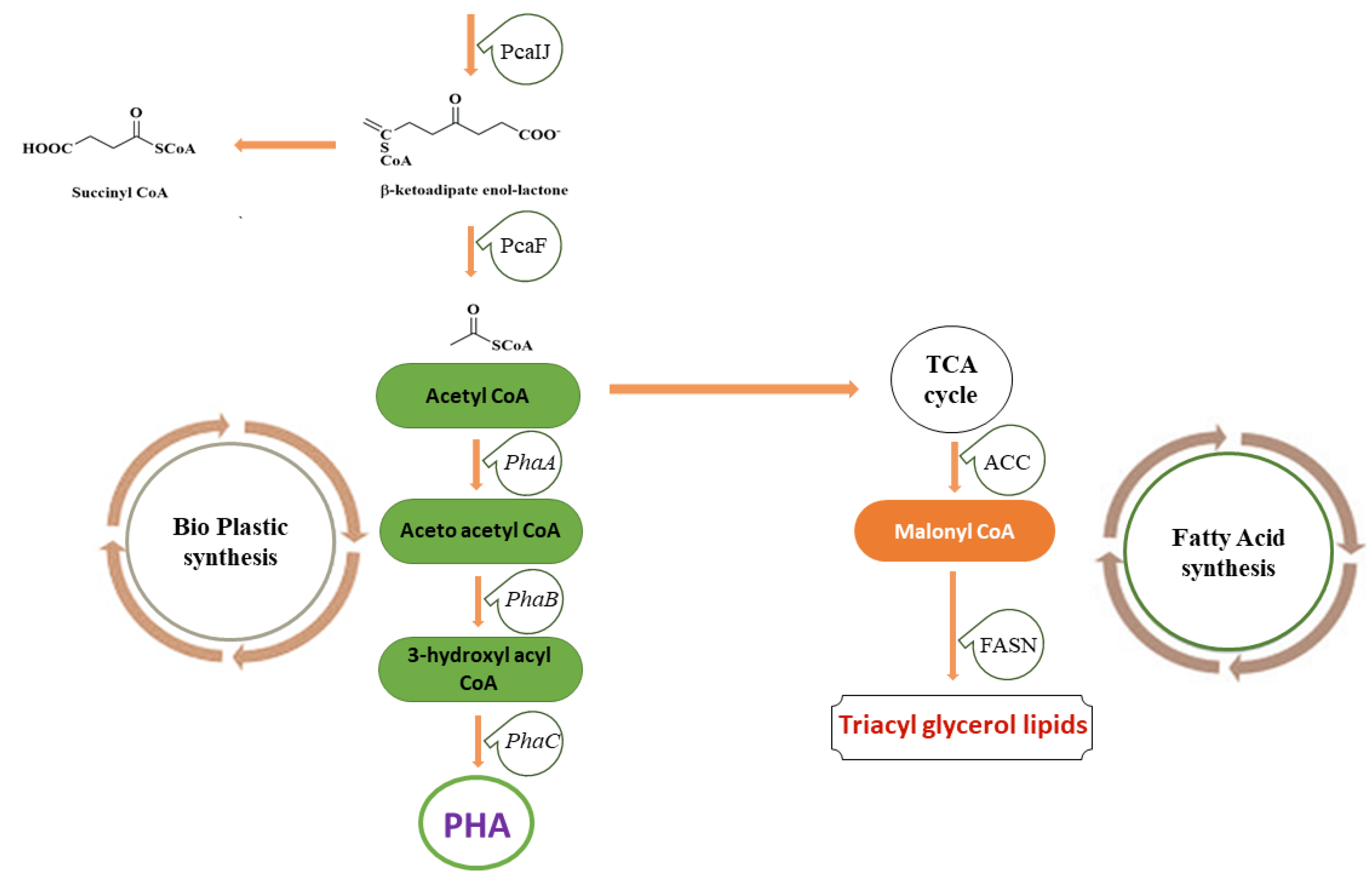
Figure 1. (A) Composition of the plant cell wall. Arrangement of hydrophobic lignin and hydrophilic cellulose along with other cell wall structure components. (B) Value-added intermediate products synthesis from lignin. In step 1, the pretreatment of the lignocellulosic biomass occurs, separating cellulose, hemicellulose and lignin. The enzyme depolymerization of the lignin takes place in step 2, generating certain lignin-derived aromatics. The aromatics are hydrolyzed in step 3 through the microbial action of lignin-degrading white rot or bacteria, leading to the formation of key intermediates, including protocatechuate and catechol. The protocatechuate and catechol on further conversion and entering the β-ketoadipate pathway lead to value-added products like triglycerides.
