| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elizabeth Mulcahy | + 1751 word(s) | 1751 | 2020-10-21 10:22:39 | | | |
| 2 | Rita Xu | -688 word(s) | 1063 | 2020-10-23 03:31:32 | | |
Video Upload Options
A discussion of malignant brain tumors including both primary tumors (gliomas, embryonal CNS tumors) and secondary tumors (brain metastasis).
1. Malignant brain tumors
In the United States, at any given time, more than 76,000 people are living with brain and other central nervous system (CNS) tumors [1]. In one year’s time, more than 19,000 people will have died from the malignant forms of these cancers, the most common being glioblastoma. Nearly 90,000 people will be newly diagnosed with a brain tumor each year. White men in their 80s are the most susceptible group within the world population. This has been hypothesized to be attributable to hormonal differences, genetic differences, and micro-environmental changes in the cell of origin [2]. Despite significant understanding and advances over the past half century, malignant brain tumors remain largely incurable [3]. There are several reasons for this.
First, malignant brain tumors are genetically heterogeneous and contain a complex array of somatic genetic alterations. This characteristic of these tumors can significantly weaken the effectiveness of monotherapies. Some examples of genetic alterations are: recurrent mutations in the isocitrate dehydrogenase genes 1 and 2 (IDH1 and IDH2), receptor tyrosine kinase (RTK) amplification associated with genetic abnormalities, mutations in the promoter region of the telomerase reverse transcriptase (TERT) gene, mutations of the canonical “guardian of the genome” tumor protein p53 (TP53), etc [4][5][6]. Oftentimes, more than one of these alterations are present in the tumor and give rise to a heterogeneous population of cells.
Second, the brain is an immune privileged organ and is protected by the blood and brain barrier (BBB) which results from tight junctions formed between endothelial cells that make up the vasculature of the brain’s outer layer [7]. This can be a great obstacle for most available, Food and Drug Administration (FDA) approved drugs as only small molecules can cross [8][9].
Third, surgical resection within the brain is difficult given the confined space and its essential contents. Studies on the effect of surgical resection for low grade gliomas showed that patients whose tumors were ≥90% resected by volume had a 91% chance of being alive 8 years post diagnosis [10]. This is contrasted with patients whose tumors were <90% resected by volume and had a 60% chance of being alive 8 years later. These studies have been adjusted for age, tumor location and subtype, and the respective Karnofsky Performance Scores (KPS) of each patient. The KPS is a measurement of the patient’s ability to carry out ordinary tasks and is reflective of the impact the disease [11]. In other words, although surgery can greatly improve disease prognosis, it does not entirely remove the tumor and does not cure the patient.
Fourth, diagnosis is complicated by the often vague symptoms that present and the expense of imaging using magnetic resonance imaging (MRI). This is because symptoms are often convolved with other pathologies. For example, up to 80% of patients may present seizures, 30% present headaches, and 15% present morning nausea and vomiting [12]. Many of these symptoms are often attributed to other more likely diseases.
2. Primary Malignant Brain Tumors
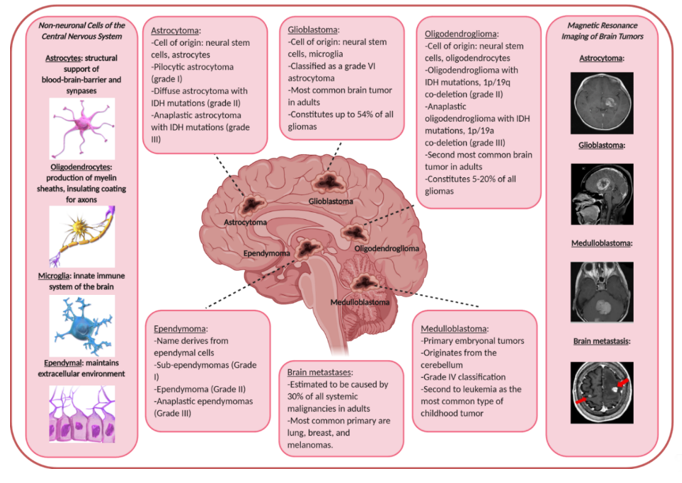
Gliomas make up nearly 80% of all primary malignant brain tumors [13]. Here, we will briefly discuss astrocytomas, glioblastomas, oligodendrogliomas, ependymomas, and embryonal central nervous system tumors (including medulloblastomas and others). See Figure 1 for a general summary of the different primary and secondary malignant brain tumors, their nomenclature-derived cell of origin, and real-life MRI images.
