
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Denis Gubin | -- | 1832 | 2022-09-22 13:55:06 | | | |
| 2 | Lindsay Dong | Meta information modification | 1832 | 2022-09-26 03:14:36 | | |
Video Upload Options
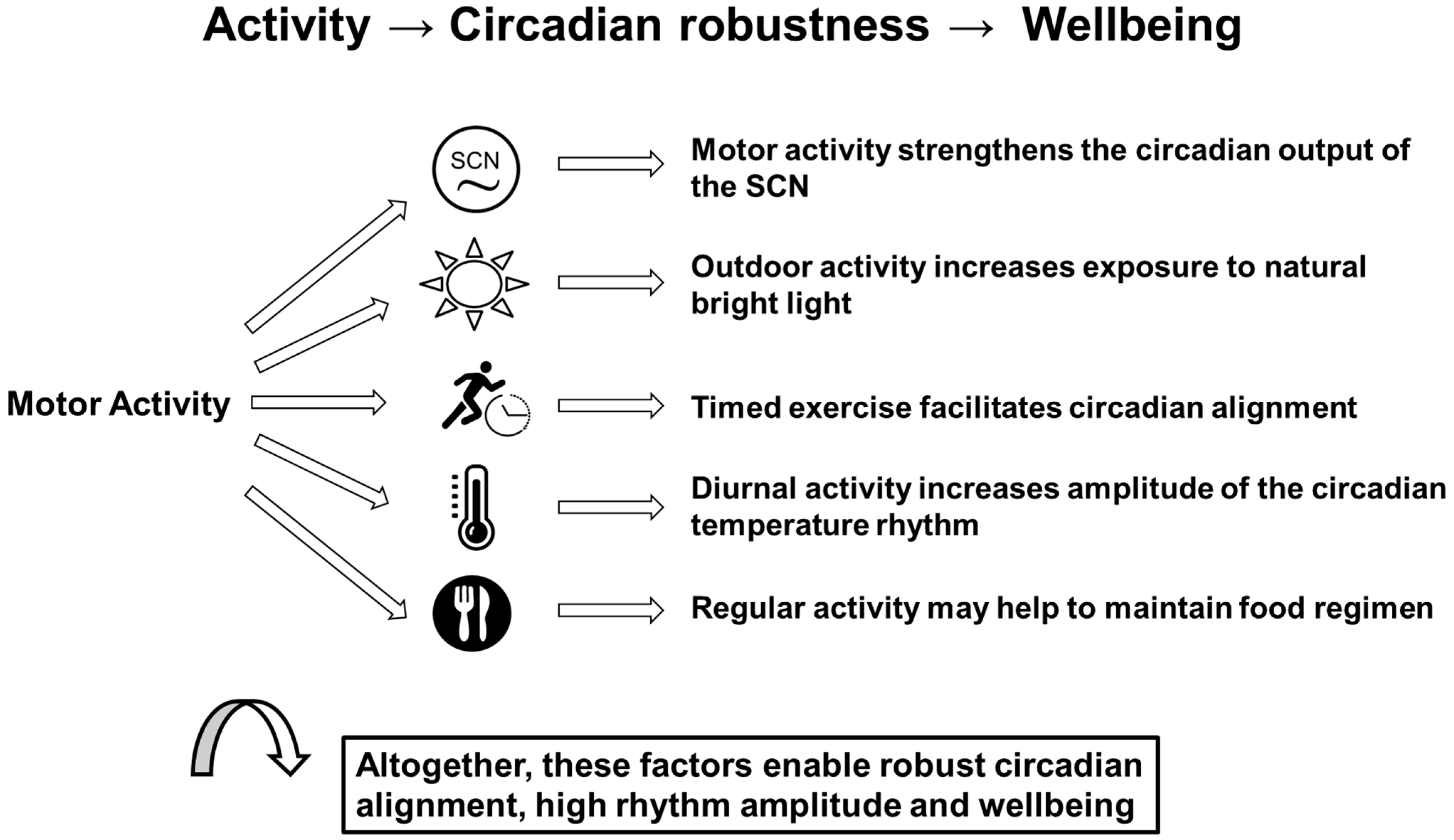
Circadian rhythms are an inherent property of all living systems and an essential part of the external and internal temporal order. They enable organisms to be synchronized with their periodic environment and guarantee the optimal functioning of organisms. Any disturbances, so-called circadian disruptions, may have adverse consequences for health, physical and mental performance, and wellbeing. The environmental light–dark cycle is the main zeitgeber for circadian rhythms. Moreover, regular physical activity is most useful. Not only does it have general favorable effects on the cardiovascular system, the energy metabolism and mental health, for example, but it may also stabilize the circadian system via feedback effects on the suprachiasmatic nuclei (SCN), the main circadian pacemaker. Regular physical activity helps to maintain high-amplitude circadian rhythms, particularly of clock gene expression in the SCN. It promotes their entrainment to external periodicities and improves the internal synchronization of various circadian rhythms. This in turn promotes health and wellbeing.