Scientists are still working to find economical and reliable methods to degrade lignin for acquiring the desired breakdown products [14][15]. Several enzymes for lignin depolymerization have been discovered, along with the essential auxiliary enzymes involved in valuable compound synthesis. The white and brown-rot fungi contributing to this degradation produce extracellular peroxidase and laccase enzymes, along with the involvement of some peripheral enzymes [15][16][17]. Certain lignin-degrading bacteria are also considered significant for contributing to the bioconversion and biocatalysis of lignin through their enzymes [18][19].
The bioconversion of lignin mainly involves lignin pretreatment, the depolymerization of lignin into small molecular aromatic compounds, their degradation into central metabolites or key intermediates, and, lastly, the value-added product synthesis by microorganisms as represented in Figure 1B. The lignin depolymerization facilitates the conversion of low-molecular-weight lignin into monomers and oligomers, the degradation of monomers/oligomers into archetypal substrates, e.g., protocatechuate (PCA), the formation of acetyl-CoA from PCA through the β-KAP pathway, and the synthesis of lipid/PHA or other important molecules [20][21][22].
2. Lignin Availability and Structure
Lignin is technically intertwined with cellulose and hemicellulose to form the main structure of plants, providing plants with strength and rigidity. The synthesis of lignin occurs via the free-radical-assisted enzymatic dehydrogenative polymerization of phenylpropanoid precursors, namely p-coumaryl alcohol (H), sinapyl alcohol (S) and coniferyl alcohol (G) [7][23] (Figure 2A). Thus, optically inactive amorphous heteropolymer (lignin) is naturally featured, with a branched and cross-linked network of phenylpropane units (C9). The ratio of lignin subunits differs among different plant species at different growth stages. The lignin monomers are conjugated by different bonds, such as β-O-4, β-5, β-1, 5-5, β-β, α-O-4 and 4-O-5. The β-aryl ethers (β-O-4) are the dominant inter-unit linkages in the native lignin structure, as shown in Figure 2B. The subunits are cross-linked with the polysaccharides present in the xylem and phloem tissue, contributing to recalcitrance by preventing microbial attract from infiltrating into cell walls [24][25]. Achievements have been made to effectively enhance lignocellulosic biomass conversion by increasing the syringl residues ratio. The lignocellulose conversion can also be enhanced by introducing more ester linkages via alternative monolignols expression [24][26]. The composition of depolymerized lignin varies significantly based on the source of the feedstock and how the feedstock is processed. Besides the biomass variations, the lignin isolation methods also have an influential role in defining the structure and nature of lignin. Like the two primary processes involved in the pulp and paper industry, separating lignin from carbohydrates includes kraft and sulfite pulping; thus, the lignin is termed kraft lignin and lignosulfonate [1][27][28]. Similarly, soda lignin involves treatment with soda or alkali, while the lignin formed due to a mixture of organic ethanol and water as solvents from lignocellulose is referred to as organosolv process lignin [29][30]. Additionally, the various other advanced isolation methods can lead to the formation of certain types of lignin, including milled wood lignin, cellulolytic enzyme lignin and enzymatic mild acidolysis lignin [31][32][33]. The types of lignin based either on the different feedstocks or pre-treatment methods along with their monomers’ molecular weights are given in Table 1, while the other advanced methods for lignin isolation, along with the average molecular weights of the extracted lignin, their advantages, and limitations are presented in Table 2.
