Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liang Yu | -- | 1802 | 2022-09-26 14:20:00 | | | |
| 2 | Peter Tang | + 25 word(s) | 1827 | 2022-09-27 03:06:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yu, L.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Amine Type in CO2 Separation. Encyclopedia. Available online: https://encyclopedia.pub/entry/27599 (accessed on 08 February 2026).
Yu L, Kanezashi M, Nagasawa H, Tsuru T. Amine Type in CO2 Separation. Encyclopedia. Available at: https://encyclopedia.pub/entry/27599. Accessed February 08, 2026.
Yu, Liang, Masakoto Kanezashi, Hiroki Nagasawa, Toshinori Tsuru. "Amine Type in CO2 Separation" Encyclopedia, https://encyclopedia.pub/entry/27599 (accessed February 08, 2026).
Yu, L., Kanezashi, M., Nagasawa, H., & Tsuru, T. (2022, September 26). Amine Type in CO2 Separation. In Encyclopedia. https://encyclopedia.pub/entry/27599
Yu, Liang, et al. "Amine Type in CO2 Separation." Encyclopedia. Web. 26 September, 2022.
Copy Citation
Efficient CO2 capture technologies such as absorption/adsorption of CO2 sorbents, cryogenic separation, and membrane separation, have been developed experimentally and industrially. Among them, membrane technology is attracting growing attention owing to its inherent advantages that include energy-efficiency, ease of operation and scale-up, and a small footprint.
carbon dioxide separation
organosilica membrane
amine type
sterical hindrance
activation energy for permeation
1. Introduction
The ever-increasing rate of greenhouse gas emissions in the atmosphere, particularly CO2 generated from the combustion of fossil fuels, has led to serious global climate concerns. Therefore, efficient CO2 capture technologies such as absorption/adsorption of CO2 sorbents, cryogenic separation, and membrane separation, have been developed experimentally and industrially over the past few decades. Among them, membrane technology is attracting growing attention owing to its inherent advantages that include energy-efficiency, ease of operation and scale-up, and a small footprint [1][2][3][4][5]. As one of the most important members of the membrane family, organosilica membranes have been applied to various membrane processes with superior separation performance and a high tolerance to harsh conditions. Typical organosilica membranes developed for CO2/N2 separation are either microporous with rigid pores or nonporous with “free-volume” pores. Separations are therefore achieved mainly by the effect of molecular sieving along with partial contributions from selective surface diffusion through rigid pores and/or solution-diffusion within a nonporous structure. The selectivity is always less arresting due to the comparable kinetic diameters of CO2 (3.3 Å) and N2 (3.64 Å) and due as well to the difficulty in precisely controlling the pore (rigid or free-volume type) sizes. Given this, various amine-functionalized silica-based membranes have been developed to accomplish CO2 separation from gas mixtures with potentially enhanced efficiency [6][7][8][9][10][11][12][13]. These membranes are expected to show great potential in simultaneously promoting CO2 permeance and permselectivity due to the reversible reactions between CO2 and amine groups. Thus far, however, only a limited number of membranes have offered appealing CO2 separation performance with both high CO2 permeance and CO2/gas permselectivity irrespective of many reports of amine-functionalized silica-based membranes. Instead, in some cases even reverse CO2/N2 selectivity under a dry state has been observed [13]. Whereas, in some cases, other types of membranes with only CO2-philic groups rather than chemical reactions, such as poly(ethylene oxide)-based membranes, have resulted in attractive CO2 separation performance [2]. Hence, further fundamental studies in terms of the effect of amine-CO2 chemical interactions on CO2 transport behaviors through amine-functionalized silica-based membranes are needed to direct the fabrication of high-performance CO2 separation membranes.