1. Introduction
2. Physical Activity and the Circadian System
2.1. The Diagnostic Value of Circadian Activity Rhythms
2.2. Beneficial Effects of Increasing the Daily Activity Level
2.3. Effects of Motor Activity on the Circadian System
2.4. Beneficial Effects of Stabilized Circadian Rhythms
References
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941.
- Dijk, D.J.; Lockley, S.W. Integration of human sleep-wake regulation and circadian rhythmicity. J. Appl. Physiol. 2002, 92, 852–862.
- Wollnik, F.; Turek, F.W. SCN lesions abolish ultradian and circadian components of activity rhythms in LEW/Ztm rats. Am. J. Physiol. 1989, 256, R1027–R1039.
- Kalsbeek, A.; Scheer, F.A.; Perreau-Lenz, S.; La Fleur, S.E.; Yi, C.X.; Fliers, E.; Buijs, R.M. Circadian disruption and SCN control of energy metabolism. FEBS Lett. 2011, 585, 1412–1426.
- Waterhouse, J.; Drust, B.; Weinert, D.; Edwards, B.; Gregson, W.; Atkinson, G.; Kao, S.; Aizawa, S.; Reilly, T. The circadian rhythm of core temperature: Origin and some implications for exercise performance. Chronobiol. Int. 2005, 22, 207–225.
- Hurd, M.W.; Ralph, M.R. The significance of circadian organization for longevity in the golden hamster. J. Biol. Rhythm. 1998, 13, 430–436.
- Müller, L.; Weinert, D. Individual recognition of social rank and social memory performance depends on a functional circadian system. Behav Process. 2016, 132, 85–93.
- Sharma, A.; Tiwari, S.; Singaravel, M. Circadian rhythm disruption: Health consequences. Biol. Rhythm Res. 2015, 47, 191–213.
- Smolensky, M.H.; Hermida, R.C.; Reinberg, A.; Sackett-Lundeen, L.; Portaluppi, F. Circadian disruption: New clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol. Int. 2016, 33, 1101–1119.
- Weinert, D.; Schöttner, K.; Müller, L.; Wienke, A. Intensive voluntary wheel running may restore circadian activity rhythms and improves the impaired cognitive performance of arrhythmic Djungarian hamsters. Chronobiol. Int. 2016, 33, 1161–1170.
- Gubin, D.G.; Weinert, D. Temporal order deterioration and circadian disruption with age. 1. Central and peripheral mechanisms. Adv. Gerontol. 2015, 5, 209–218.
- Gubin, D.G.; Weinert, D. Deterioration of Temporal Order and Circadian Disruption with Age 2: Systemic Mechanisms of Aging Related Circadian Disruption and Approaches to Its Correction. Adv. Gerontol. 2016, 6, 10–20.
- Gubin, D.G.; Weinert, D.; Bolotnova, T.V. Age-Dependent Changes of the Temporal Order—Causes and Treatment. Curr. Aging Sci. 2016, 9, 14–25.
- Weinert, D. Age-dependent changes of the circadian system. Chronobiol. Int. 2000, 17, 261–283.