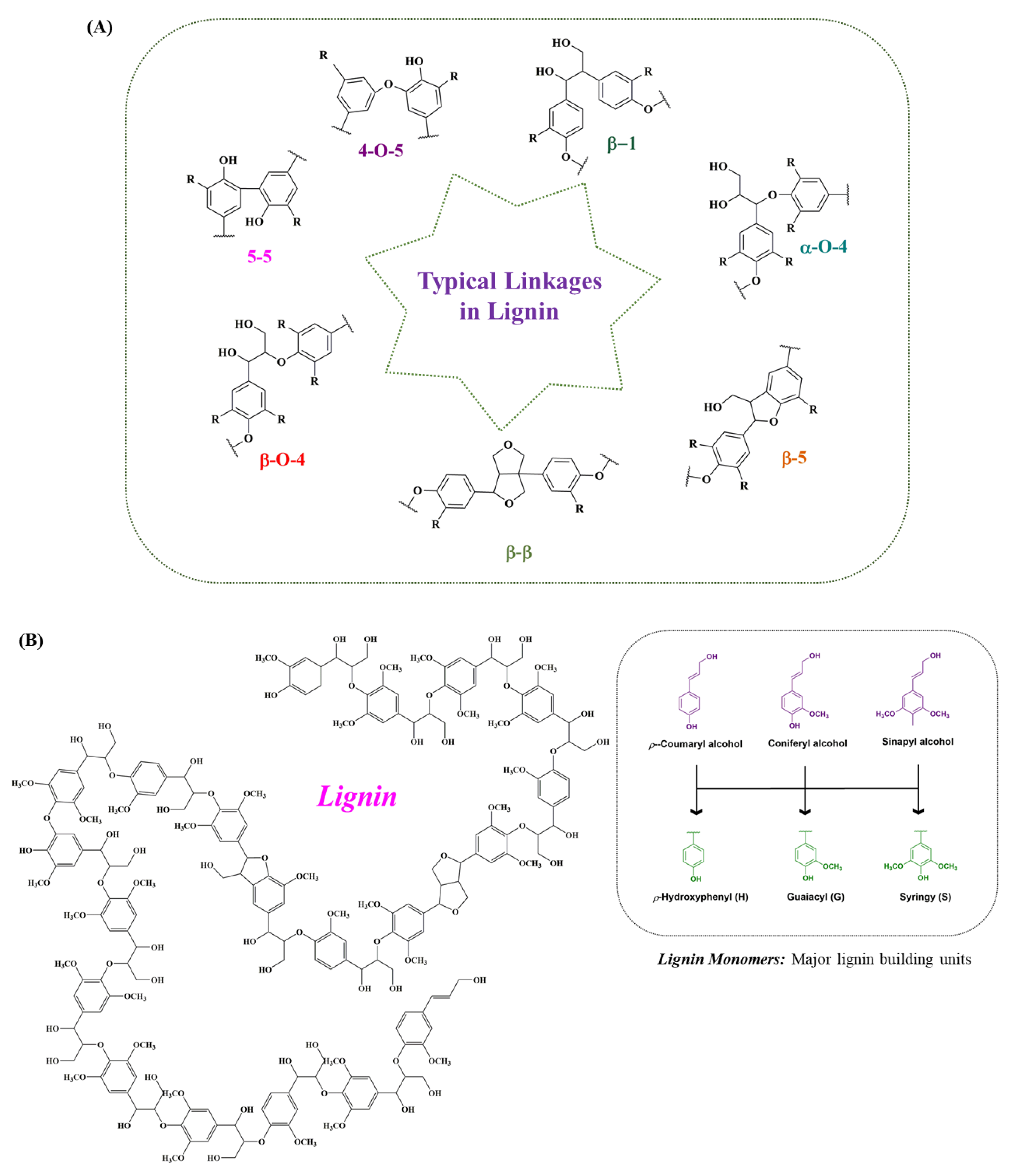
Figure 2. Typical linkages present in lignin. (A) The hetero-polymer lignin is naturally featured with a branched and cross-linked network of phenylpropane units. These lignin-forming units are conjugated by different linkages, such as β-O-4, α-O-4, β-1, β-5, 5-5, β-β, and 4-O-5. From the lignin structure, it is observed that the dominant inter-unit linkages present in the native lignin structure are the β-aryl ether (β-O-4). (B) Structure of lignin. The combination of different lignin monomers, mainly sinapyl alcohol, coniferyl alcohol and p-coumaryl alcohol, forms a stable lignin structure through energy-rich bonds.
Table 1. Different types of lignin, their monomer’s molecular weight, lignin content and chemicals.
| Types of Lignin | Source | Monomer Molecular Weight (g/mol) | Lignin Content (mmol g−1) | Chemicals/Catalysts | Reference | |
|---|---|---|---|---|---|---|
| Sulfur process | Kraft lignin | Wood chips, softwoods, hardwoods | 2000–3000 | 1.25 | NaOH, Na2S | [34] |
| Lignosulfonates | Softwoods, hardwoods, annual plants | 20,000–50,000 | 1.25–2.5 | Ca(HSO3)2 or Mg(HSO3)2 | [34] | |
| Sulfur free process | Organosolv lignin | Hardwood, softwood and wheat straw | 2000–5000 | 0 | Methanol, ethanol, various bronsted acid catalysts (H2SO4) | [28][35] |
| Alkali/soda lignin | Hardwood, bagasse, wheat straw and flax | 5000–6000 | 0 | NaOH, NH4OH, Ca(OH)2 | [36][37] |
Table 2. Comparison of various lignin isolation methods.