In general, how fast and selectively the species to be separated can enter into, transport through, and exit from the membrane govern the membrane separation properties in terms of both flux and selectivity. Therefore, the reaction activities of amine groups (or membrane affinity) should be regarded in a broad sense, comprising not only the favorable and rapid reaction/interaction with CO2 molecules but also the promoted/inhibited motion of CO2 within amine-containing membranes. Consequently, to push the CO2 separation performance (or transport efficiency) of these membranes to the limit, the first idea to keep in mind in the design process should be to choose an amine-containing material with a convenient affinity for CO2 molecules. In fact, the effects that amine type (primary, secondary, and tertiary), basicity (or pKa), and steric hindrance can exert on CO2 sorption performance in terms of absorption/adsorption capacity and rate, as well as energy cost during the CO2 regeneration/release process, have been extensively studied in the field of amine-based CO2 sorbents (solid adsorbents or aqueous alkylamine solutions) [14][15][16][17]. It is common knowledge that tertiary and/or sterically hindered amines in some cases could exceed the trade-off between the heat of reaction and the CO2 absorption rate (see Figure 1), which thus enables energy-saving CO2 sorption and release processes. Theoretically, this phenomenon could offer innovations for the design and development of amine-containing CO2 separation membranes that integrate CO2 adsorption, diffusion, and desorption processes into a thin membrane layer. Indeed, Prof. Ho’s group developed and studied a series of CO2-selective polymeric membranes containing sterically hindered polyamines [18][19][20]. They found that the steric hindrance effect of polyamines could significantly promote CO2 transport performance in both CO2 permeability and CO2/gas (gas = H2, N2) selectivity, and this trend was more distinct in the case of moderately hindered polyamines (see Figure 2) [18]. Unfortunately, only a very limited number of systematic studies providing simultaneous CO2 adsorption and diffusion/desorption kinetic properties have been devoted to amine-functionalized, silica-based membranes. Given this, in the earlier studies, the researchers developed a series of amine-containing organosilica membranes including unhindered (PA-Si, SA-Si) and sterically hindered (TA-Si, BTPP) amines, as well as a quaternary ammonium salt (QA-Si), and investigated the effect that amine type exerted on the CO2 adsorption/desorption properties of solid powders and the related membrane performance (see Figure 3) [21][22][23][24]. It seems really important to more systematically compare the CO2 separation performance of amine-functionalized silica-based membranes, despite many review papers for CO2 separation membranes in the literature, which mostly review hydrocarbon-based membranes [4][25].