- Casiraghi, L.P.; Oda, G.A.; Chiesa, J.J.; Friesen, W.O.; Golombek, D.A. Forced Desynchronization of Activity Rhythms in a Model of Chronic Jet Lag in Mice. J. Biol. Rhythm. 2012, 27, 59–69.
- Waterhouse, J.; Kao, S.; Edwards, B.; Weinert, D.; Atkinson, G.; Reilly, T. Transient changes in the pattern of food intake following a simulated time-zone transition to the east across eight time zones. Chronobiol. Int. 2005, 22, 299–319.
- Waterhouse, J.; Reilly, T.; Atkinson, G.; Edwards, B. Jet lag: Trends and coping strategies. Lancet 2007, 369, 1117–1129.
- Shurkevich, N.P.; Vetoshkin, A.S.; Gapon, L.I.; Dyachkov, S.M.; Gubin, D.G. Prognostic value of blood pressure circadian rhythm disturbances in normotensive shift workers of the Arctic polar region. Arter. Hypertens. 2017, 23, 36–46. (In Russian)
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106.
- Gubin, D.G.; Nelaeva, A.A.; Uzhakova, A.E.; Hasanova, Y.V.; Cornelissen, G.; Weinert, D. Disrupted circadian rhythms of body temperature, heart rate and fasting blood glucose in prediabetes and type 2 diabetes mellitus. Chronobiol. Int. 2017, 34, 1136–1148.
- Kent, B.A. Synchronizing an aging brain: Can entraining circadian clocks by food slow Alzheimer’s disease? Front. Aging Neurosci. 2014, 6, 234.
- Neroev, V.; Malishevskaya, T.; Weinert, D.; Astakhov, S.; Kolomeichuk, S.; Cornelissen, G.; Kabitskaya, Y.; Boiko, E.; Nemtsova, I.; Gubin, D. Disruption of 24-Hour Rhythm in Intraocular Pressure Correlates with Retinal Ganglion Cell Loss in Glaucoma. Int. J. Mol. Sci. 2021, 22, 359.
- Yilmaz, A.; Kalsbeek, A.; Buijs, R.M. Functional changes of the SCN in spontaneous hypertension but not after the induction of hypertension. Chronobiol. Int. 2018, 35, 1221–1235.
- Cardinali, D.P. Melatonin as a chronobiotic/cytoprotector: Its role in healthy aging. Biol. Rhythm Res. 2019, 50, 28–45.
- Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients? Diseases 2020, 8, 44.
- Hardeland, R. Divergent Importance of Chronobiological Considerations in High- and Low-dose Melatonin Therapies. Diseases 2021, 9, 18.
- Lewy, A.J.; Ahmed, S.; Jackson, J.M.; Sack, R.L. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol. Int. 1992, 9, 380–392.
- Gubin, D.G.; Gubin, G.D.; Gapon, L.I.; Weinert, D. Daily Melatonin Administration Attenuates Age-Dependent Disturbances of Cardiovascular Rhythms. Curr. Aging Sci. 2016, 9, 5–13.
- Gubin, D.G.; Gubin, G.D.; Waterhouse, J.; Weinert, D. The circadian body temperature rhythm in the elderly: Effect of single daily melatonin dosing. Chronobiol. Int. 2006, 23, 639–658.
- Daan, S. The Colin S. Pittendrigh Lecture. Colin Pittendrigh, Jurgen Aschoff, and the natural entrainment of circadian systems. J. Biol. Rhythm. 2000, 15, 195–207.
- Golombek, D.A.; Rosenstein, R.E. Physiology of circadian entrainment. Physiol. Rev. 2010, 90, 1063–1102.
- Cho, Y.; Ryu, S.-H.; Lee, B.R.; Kim, K.H.; Lee, E.; Choi, J. Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol. Int. 2015, 32, 1294–1310.
- Green, A.; Cohen-Zion, M.; Haim, A.; Dagan, Y. Evening light exposure to computer screens disrupts human sleep, biological rhythms, and attention abilities. Chronobiol. Int. 2017, 34, 855–865.