| Method | Source | Extraction Process |
Approximate Average Mw (g/mol) | Approximate Average Mn (g/mol) | Advantages | Challenges | References |
|---|---|---|---|---|---|---|---|
| Milled wood lignin (MWL) | Milled sample particles and wood chips from LCB, wheat straw, redwood, white fir, | Extraction with a neutral organic solvent | 9880 | 3367 | Requires mild conditions and room temperature | Relatively low yield and time-consuming | [38][39][40] |
| Cellulolytic enzyme lignin (CEL) | MWL residue, redwood, wheat straw, white fir | Cellulolytic enzyme hydrolysis before aqueous dioxane extraction | 19,830 | 5967 | Requires mild conditions, no impurity and less inhibitor formation | Low yield of soluble and fragmented lignin | [39][41][42] |
| Enzymatic mild acidolysis lignin (EMAL) | Milled wood, hardwood, softwood, wheat straw, redwood, white fir | Cleaving lignin–carbohydrate bonds with the combined effect of enzymatic and mild acid hydrolysis | 45,530 | 7717 | Comparatively higher yield than MWL and CEL, with low severity | High concentration of HCl may compromise the structure of the isolated lignin | [39][40][43] |
3. Challenges and Strategies for Lignin Valorization
Even though some progress has been made in lignin valorization, effectively harnessing the intrinsic capabilities of biology to valorize lignin will require substantial research and developmental efforts. Some of the challenges that are of primary concerns and that need to be considered during the lignin valorization process and the possible strategic solutions to enhance the microbial biotransformation of lignin are discussed below.
3.1. Selection of Lignin Source and Depolymerization Capacity
The first and most challenging step is to depolymerize lignin to monomeric or dimeric/oligomeric compounds for further utilization by microorganisms. Recent literature has reported that bacteria can depolymerize and convert renewable lignin from different sources. However, the depolymerization and utilization efficiency is low due to the lignin macromolecule’s severe recalcitrance. Even though some bacteria can use lignin-derived aromatic compounds, the lignin-depolymerization capacity of most of these bacteria is relatively weak compared to white-rot fungus [44][45][46]. For achieving high product titer and efficient lignin utilization, it is critical to conduct efficient lignin-depolymerization that could degrade heteropolymers into aromatic compounds [47][48]. Lignin sources affect lignin components in the process of depolymerization, so adjusting the source of lignin and lignin manipulation is the first strategy for promoting the efficiency of lignin depolymerization. Previous research indicated that the efficiency of lignin depolymerization could be significantly affected by the origins and types of lignin used. Improvements could be made to approve the associated relationship between lignin characteristic features and their conversion yield, as well as the final production efficiency [49][50].
3.2. Selection of Ideal Microorganisms
Depolymerization approaches will result in many heterogeneous aromatic compounds. Many of these products are likely to be detrimental to the growth of bacteria, such as phenol, vanillin, 4-hydroxybenzoic acid, and coumaric acid [51][52][53]. One of the challenges is reducing or avoiding the influence of lignin-derived phenolic monomers and tannin derivatives on microbial growth. To address this challenge, it is important to screen and select appropriate microorganisms for utilizing and tolerating a broad range of lignin-derived aromatic compounds. The ideal microorganisms need not only be capable of utilizing these heterogeneous compounds but also should adapt to the potentially unfavorable compounds derived from lignin depolymerization and must be domesticated for use in industrial bioreactors [54].
Oceanimonas doudoroffii was recognized as a functional strain that was isolated from a contaminated marine environment and revealed the capacity of consuming lignin and its byproducts as the solitary carbon source for the synthesis of PHA [55]. Similarly, another vigorous bacterium, Cupriavidus basilensis B-8, screened from the steeping fluid of the erosive bamboo slips has been reported for its high capacity for lignin-derived aromatic compound catabolism [54].