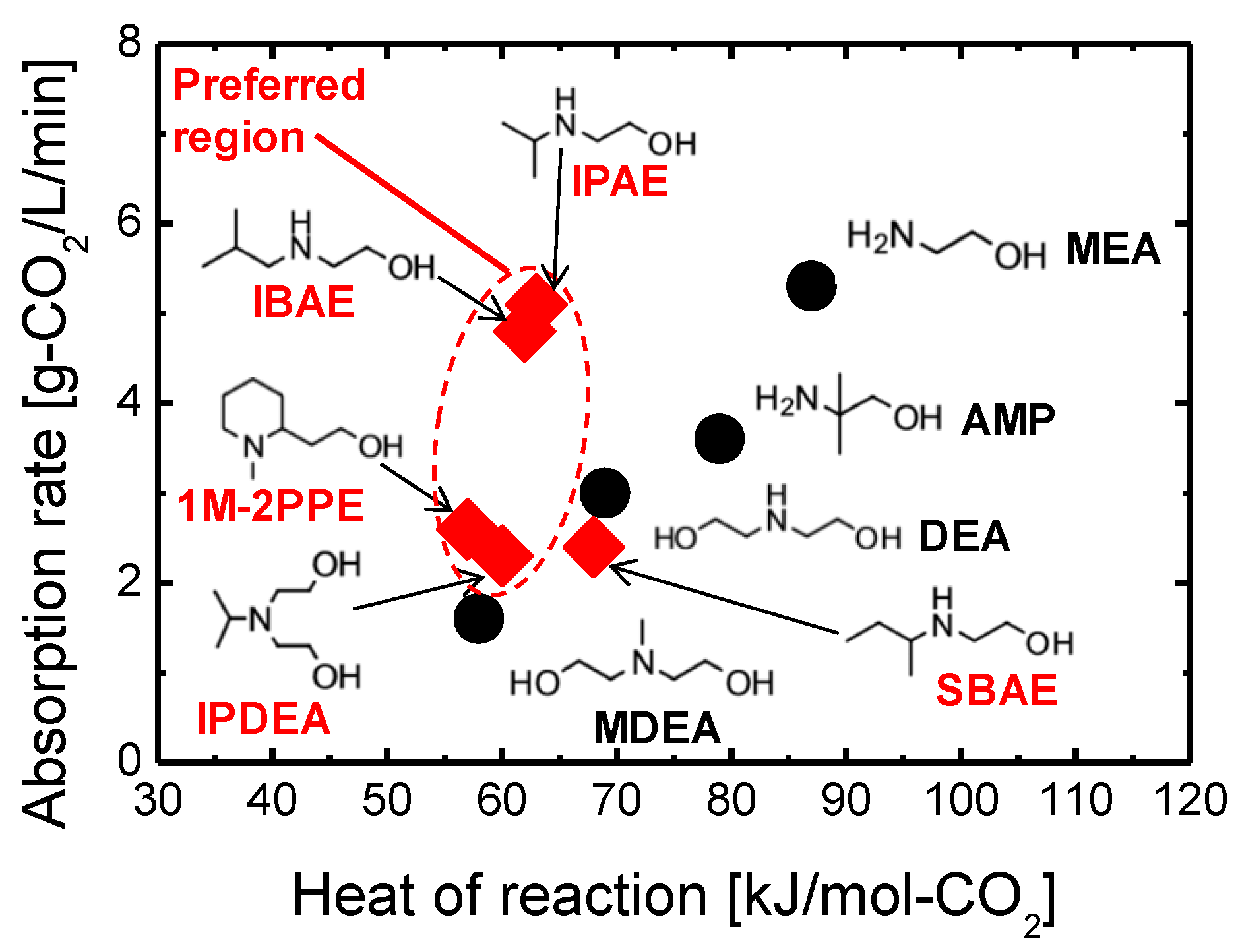
Figure 1. Relationships of absorption rate and heat of reaction between conventional and synthesized amines employed for CO2 absorbents. Solid circles (  ): conventional amines with no/low steric hindrance. Solid diamond (
): conventional amines with no/low steric hindrance. Solid diamond (  ): synthesized amines with sterical hindrance. Abbreviations: MEA, 2-aminoethanol; AMP, 2-amino-2-methyl-1-propanol; DEA, diethanolamine; MDEA, methyldiethanolamine; IPAE, 2-(isopropylamino)ethanol; IBAE, 2-(isobutylamino)ethanol; SBAE, 2-(secondarybutyamino)ethanol; IPDEA, 2-(isopropyl)diethanolamine; 1M-2PPE, 1-Methyl-2-piperidineethanol. Generally, there is a trade-off relationship between the heat of reaction and the absorption rate for conventional amines. However, the introduction of steric hindrance creates a unique performance that features a low heat of reaction but results in a moderately high absorption rate. The data were adapted from ref. [15].
): synthesized amines with sterical hindrance. Abbreviations: MEA, 2-aminoethanol; AMP, 2-amino-2-methyl-1-propanol; DEA, diethanolamine; MDEA, methyldiethanolamine; IPAE, 2-(isopropylamino)ethanol; IBAE, 2-(isobutylamino)ethanol; SBAE, 2-(secondarybutyamino)ethanol; IPDEA, 2-(isopropyl)diethanolamine; 1M-2PPE, 1-Methyl-2-piperidineethanol. Generally, there is a trade-off relationship between the heat of reaction and the absorption rate for conventional amines. However, the introduction of steric hindrance creates a unique performance that features a low heat of reaction but results in a moderately high absorption rate. The data were adapted from ref. [15].
 ): conventional amines with no/low steric hindrance. Solid diamond (
): conventional amines with no/low steric hindrance. Solid diamond (  ): synthesized amines with sterical hindrance. Abbreviations: MEA, 2-aminoethanol; AMP, 2-amino-2-methyl-1-propanol; DEA, diethanolamine; MDEA, methyldiethanolamine; IPAE, 2-(isopropylamino)ethanol; IBAE, 2-(isobutylamino)ethanol; SBAE, 2-(secondarybutyamino)ethanol; IPDEA, 2-(isopropyl)diethanolamine; 1M-2PPE, 1-Methyl-2-piperidineethanol. Generally, there is a trade-off relationship between the heat of reaction and the absorption rate for conventional amines. However, the introduction of steric hindrance creates a unique performance that features a low heat of reaction but results in a moderately high absorption rate. The data were adapted from ref. [15].