- Gubin, D.G.; Weinert, D.; Rybina, S.V.; Danilova, L.A.; Solovieva, S.V.; Durov, A.M.; Prokopiev, N.Y.; Ushakov, P.A. Activity, sleep and ambient light have a different impact on circadian blood pressure, heart rate and body temperature rhythms. Chronobiol. Int. 2017, 34, 632–649.
- Smolensky, M.H.; Sackett-Lundeen, L.L.; Portaluppi, F. Nocturnal light pollution and underexposure to daytime sunlight: Complementary mechanisms of circadian disruption and related diseases. Chronobiol. Int. 2015, 32, 1029–1048.
- Castillo, M.R.; Hochstetler, K.J.; Tavernier, R.J., Jr.; Greene, D.M.; Bult-Ito, A. Entrainment of the master circadian clock by scheduled feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R551–R555.
- Froy, O.; Miskin, R. Effect of feeding regimens on circadian rhythms: Implications for aging and longevity. Aging 2010, 2, 7–27.
- Lamont, E.W.; Diaz, L.R.; Barry-Shaw, J.; Stewart, J.; Amir, S. Daily restricted feeding rescues a rhythm of period2 expression in the arrhythmic suprachiasmatic nucleus. Neuroscience 2005, 132, 245–248.
- Stokkan, K.A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 2001, 291, 490–493.
- Acosta-Rodriguez, V.A.; Rijo-Ferreira, F.; Green, C.B.; Takahashi, J.S. Importance of circadian timing for aging and longevity. Nat. Commun. 2021, 12, 2862.
- Mistlberger, R.E.; Antle, M.C. Entrainment of circadian clocks in mammals by arousal and food. Essays Biochem. 2011, 49, 119–136.
- Minors, D.; Atkinson, G.; Bent, N.; Rabbitt, P.; Waterhouse, J. The effects of age upon some aspects of lifestyle and implications for studies on circadian rhythmicity. Age Ageing 1998, 27, 67–72.
- Monk, T.H.; Reynolds, C.F., III; Kupfer, D.J.; Hoch, C.C.; Carrier, J.; Houck, P.R. Differences over the life span in daily life-style regularity. Chronobiol. Int. 1997, 14, 295–306.
- Hallal, P.C.; Andersen, L.B.; Bull, F.C.; Guthold, R.; Haskell, W.; Ekelund, U. Lancet Physical Activity Series Working G. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 2012, 380, 247–257.
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229.
- de Souza Teixeira, A.A.; Lira, F.S.; Rosa-Neto, J.C. Aging with rhythmicity. Is it possible? Physical exercise as a pacemaker. Life Sci. 2020, 261, 118453.
- Weinert, D.; Waterhouse, J. The circadian rhythm of core temperature: Effects of physical activity and aging. Physiol. Behav. 2007, 90, 246–256.
- Bellone, G.J.; Plano, S.A.; Cardinali, D.P.; Chada, D.P.; Vigo, D.E.; Golombek, D.A. Comparative analysis of actigraphy performance in healthy young subjects. Sleep Sci. 2016, 9, 272–279.
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003, 26, 342–392.
- Carvalho-Bos, S.S.; Riemersma-van der Lek, R.F.; Waterhouse, J.; Reilly, T.; Van Someren, E.J. Strong association of the rest-activity rhythm with well-being in demented elderly women. Am. J. Geriatr. Psychiatry 2007, 15, 92–100.
- Chevalier, V.; Mormont, M.C.; Cure, H.; Chollet, P. Assessment of circadian rhythms by actimetry in healthy subjects and patients with advanced colorectal cancer. Oncol. Rep. 2003, 10, 733–737.
- Weinert, D.; Sturm, J.; Waterhouse, J. Different behavior of the circadian rhythms of activity and body temperature during resynchronization following an advance of the LD cycle. Biol. Rhythm Res. 2002, 33, 187–197.
- Weinert, D.; Weiss, T. A nonlinear interrelationship between period length and the amount of activity—Age-dependent changes. Biol. Rhythm Res. 1997, 28, 105–120.
- Gubin, D.; Weinert, D.; Cornélissen, G. Chronotheranostics and chronotherapy—Frontiers for personalized medicine. J. Chronomed. 2020, 22, 3–23.