3.3. Enzyme–Microbe and Microbe–Microbe Synergy
Commonly, depolymerizing lignin into solely monomeric or dimeric aromatic compounds is challenging. Despite the unveiling of fungal and bacterial enzymatic machinery for lignin depolymerization, the genetic manipulation to engineer lignin bioprocess streams is still not much developed. Hence, the efficient depolymerization and conversion of lignin always requires the synergistic effects of different enzymes [56]. A recent report demonstrated that combining the commercial laccase from Trametes versicolor with an oleaginous bacterium, Rhodococcus opacus PD630, can selectively degrade different chemical linkages to synergize lignin depolymerization. Thus, the development of a simultaneous depolymerization and fermentation process results in the fast growth of cells and higher lipid yields on kraft lignin [57]. Some other work has reported that MnP can accelerate lignin depolymerization when applied in the anaerobic digestion of municipal solid waste [58]. Besides laccase and MnP, some fungi could produce a hydroxyl radical via Fenton’s reaction; thus, the lignin structure will be more accessible for the lignin-degrading enzymes through this chemical oxidation generated by Fenton’s reaction [59]. Meanwhile, some other lignin-degradation enzymes, such as lignin peroxidase, versatile peroxidase and the novel dye-decolorizing peroxidase, could be explored for further potential development to synergize lignin degradation. Another significant provision of lignin-degrading enzymes is engineering microorganisms to secrete the main heterologous enzymes in the target microbe to enable efficient lignin depolymerization.
A biological team reported up to 20% lignin decomposition from some basidiomycetes, including Coriolus versicolor, Trametes gallica, lignin-degrading Microbacterium sp., and Streptomyces sp. for the generation of valuable chemicals due to potential synergy [57][60]. Similarly, lignin-degrading enzymes like laccase and MnP have been successfully expressed in Escherichia coli and yeast. However, the value of ligninolytic enzymes and its optimal mixture to degrade lignin synergistically for the synthesis of value-added products is currently unclear [15][61]. Therefore, advanced study is required to gain knowledge of potential interaction, which will help in the development of a lignin-degrading enzymes cocktail for lignin conversion to useful target products. Moreover, the application of biological omics-based techniques and advanced chemical analytics will not only provide an efficient tool to search for new enzymes but also new clues about how natural systems utilize these complex substrates.
3.4. Optimization of Catabolic Pathways and Yield of Desire Product
The second essential and challenging step is to catabolize lignin-derived aromatic compounds into other value-added intermediates by microorganisms. Numerous researchers have proven the possibility of the biological valorization of lignin; however, a common problem is still the low yield of the final chemical products [62][63]. Despite lignin-degraders being continuously explored, there is still a lack of particular traits responsible for producing target products from lignin at a predicted level. Thus, the mechanism of their catabolic pathways suggests a possible approach to tackle the low yield of target products [64]. To obtain a higher yield of natural intermediates in the upper pathways of lignin catabolism, one effort could be made to block the advance metabolism of a desire product [65]. Several studies have unveiled opportunities for optimizing the yield of the final product via microbial pathway engineering; however, research on aromatic catabolism is solely concentrated on previously reported strains, such as P. putida KT2240, R. jostii RHA1 and Cupriavidus Necator H16. Therefore, it is necessary to explore those microorganisms that are efficient t changing lignin into value-added products and that eventually may reveal new pathways to deliver novel value-added products. Finally, a fundamental understanding of the lignin conversion process could optimize the conversion process of lignin derived from biorefinery into value-added products.
References
- Al-Battashi, H.S.; Annamalai, N.; Sivakumar, N.; Al-Bahry, S.; Tripathi, B.N.; Nguyen, Q.D.; Gupta, V.K. Lignocellulosic Biomass (LCB): A Potential Alternative Biorefinery Feedstock for Polyhydroxyalkanoates Production. Rev. Environ. Sci. Bio/Technol. 2019, 18, 183–205.
- Yousuf, A.; Pirozzi, D.; Sannino, F. Fundamentals of Lignocellulosic Biomass. In Lignocellulosic Biomass to Liquid Biofuels; Yousuf, A., Pirozzi, D., Sannino, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 1; pp. 1–15. ISBN 978-0-12-815936-1.
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin Structure and Its Engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249.
- Huang, J.; Fu, S.; Gan, L. Structure and Characteristics of Lignin. In Lignin Chemistry and Applications; Huang, J., Fu, S., Gan, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 2; pp. 25–50. ISBN 978-0-12-813941-7.
- Lourenço, A.; Rencoret, J.; Chemetova, C.; Gominho, J.; Gutiérrez, A.; del Río, J.C.; Pereira, H. Lignin Composition and Structure Differs between Xylem, Phloem and Phellem in Quercus Suber L. Front. Plant Sci. 2016, 7, 1612.