): synthesized amines with sterical hindrance. Abbreviations: MEA, 2-aminoethanol; AMP, 2-amino-2-methyl-1-propanol; DEA, diethanolamine; MDEA, methyldiethanolamine; IPAE, 2-(isopropylamino)ethanol; IBAE, 2-(isobutylamino)ethanol; SBAE, 2-(secondarybutyamino)ethanol; IPDEA, 2-(isopropyl)diethanolamine; 1M-2PPE, 1-Methyl-2-piperidineethanol. Generally, there is a trade-off relationship between the heat of reaction and the absorption rate for conventional amines. However, the introduction of steric hindrance creates a unique performance that features a low heat of reaction but results in a moderately high absorption rate. The data were adapted from ref. [15].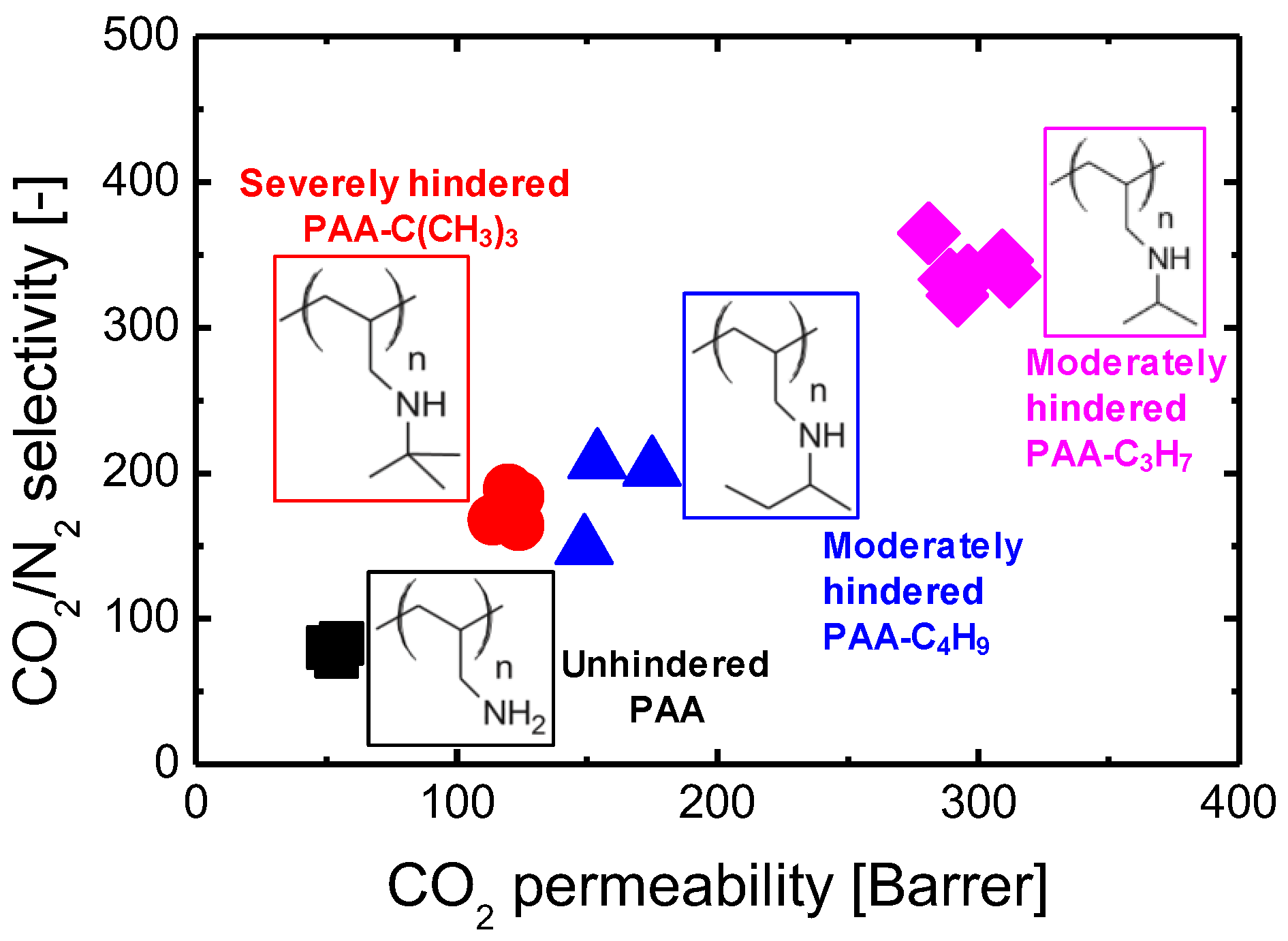
Figure 2. CO2 separation performance comparison regarding the steric hindrance effect of polyamines for polyallylamine (PAA) membranes. CO2/N2 separation was performed at 110 °C and a feed pressure of 2 bar with water injection rates = 0.03/0.03 cm3/min (feed/sweep) using a feed gas composition of 20% CO2, 40% H2, and 40% N2 (on dry basis). The data was adapted from ref. [18].