- Martinez-Nicolas, A.; Madrid, J.A.; García, F.J.; Campos, M.; Moreno-Casbas, M.T.; Almaida-Pagán, P.F.; Lucas-Sánchez, A.; Rol, M.A. Circadian monitoring as an aging predictor. Sci. Rep. 2018, 8, 15027.
- Vitale, J.A.; Banfi, G.; Tivolesi, V.; Pelosi, C.; Borghi, S.; Negrini, F. Rest-activity daily rhythm and physical activity levels after hip and knee joint replacement: The role of actigraphy in orthopedic clinical practice. Chronobiol. Int. 2021, 38, 1692–1701.
- Minors, D.; Akerstedt, T.; Atkinson, G.; Dahlitz, M.; Folkard, S.; Levi, F.; Mormont, C.; Parkes, D.; Waterhouse, J. The difference between activity when in bed and out of bed. I. Healthy subjects and selected patients. Chronobiol. Int. 1996, 13, 27–34.
- Mormont, M.C.; Langouet, A.M.; Claustrat, B.; Bogdan, A.; Marion, S.; Waterhouse, J.; Touitou, Y.; Levi, F. Marker rhythms of circadian system function: A study of patients with metastatic colorectal cancer and good performance status. Chronobiol. Int. 2002, 19, 141–155.
- Mormont, M.C.; Waterhouse, J. Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiol. Int. 2002, 19, 313–323.
- Mormont, M.C.; Waterhouse, J.; Bleuzen, P.; Giacchetti, S.; Jami, A.; Bogdan, A.; Lellouch, J.; Misset, J.L.; Touitou, Y.; Levi, F. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin. Cancer Res. 2000, 6, 3038–3045.
- Hoopes, E.K.; Witman, M.A.; D’Agata, M.N.; Berube, F.R.; Brewer, B.; Malone, S.K.; Grandner, M.A.; Patterson, F. Rest-activity rhythms in emerging adults: Implications for cardiometabolic health. Chronobiol. Int. 2021, 38, 543–556.
- Huang, T.; Mariani, S.; Redline, S. Sleep Irregularity and Risk of Cardiovascular Events. J. Am. Coll. Cardiol. 2020, 75, 991–999.
- Borisenkov, M.; Tserne, T.; Bakutova, L.; Gubin, D. Food Addiction and Emotional Eating Are Associated with Intradaily Rest–Activity Rhythm Variability. Eat Weight Disord. 2022.
- Gubin, D.G.; Malishevskaya, T.N.; Astakhov, Y.S.; Astakhov, S.Y.; Cornelissen, G.; Kuznetsov, V.A.; Weinert, D. Progressive retinal ganglion cell loss in primary open-angle glaucoma is associated with temperature circadian rhythm phase delay and compromised sleep. Chronobiol. Int. 2019, 36, 564–577.
- Charansonney, O.L. Physical activity and aging: A life-long story. Discov. Med. 2011, 12, 177–185.
- Choi, Y.; Cho, J.; No, M.-H.; Heo, J.-W.; Cho, E.-J.; Chang, E.; Park, D.-H.; Kang, J.-H.; Kwak, H.-B. Re-Setting the Circadian Clock Using Exercise against Sarcopenia. Int. J. Mol. Sci. 2020, 21, 3106.
- Kume, Y.; Kodama, A.; Maekawa, H. Preliminary report; Comparison of the circadian rest-activity rhythm of elderly Japanese community-dwellers according to sarcopenia status. Chronobiol. Int. 2020, 37, 1099–1105.
- Balbus, J.M.; Barouki, R.; Birnbaum, L.S.; Etzel, R.A.; Gluckman, P.D.; Grandjean, P.; Hancock, C.; Hanson, M.A.; Heindel, J.J.; Hoffman, K.; et al. Early-life prevention of non-communicable diseases. Lancet 2013, 38, 3–4.
- Kelly, S.A.; Pomp, D. Genetic determinants of voluntary exercise. Trends Genet. 2013, 29, 348–357.