- Neutelings, G. Lignin Variability in Plant Cell Walls: Contribution of New Models. Plant Sci. 2011, 181, 379–386.
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Its Integration into Metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239.
- Rajesh Banu, J.; Preethi; Kavitha, S.; Tyagi, V.K.; Gunasekaran, M.; Karthikeyan, O.P.; Kumar, G. Lignocellulosic Biomass Based Biorefinery: A Successful Platform towards Circular Bioeconomy. Fuel 2021, 302, 121086.
- Velvizhi, G.; Balakumar, K.; Shetti, N.P.; Ahmad, E.; Kishore Pant, K.; Aminabhavi, T.M. Integrated Biorefinery Processes for Conversion of Lignocellulosic Biomass to Value Added Materials: Paving a Path towards Circular Economy. Bioresour. Technol. 2022, 343, 126151.
- Ma, R.; Xu, Y.; Zhang, X. Catalytic Oxidation of Biorefinery Lignin to Value-Added Chemicals to Support Sustainable Biofuel Production. ChemSusChem 2015, 8, 24–51.
- Silva, J.P.; Ticona, A.R.P.; Hamann, P.R.V.; Quirino, B.F.; Noronha, E.F. Deconstruction of Lignin: From Enzymes to Microorganisms. Molecules 2021, 26, 2299.
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874.
- Brink, D.P.; Ravi, K.; Lidén, G.; Gorwa-Grauslund, M.F. Mapping the Diversity of Microbial Lignin Catabolism: Experiences from the ELignin Database. Appl. Microbiol. Biotechnol. 2019, 103, 3979–4002.
- Poveda-Giraldo, J.A.; Solarte-Toro, J.C.; Cardona Alzate, C.A. The Potential Use of Lignin as a Platform Product in Biorefineries: A Review. Renew. Sustain. Energy Rev. 2021, 138, 110688.
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczynski, A. Lignin Degradation: Microorganisms, Enzymes Involved, Genomes Analysis and Evolution. FEMS Microbiol. Rev. 2017, 41, 941–962.
- Cagide, C.; Castro-Sowinski, S. Technological and Biochemical Features of Lignin-Degrading Enzymes: A Brief Review. Environ. Sustain. 2020, 3, 371–389.
- Singhania, R.R.; Patel, A.K.; Raj, T.; Chen, C.-W.; Ponnusamy, V.K.; Tahir, N.; Kim, S.-H.; Dong, C.-D. Lignin Valorisation via Enzymes: A Sustainable Approach. Fuel 2022, 311, 122608.
- Bugg, T.D.H.; Williamson, J.J.; Rashid, G.M.M. Bacterial Enzymes for Lignin Depolymerisation: New Biocatalysts for Generation of Renewable Chemicals from Biomass. Curr. Opin. Chem. Biol. 2020, 55, 26–33.
- De Gonzalo, G.; Colpa, D.I.; Habib, M.H.M.; Fraaije, M.W. Bacterial Enzymes Involved in Lignin Degradation. J. Biotechnol. 2016, 236, 110–119.
- Radhika, N.L.; Sachdeva, S.; Kumar, M. Lignin Depolymerization and Biotransformation to Industrially Important Chemicals/Biofuels. Fuel 2022, 312, 122935.
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937.
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl Carbonate: A Versatile Reagent for a Sustainable Valorization of Renewables. Green Chem. 2018, 20, 288–322.
- De Vries, L.; Guevara-Rozo, S.; Cho, M.; Liu, L.-Y.; Renneckar, S.; Mansfield, S.D. Tailoring Renewable Materials via Plant Biotechnology. Biotechnol. Biofuels 2021, 14, 167.
- Mottiar, Y.; Vanholme, R.; Boerjan, W.; Ralph, J.; Mansfield, S.D. Designer Lignins: Harnessing the Plasticity of Lignification. Curr. Opin. Biotechnol. 2016, 37, 190–200.
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current Advancement on the Isolation, Characterization and Application of Lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024.
- Mahon, E.L.; Mansfield, S.D. Tailor-Made Trees: Engineering Lignin for Ease of Processing and Tomorrow’s Bioeconomy. Curr. Opin. Biotechnol. 2019, 56, 147–155.
- Matsushita, Y. Conversion of Technical Lignins to Functional Materials with Retained Polymeric Properties. J. Wood Sci. 2015, 61, 230–250.