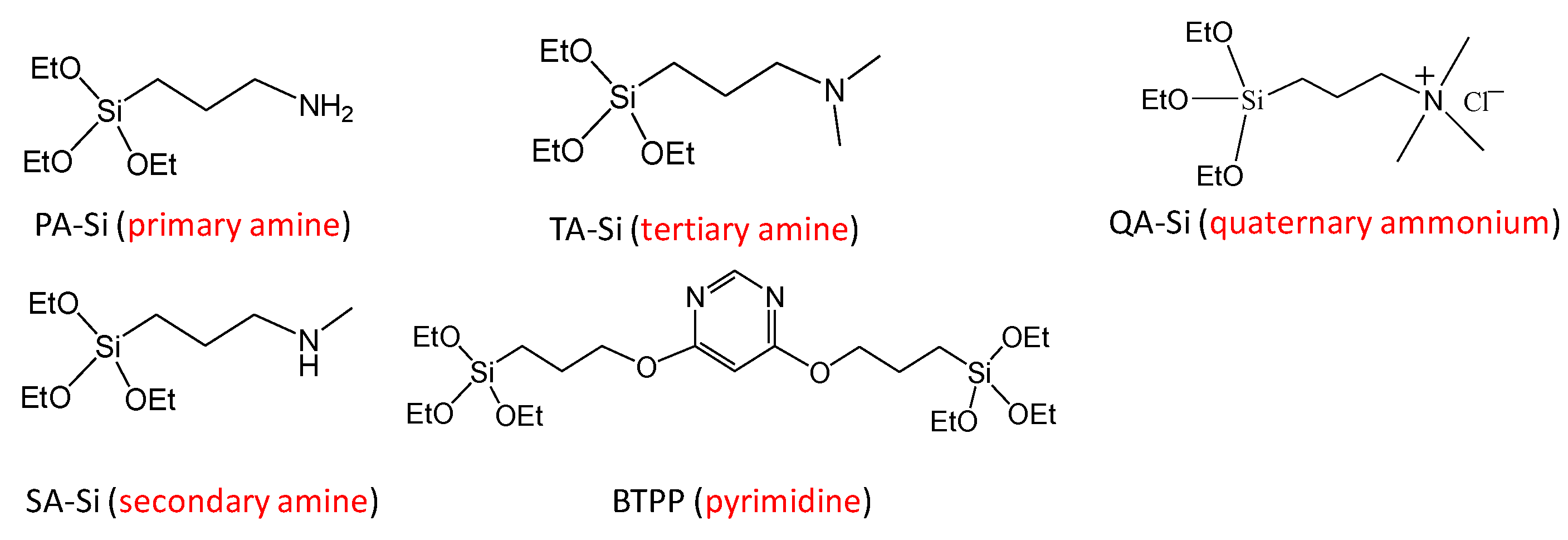
Figure 3. Chemical structures of amine-containing organosilica precursors employed in Prof. Tsuru’s group.
2. Classifications and Reaction Activities between CO2 and Amines
Amines are typically sub-classified as primary, secondary, or tertiary based on the degree of hydrocarbon substitution, while they also can be sub-classified as aliphatic, cycloaliphatic, aromatic, or heterocyclic. The diverse structures of amines confer basicity and a reaction activity that varies with acidic molecules (e.g., CO2, H2S). Generally, there are three factors that influence amine basicity: alkyl group substitution (including solvation effects and steric hindrance), resonance effect, and hybridization effect. The interlaced influences complicate the actual situation and make it more difficult to predict the down-to-earth basicity (or even the basicity order), which, however, can be reflected by the actual measurement of pKa. Consequently, the normal aliphatic amines of primary, secondary and tertiary types are all of approximately equal basicity, which is generally stronger than aromatic amines. Notwithstanding, it is the steric hindrance effect rather than amine basicity that plays a key role in impacting their reaction activities with CO2. Usually, a normal aliphatic primary or secondary amine can form a zwitterion upon encountering a CO2 molecule (Equation (1)) and can then proceed with the formation of a fairly stable carbamate complex with another amine group (Equation (2)). Under humid conditions, this carbamate complex can further react with a water molecule and form a bicarbonate ion and a free amine group (Equation (3)) [26].
RR*NH + CO2 ↔ RR*NH+COO− (1)
RR*NH+COO− + RR*NH ↔ RR*NCOO− + RR*NH2+ (2)
RR*NCOO− + H2O ↔RR*NH + HCO3− (3)
In Equations (1) to (3), R* represents a H atom for a primary amine or another alkyl group. Equation (1) is a nucleophilic addition reaction, which is the rate-limiting process regardless of whether water is involved. Equations (2) and (3), however, represent rapid proton transfer reactions [27]. Tertiary amines, sterically hindered or not, on the other hand, owing to the lack of free hydrogen atom, are unable to react directly with CO2 to form a carbamate. Instead, an alternative reaction occurs preferably to form a bicarbonate ion under humid conditions (Equation (4)) [28].