- Stensel, D. Primary prevention of CVD: Physical activity. BMJ Clin. Evid. 2009, 2009, 0218.
- Gallanagh, S.; Quinn, T.J.; Alexander, J.; Walters, M.R. Physical activity in the prevention and treatment of stroke. ISRN Neurol. 2011, 2011, 953818.
- Denlinger, C.S.; Engstrom, P.F. Colorectal cancer survivorship: Movement matters. Cancer Prev. Res. 2011, 4, 502–511.
- Wadden, T.A.; Webb, V.L.; Moran, C.H.; Bailer, B.A. Lifestyle modification for obesity: New developments in diet, physical activity, and behavior therapy. Circulation 2012, 125, 1157–1170.
- Matura, S.; Carvalho, A.F.; Alves, G.S.; Pantel, J. Physical Exercise for the Treatment of Neuropsychiatric Disturbances in Alzheimer’s Dementia: Possible Mechanisms, Current Evidence and Future Directions. Curr. Alzheimer Res. 2016, 13, 1112–1123.
- Vyazovskiy, V.V.; Ruijgrok, G.; Deboer, T.; Tobler, I. Running wheel accessibility affects the regional electroencephalogram during sleep in mice. Cereb. Cortex. 2006, 16, 328–336.
- Greenwood, B.N.; Fleshner, M. Exercise, learned helplessness, and the stress-resistant brain. Neuromol. Med. 2008, 10, 81–98.
- Kingston, R.C.; Smith, M.; Lacey, T.; Edwards, M.; Best, J.N.; Markham, C.M. Voluntary exercise increases resilience to social defeat stress in Syrian hamsters. Physiol. Behav. 2018, 188, 194–198.
- Groot, C.; Hooghiemstra, A.M.; Raijmakers, P.G.H.M.; van Berckel, B.N.M.; Scheltens, P.; Scherder, E.J.A.; van der Flier, W.M.; Ossenkoppele, R. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res. Rev. 2016, 25, 13–23.
- Winchester, J.; Dick, M.B.; Gillen, D.; Reed, B.; Miller, B.; Tinklenberg, J.; Mungas, D.; Chui, H.; Galasko, D.; Hewett, L.; et al. Walking stabilizes cognitive functioning in Alzheimer’s disease (AD) across one year. Arch. Gerontol. Geriatr. 2013, 56, 96–103.
- Fabel, K.; Kempermann, G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromol. Med. 2008, 10, 59–66.
- Feter, N.; Spanevello, R.M.; Soares, M.S.P.; Spohr, L.; Pedra, N.S.; Bona, N.P.; Freitas, M.P.; Gonzales, N.G.; Ito, L.G.M.S.; Stefanello, F.M.; et al. How does physical activity and different models of exercise training affect oxidative parameters and memory? Physiol. Behav. 2019, 201, 42–52.
- Mustroph, M.L.; Chen, S.; Desai, S.C.; Cay, E.B.; DeYoung, E.K.; Rhodes, J.S. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience 2012, 219, 62–71.
- Rajizadeh, M.A.; Esmaeilpour, K.; Masoumi-Ardakani, Y.; Bejeshk, M.A.; Shabani, M.; Nakhaee, N.; Ranjbar, M.P.; Borzadaran, F.M.; Sheibani, V. Voluntary exercise impact on cognitive impairments in sleep-deprived intact female rats. Physiol. Behav. 2018, 188, 58–66.
- van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431.
- Edgar, D.M.; Kilduff, T.S.; Martin, C.E.; Dement, W.C. Influence of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice. Physiol. Behav. 1991, 50, 373–378.
- Weinert, D.; Schöttner, K. An inbred lineage of Djungarian hamsters with a strongly attenuated ability to synchronize. Chronobiol. Int. 2007, 24, 1065–1079.
- Antle, M.C.; Sterniczuk, R.; Smith, V.M.; Hagel, K. Non-photic modulation of phase shifts to long light pulses. J. Biol. Rhythm. 2007, 22, 524–533.
- Ralph, M.R.; Mrosovsky, N. Behavioral inhibition of circadian responses to light. J. Biol. Rhythm. 1992, 7, 353–359.