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The Critical Role of Lignin in Lignocellulosic Biomass Conversion and Recent Pretreatment Strategies: A Comprehensive Review. Bioresour. Technol. 2020, 301, 122784.
- Graglia, M.; Kanna, N.; Esposito, D. Lignin Refinery: Towards the Preparation of Renewable Aromatic Building Blocks. ChemBioEng Rev. 2015, 2, 377–392.
- Fernández-Rodríguez, J.; Erdocia, X.; Hernández-Ramos, F.; Gordobil, O.; González Alriols, M.; Labidi, J. Direct Lignin Depolymerization Process from Sulfur-Free Black Liquors. Fuel Process. Technol. 2020, 197, 106201.
- Nitsos, C.K.; Lazaridis, P.A.; Mach-Aigner, A.; Matis, K.A.; Triantafyllidis, K.S. Enhancing Lignocellulosic Biomass Hydrolysis by Hydrothermal Pretreatment, Extraction of Surface Lignin, Wet Milling and Production of Cellulolytic Enzymes. ChemSusChem 2019, 12, 1179–1195.
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From Lignin to Valuable Products–Strategies, Challenges, and Prospects. Bioresour. Technol. 2019, 271, 449–461.
- Xu, J.; Li, C.; Dai, L.; Xu, C.; Zhong, Y.; Yu, F.; Si, C. Biomass Fractionation and Lignin Fractionation towards Lignin Valorization. ChemSusChem 2020, 13, 4284–4295.
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem. 2017, 10, 1861–1877.
- Başakçılardan Kabakcı, S.; Tanış, M.H. Pretreatment of Lignocellulosic Biomass at Atmospheric Conditions by Using Different Organosolv Liquors: A Comparison of Lignins. Biomass Convers. Biorefinery 2021, 11, 2869–2880.
- Xu, L.; Zhang, S.-J.; Zhong, C.; Li, B.-Z.; Yuan, Y.-J. Alkali-Based Pretreatment-Facilitated Lignin Valorization: A Review. Ind. Eng. Chem. Res. 2020, 59, 16923–16938.
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48.
- Crestini, C.; Melone, F.; Sette, M.; Saladino, R. Milled Wood Lignin: A Linear Oligomer. Biomacromolecules 2011, 12, 3928–3935.
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and Analysis of the Molecular Weight of Lignin for Biorefining Studies. Biofuels Bioprod. Biorefining 2014, 8, 836–856.
- Guerra, A.; Filpponen, I.; Lucia, L.A.; Saquing, C.; Baumberger, S.; Argyropoulos, D.S. Toward a Better Understanding of the Lignin Isolation Process from Wood. J. Agric. Food Chem. 2006, 54, 5939–5947.
- Zhang, A.; Lu, F.; Sun, R.-C.; Ralph, J. Isolation of Cellulolytic Enzyme Lignin from Wood Preswollen/Dissolved in Dimethyl Sulfoxide/N-Methylimidazole. J. Agric. Food Chem. 2010, 58, 3446–3450.
- Chen, Z.; Bai, X.; Zhang, H.; Wan, C. Insights into Structural Changes of Lignin toward Tailored Properties during Deep Eutectic Solvent Pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 9783–9793.
- Wang, S.; Li, H.; Xiao, L.-P.; Song, G. Unraveling the Structural Transformation of Wood Lignin during Deep Eutectic Solvent Treatment. Front. Energy Res. 2020, 8, 48.
- Rahimi, A.; Ulbrich, A.; Coon, J.J.; Stahl, S.S. Formic-Acid-Induced Depolymerization of Oxidized Lignin to Aromatics. Nature 2014, 515, 249–252.
- Xu, R.; Zhang, K.; Liu, P.; Han, H.; Zhao, S.; Kakade, A.; Khan, A.; Du, D.; Li, X. Lignin Depolymerization and Utilization by Bacteria. Bioresour. Technol. 2018, 269, 557–566.
- Barnhart-Dailey, M.C.; Ye, D.; Hayes, D.C.; Maes, D.; Simoes, C.T.; Appelhans, L.; Carroll-Portillo, A.; Kent, M.S.; Timlin, J.A. Internalization and Accumulation of Model Lignin Breakdown Products in Bacteria and Fungi. Biotechnol. Biofuels 2019, 12, 175.