RR*R**N + CO2 + H2O ↔ RR*R**NH+ + HCO3− (4)
where R, R* and R** could be either different or identical alkyl groups.
Sterically hindered amines (SHAs), as originally defined by Sartori and Savage, can be identified from either of the following: (i) a primary amine in which the amino group is attached to a tertiary carbon atom or (ii) a secondary amine in which the amino group is attached to at least one secondary or tertiary carbon atom [29]. Based on this definition, it is difficult to identify tertiary amines with small bulkiness. Herein, the researchers regard tertiary amines as SHAs due to their similar performance. Unlike unhindered amines, SHAs are characterized by the formation of carbamates with intermediate-to-low stability due to the bulkiness of the substitutions attached to the amino groups. In this way, SHAs may provide a faster reaction with CO2 while they lower the heat of reaction. As such, membranes with SHAs might proceed with CO2 transport (adsorption-hop-desorption) more effectively across the membrane thickness. In addition, as shown in Equations (3) and (4), humidity or water vapor is also an important factor that impacts the CO2 transport behaviors within amine-containing membranes, which might make it more complicated to investigate the role of amine type in CO2 transport behaviors. The effect of humidity on CO2 transport behaviors was therefore excluded here.
3. Synthesis of Amine-Functionalized Silica/Organosilica Membranes
In general, amine-functionalized silica-based membranes are mainly fabricated via either (i) direct sol-gel processing from amine-functionalized silica precursors [6][7][30] or (ii) post-grafting or impregnation of mesoporous silica membranes [11][12][13]. In addition, the chemical vapor deposition (CVD) method is also sometimes used for the direct deposition of amine-functionalized silica precursor on the substrates, which suggests advantages to prepare defect-free membranes compared with the sol-gel route [10]. However, this method consists of a thermal decomposition step for the silica precursor at higher temperatures (generally > 400°C) prior to the deposition process, which could result in the degradation of most amine groups.
4. Role of Amine Type in CO2 Separation Performance