Astrocytomas derive their name from astrocytes, a star-shaped cell that is the most abundant glial cell type in the CNS [14]. They help support neuronal cells by mediating synapses and providing nutrients. The cells of origin of astrocytomas are probably neural stem and/or oligodendrocyte progenitor cells in mouse models [15]. Glioblastomas arise from several different cell types. Over the past many years, the cells of origin for this particular tumor type has been the source of dissension and heated discussion for many. While its name is derived from glial cells which are the resident immune cells of the brain, glioblastomas likely arise from neural stem cells or perhaps even oligodendrocyte progenitor precursor cells [2][16]. In fact, many studies, prompted by this heated discussion, have given rise to a wealth of evidence showing the presence of glioblastoma stem cells in both mouse models as well as human tumors [17][18][19][20]. One of the most cited studies showing this was performed by Liu et al in 2006[19]. In this research paper, the authors used sorting methods to separate different population of cells from primary cultured cell lines established from glioblastoma patient tissue samples. They found that more than 10% of the cells from the human tumors harbored the CD133 marker, a major stem cell marker. These cells were further isolated and shown significant resistance to chemotherapy. Furthermore, the expression of this marker was significantly higher in recurrent glioblastoma tissues samples of patients compared to the same patient’s newly diagnosed tumors.
Oligodendrogliomas, despite the name, likely also arise from neural stem cells or glial progenitor cells [21]. Ependymomas develop from ependymal cells that make up the ventricular system of the brain [22].
Embryonal central nervous system tumors are formed from embryonic cells in the brain after birth [23]. Brain tumors in children are the second most common type of cancer in children after leukemia. There are two main types of central nervous system embryonal tumors: medulloblastomas and non-medulloblastoma embryonal tumors. Medulloblastomas arise in the cerebellum, likely from granule progenitor cells [24]. It is the most common type of primary brain tumor in children. Non-medulloblastomas are rare and include tumor types such as pineoblastoma, atypical teratoid/rhabdoid and others [25][26].
Figure 1. Summary of the different malignant brain tumors that are discussed in this review, their name-derived cell of origin, and real-life images taken from patients. Classifications are adapted from the World Health Organization [27]. Drawings of cells and patient tumor magnetic resonance imaging images are taken from the public domain [28].
3. Secondary Malignant Brain Tumors (Brain metastasis)
Almost every malignant primary tumor is capable of metastasizing to the brain [29]. However, the incidence of brain metastases is difficult to quantify because some remain asymptomatic or are ignored because the patient is often severely ill with advanced primary disease. Autopsy studies suggest that as many as 30% of adult cancer patients with systemic malignancies have brain metastases at the time of death [30]. In adults, lung, breast, and melanomas account for 75% of the primary tumor of brain metastases [31]. In children and young adults, sarcomas such as Ewing’s sarcoma and germ cell tumors are the most likely to metastasize to the brain [32].
References
- Ostrom, Q.T., et al., CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol, 2019. 21(Suppl 5): p. v1-v100.
- Cahill, D. and S. Turcan, Origin of Gliomas. Semin Neurol, 2018. 38(1): p. 5-10.
- Kelly, P.J., Gliomas: Survival, origin and early detection. Surg Neurol Int, 2010. 1: p. 96.
- Yan, H., et al., IDH1 and IDH2 mutations in gliomas. N Engl J Med, 2009. 360(8): p. 765-73.