- Tsegaye, B.; Balomajumder, C.; Roy, P. Microbial Delignification and Hydrolysis of Lignocellulosic Biomass to Enhance Biofuel Production: An Overview and Future Prospect. Bull. Natl. Res. Cent. 2019, 43, 51.
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678.
- Evstigneyev, E.I.; Shevchenko, S.M. Structure, Chemical Reactivity and Solubility of Lignin: A Fresh Look. Wood Sci. Technol. 2019, 53, 7–47.
- Li, M.; Pu, Y.; Ragauskas, A.J. Current Understanding of the Correlation of Lignin Structure with Biomass Recalcitrance. Front. Chem. 2016, 4, 45.
- Pineda, A.; Lee, A.F. Heterogeneously Catalyzed Lignin Depolymerization. Appl. Petrochem. Res. 2016, 6, 243–256.
- Kurosawa, K.; Laser, J.; Sinskey, A.J. Tolerance and Adaptive Evolution of Triacylglycerol-Producing Rhodococcus Opacus to Lignocellulose-Derived Inhibitors. Biotechnol. Biofuels 2015, 8, 76.
- Wang, W.; Yang, S.; Hunsinger, G.B.; Pienkos, P.T.; Johnson, D.K. Connecting Lignin-Degradation Pathway with Pre-Treatment Inhibitor Sensitivity of Cupriavidus Necator. Front. Microbiol. 2014, 5, 247.
- Moraes, E.C.; Alvarez, T.M.; Persinoti, G.F.; Tomazetto, G.; Brenelli, L.B.; Paixão, D.A.A.; Ematsu, G.C.; Aricetti, J.A.; Caldana, C.; Dixon, N. Lignolytic-Consortium Omics Analyses Reveal Novel Genomes and Pathways Involved in Lignin Modification and Valorization. Biotechnol. Biofuels 2018, 11, 75.
- Numata, K.; Morisaki, K. Screening of Marine Bacteria to Synthesize Polyhydroxyalkanoate from Lignin: Contribution of Lignin Derivatives to Biosynthesis by Oceanimonas Doudoroffii. ACS Sustain. Chem. Eng. 2015, 3, 569–573.
- Hou, L.; Ji, D.; Dong, W.; Yuan, L.; Zhang, F.; Li, Y.; Zang, L. The Synergistic Action of Electro-Fenton and White-Rot Fungi in the Degradation of Lignin. Front. Bioeng. Biotechnol. 2020, 8, 99.
- Zhao, C.; Xie, S.; Pu, Y.; Zhang, R.; Huang, F.; Ragauskas, A.J.; Yuan, J.S. Synergistic Enzymatic and Microbial Lignin Conversion. Green Chem. 2016, 18, 1306–1312.
- Jayasinghe, P.A.; Hettiaratchi, J.P.A.; Mehrotra, A.K.; Kumar, S. Effect of Enzyme Additions on Methane Production and Lignin Degradation of Landfilled Sample of Municipal Solid Waste. Bioresour. Technol. 2011, 102, 4633–4637.
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 2018, 6, 141.
- Brzonova, I.; Kozliak, E.; Kubátová, A.; Chebeir, M.; Qin, W.; Christopher, L.; Ji, Y. Kenaf Biomass Biodecomposition by Basidiomycetes and Actinobacteria in Submerged Fermentation for Production of Carbohydrates and Phenolic Compounds. Bioresour. Technol. 2014, 173, 352–360.
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Insights into Lignin Degradation and Its Potential Industrial Applications. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 82, pp. 1–28.
- Xu, Z.; Lei, P.; Zhai, R.; Wen, Z.; Jin, M. Recent Advances in Lignin Valorization with Bacterial Cultures: Microorganisms, Metabolic Pathways, and Bio-Products. Biotechnol. Biofuels 2019, 12, 32.
- Xie, S.; Ragauskas, A.J.; Yuan, J.S. Lignin Conversion: Opportunities and Challenges for the Integrated Biorefinery. Ind. Biotechnol. 2016, 12, 161–167.
- Lee, S.; Kang, M.; Bae, J.-H.; Sohn, J.-H.; Sung, B.H. Bacterial Valorization of Lignin: Strains, Enzymes, Conversion Pathways, Biosensors, and Perspectives. Front. Bioeng. Biotechnol. 2019, 7, 209.
- Li, X.; Zheng, Y. Biotransformation of Lignin: Mechanisms, Applications and Future Work. Biotechnol. Prog. 2020, 36, e2922.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
27 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