As mentioned above, this research pays special attention to surveys of the role of amine type in CO2 transport behaviors through amine-functionalized silica-based membranes. Generally, CO2 transport through a membrane involves adsorption in the membrane surface or pore walls, diffusion across the membrane thickness, and desorption from the membrane permeate side (see Figure 4a). All the processes to some extent can be affected by the materials’ chemistry, while the diffusion process can be generally governed by both the materials’ chemistry and the membrane microstructure (e.g., pore size, pore length, and porosity) (see Figure 4b). Therefore, to systematically investigate the role of amine type in CO2 separation performance through a membrane, both materials’ chemistry in terms of CO2 adsorption/desorption properties and membrane microstructure should be involved simultaneously.
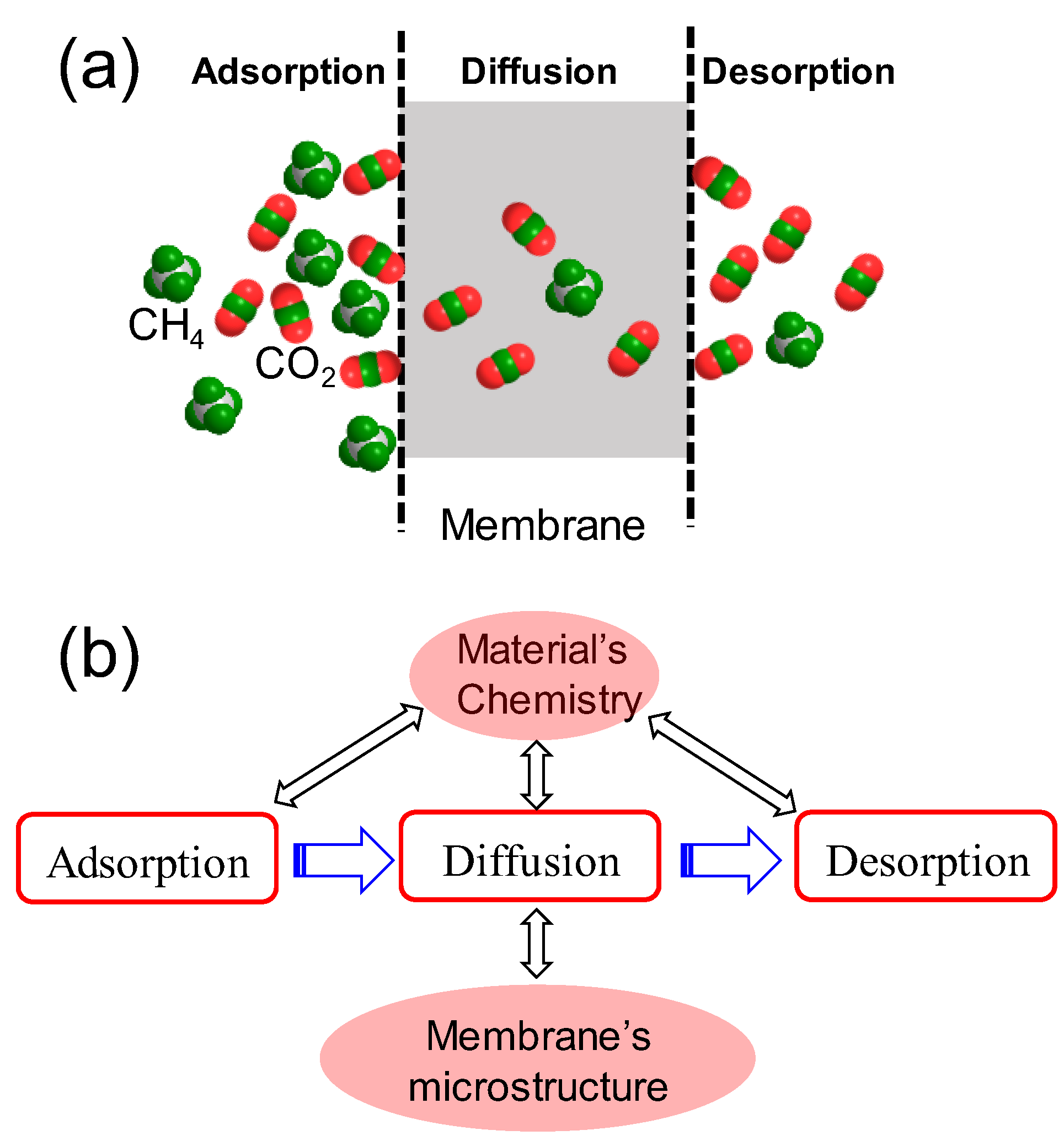
Figure 4. Schemes of (a) CO2 separation through a membrane and (b) the factors impacting CO2 transport behaviors including adsorption on the membrane surface, diffusion across the membrane thickness, and desorption from the membrane permeate surface.
References
- Gin, D.L.; Noble, R.D. Designing the next generation of chemical separation membranes. Science 2011, 332, 674–676.
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.V. Tailor-made polymeric membranes based on segmented block copolymers for CO2 separation. Adv. Funct. Mater. 2008, 18, 2815–2823.
- He, W.; Wang, Z.; Li, W.; Li, S.; Bai, Z.; Wang, J.; Wang, S. Cyclic tertiary amino group containing fixed carrier membranes for CO2 separation. J. Membr. Sci. 2015, 476, 171–181.
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (mmms) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507.
- Hillock, A.M.; Koros, W.J. Cross-linkable polyimide membrane for natural gas purification and carbon dioxide plasticization reduction. Macromolecules 2007, 40, 583–587.
- Xomeritakis, G.; Tsai, C.-Y.; Brinker, C.J. Microporous sol–gel derived aminosilicate membrane for enhanced carbon dioxide separation. Sep. Purif. Technol. 2005, 42, 249–257.
- Paradis, G.G.; Kreiter, R.; van Tuel, M.M.; Nijmeijer, A.; Vente, J.F. Amino-functionalized microporous hybrid silica membranes. J. Mater. Chem. 2012, 22, 7258–7264.
- Suzuki, S.; Messaoud, S.B.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Development of inorganic–organic hybrid membranes for carbon dioxide/methane separation. J. Membr. Sci. 2014, 471, 402–411.
- Jang, K.-S.; Kim, H.-J.; Johnson, J.; Kim, W.-G.; Koros, W.J.; Jones, C.W.; Nair, S. Modified mesoporous silica gas separation membranes on polymeric hollow fibers. Chem. Mater. 2011, 23, 3025–3028.
- Messaoud, S.B.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Alkylamine–silica hybrid membranes for carbon dioxide/methane separation. J. Membr. Sci. 2015, 477, 161–171.