- Steinlechner, S.; Stieglitz, A.; Ruf, T. Djungarian hamsters: A species with a labile circadian pacemaker? Arrhythmicity under a light-dark cycle induced by short light pulses. J. Biol. Rhythm. 2002, 17, 248–258.
- Mrosovsky, N.; Salmon, P.A.; Menaker, M.; Ralph, M.R. Nonphotic phase shifting in hamster clock mutants. J. Biol. Rhythm. 1992, 7, 41–49.
- Youngstedt, S.D.; Elliott, J.A.; Kripke, D.F. Human circadian phase-response curves for exercise. J. Physiol. 2019, 597, 2253–2268.
- Deboer, T.; Vansteensel, M.J.; Detari, L.; Meijer, J.H. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat. Neurosci. 2003, 6, 1086–1090.
- Maywood, E.S.; Okamura, H.; Hastings, M.H. Opposing actions of neuropeptide Y and light on the expression of circadian clock genes in the mouse suprachiasmatic nuclei. Eur. J. Neurosci. 2002, 15, 216–220.
- Schaap, J.; Meijer, J.H. Opposing effects of behavioural activity and light on neurons of the suprachiasmatic nucleus. Eur. J. Neurosci. 2001, 13, 1955–1962.
- Song, Y.; Choi, G.; Jang, L.; Kim, S.-W.; Jung, K.-H.; Park, H. Circadian rhythm gene expression and daily melatonin levels vary in athletes and sedentary males. Biol. Rhythm Res. 2018, 49, 237–245.
- Hower, I.M.; Harper, S.A.; Buford, T.W. Circadian Rhythms, Exercise, and Cardiovascular Health. J. Circadian Rhythm. 2018, 16, 7.
- Rubio-Sastre, P.; Gómez-Abellán, P.; Martinez-Nicolas, A.; Ordovás, J.M.; Madrid, J.A.; Garaulet, M. Evening physical activity alters wrist temperature circadian rhythmicity. Chronobiol. Int. 2014, 31, 276–282.
- Duglan, D.; Lamia, K.A. Clocking In, Working Out: Circadian Regulation of Exercise Physiology. Trends Endocrinol. Metab. 2019, 30, 347–356.
- Thomas, J.M.; Kern, P.A.; Bush, H.M.; McQuerry, K.J.; Black, W.S.; Clasey, J.L.; Pendergast, J.S. Circadian rhythm phase shifts caused by timed exercise vary with chronotype. JCI Insight 2020, 5, e134270.
- Heden, T.D.; Kanaley, J.A. Syncing Exercise with Meals and Circadian Clocks. Exerc. Sport Sci. Rev. 2019, 47, 22–28.
- Lewis, P.; Korf, H.W.; Kuffer, L.; Groß, J.V.; Erren, T.C. Exercise time cues (zeitgebers) for human circadian systems can foster health and improve performance: A systematic review. BMJ Open Sport Exerc. Med. 2018, 4, e000443.
- Beyer, K.M.M.; Szabo, A.; Hoormann, K.; Stolley, M. Time spent outdoors, activity levels, and chronic disease among American adults. J. Behav. Med. 2018, 41, 494–503.
- Korman, M.; Tkachev, V.; Reis, C.; Komada, Y.; Kitamura, S.; Gubin, D.; Kumar, V.; Roenneberg, T. Outdoor daylight exposure and longer sleep promote wellbeing under COVID-19 mandated restrictions. J. Sleep Res. 2022, 31, e13471.
- Leise, T.L.; Harrington, M.E.; Molyneux, P.C.; Song, I.; Queenan, H.; Zimmerman, E.; Lall, G.S.; Biello, S.M. Voluntary exercise can strengthen the circadian system in aged mice. Age 2013, 35, 2137–2152.
- Power, A.; Hughes, A.T.; Samuels, R.E.; Piggins, H.D. Rhythm-promoting actions of exercise in mice with deficient neuropeptide signaling. J. Biol. Rhythm. 2010, 25, 235–246.