- Szerlip, N.J., et al., Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci U S A, 2012. 109(8): p. 3041-6.
- Suzuki, H., et al., Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet, 2015. 47(5): p. 458-68.
- Huber, J.D., R.D. Egleton, and T.P. Davis, Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci, 2001. 24(12): p. 719-25.
- Dong, X., Current Strategies for Brain Drug Delivery. Theranostics, 2018. 8(6): p. 1481-1493.
- Garg, T., et al., Current strategies for targeted delivery of bio-active drug molecules in the treatment of brain tumor. J Drug Target, 2015. 23(10): p. 865-87.
- Sanai, N. and M.S. Berger, Surgical oncology for gliomas: the state of the art. Nat Rev Clin Oncol, 2018. 15(2): p. 112-125.
- Chambless, L.B., et al., The relative value of postoperative versus preoperative Karnofsky Performance Scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neurooncol, 2015. 121(2): p. 359-64.
- Lapointe, S., A. Perry, and N.A. Butowski, Primary brain tumours in adults. Lancet, 2018. 392(10145): p. 432-446.
- Bush, N.A., S.M. Chang, and M.S. Berger, Current and future strategies for treatment of glioma. Neurosurg Rev, 2017. 40(1): p. 1-14.
- Sofroniew, M.V. and H.V. Vinters, Astrocytes: biology and pathology. Acta Neuropathol, 2010. 119(1): p. 7-35.
- Alcantara Llaguno, S., et al., Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell, 2009. 15(1): p. 45-56.
- Goffart, N., J. Kroonen, and B. Rogister, Glioblastoma-initiating cells: relationship with neural stem cells and the micro-environment. Cancers (Basel), 2013. 5(3): p. 1049-71.
- Bao, S., et al., Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature, 2006. 444(7120): p. 756-60.
- Cheng, L., et al., Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell, 2013. 153(1): p. 139-52.
- Liu, G., et al., Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer, 2006. 5: p. 67.
- Li, Y., et al., c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proc Natl Acad Sci U S A, 2011. 108(24): p. 9951-6.
- Wesseling, P., M. van den Bent, and A. Perry, Oligodendroglioma: pathology, molecular mechanisms and markers. Acta Neuropathol, 2015. 129(6): p. 809-27.
- Mack, S.C., et al., Therapeutic targeting of ependymoma as informed by oncogenic enhancer profiling. Nature, 2018. 553(7686): p. 101-105.
- Ostrom, Q.T., et al., Alex's Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro Oncol, 2015. 16 Suppl 10: p. x1-x36.
- Millard, N.E. and K.C. De Braganca, Medulloblastoma. J Child Neurol, 2016. 31(12): p. 1341-53.
- Biswas, A., et al., Atypical teratoid/rhabdoid tumors: challenges and search for solutions. Cancer Manag Res, 2016. 8: p. 115-125.
- Abdelbaki, M.S., et al., Pineoblastoma in children less than six years of age: The Head Start I, II, and III experience. Pediatr Blood Cancer, 2020. 67(6): p. e28252.
- Louis, D.N., et al., The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol, 2016. 131(6): p. 803-20.
- Blausen.com-staff, Medical gallery of Blausen Medical. WikiJournal of Medicine, 2014.
- Gallego Perez-Larraya, J. and J. Hildebrand, Brain metastases. Handb Clin Neurol, 2014. 121: p. 1143-57.
- Schouten, L.J., et al., Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer, 2002. 94(10): p. 2698-705.
- DeAngelis, L.M., J.B. Posner, and J.B. Posner, Neurologic complications of cancer. 2nd ed. Contemporary neurology series. 2009, Oxford ; New York: Oxford University Press. xv, 634 p.
- Kebudi, R., et al., Brain metastasis in pediatric extracranial solid tumors: survey and literature review. J Neurooncol, 2005. 71(1): p. 43-8.