- Ostwal, M.; Singh, R.P.; Dec, S.F.; Lusk, M.T.; Way, J.D. 3-aminopropyltriethoxysilane functionalized inorganic membranes for high temperature CO2/N2 separation. J. Membr. Sci. 2011, 369, 139–147.
- Sakamoto, Y.; Nagata, K.; Yogo, K.; Yamada, K. Preparation and CO2 separation properties of amine-modified mesoporous silica membranes. Microporous Mesoporous Mat. 2007, 101, 303–311.
- Kim, H.-J.; Chaikittisilp, W.; Jang, K.-S.; Didas, S.A.; Johnson, J.R.; Koros, W.J.; Nair, S.; Jones, C.W. Aziridine-functionalized mesoporous silica membranes on polymeric hollow fibers: Synthesis and single-component CO2 and N2 permeation properties. Ind. Eng. Chem. Res. 2014, 54, 4407–4413.
- Sartori, G.; Ho, W.; Savage, D.; Chludzinski, G.; Wlechert, S. Sterically-hindered amines for acid-gas absorption. Sep. Purif. Methods 1987, 16, 171–200.
- Chowdhury, F.A.; Okabe, H.; Yamada, H.; Onoda, M.; Fujioka, Y. Synthesis and selection of hindered new amine absorbents for CO2 capture. Energy Procedia 2011, 4, 201–208.
- Idris, Z.; Eimer, D.A. Representation of CO2 absorption in sterically hindered amines. Energy Procedia 2014, 51, 247–252.
- Chowdhury, F.A.; Yamada, H.; Higashii, T.; Goto, K.; Onoda, M. CO2 capture by tertiary amine absorbents: A performance comparison study. Ind. Eng. Chem. Res. 2013, 52, 8323–8331.
- Zhao, Y.; Ho, W.W. Steric hindrance effect on amine demonstrated in solid polymer membranes for CO2 transport. J. Membr. Sci. 2012, 415, 132–138.
- Zhao, Y.; Ho, W.W. CO2-selective membranes containing sterically hindered amines for CO2/H2 separation. Ind. Eng. Chem. Res. 2012, 52, 8774–8782.
- Tong, Z.; Ho, W.W. New sterically hindered polyvinylamine membranes for CO2 separation and capture. J. Membr. Sci. 2017, 543, 202–211.
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Oshita, J.; Naka, A.; Tsuru, T. Pyrimidine-bridged organoalkoxysilane membrane for high-efficiency CO2 transport via mild affinity. Sep. Purif. Technol. 2017, 178, 232–241.
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Fabrication and CO2 permeation properties of amine-silica membranes using a variety of amine types. J. Membr. Sci. 2017, 541, 447–456.
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Moriyama, N.; Tsuru, T.; Ito, K. Enhanced CO2 separation performance for tertiary amine-silica membranes via thermally induced local liberation of CH3Cl. AIChE J. 2018, 64, 1528–1539.
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Ohshita, J.; Naka, A.; Tsuru, T. Fabrication and microstructure tuning of a pyrimidine-bridged organoalkoxysilane membrane for CO2 separation. Ind. Eng. Chem. Res. 2017, 56, 1316–1326.
- Pera-Titus, M. Porous inorganic membranes for CO2 capture: Present and prospects. Chem. Rev. 2013, 114, 1413–1492.
- Caplow, M. Kinetics of carbamate formation and breakdown. J. Am. Chem. Soc. 1968, 90, 6795–6803.
- Li, Y.; Wang, S.; He, G.; Wu, H.; Pan, F.; Jiang, Z. Facilitated transport of small molecules and ions for energy-efficient membranes. Chem. Soc. Rev. 2015, 44, 103–118.
- Donaldson, T.L.; Nguyen, Y.N. Carbon dioxide reaction kinetics and transport in aqueous amine membranes. Ind. Eng. Chem. Fund. 1980, 19, 260–266.
- Sartori, G.; Savage, D.W. Sterically hindered amines for carbon dioxide removal from gases. Ind. Eng. Chem. Fund. 1983, 22, 239–249.
- Xomeritakis, G.; Tsai, C.; Jiang, Y.; Brinker, C. Tubular ceramic-supported sol–gel silica-based membranes for flue gas carbon dioxide capture and sequestration. J. Membr. Sci. 2009, 341, 30–36.
More
Information
Subjects:
Materials Science, Composites
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
27 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




