
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Byung Kwan Park | -- | 1992 | 2022-09-22 09:36:45 | | | |
| 2 | Lindsay Dong | Meta information modification | 1992 | 2022-09-22 11:30:13 | | | | |
| 3 | Lindsay Dong | + 1 word(s) | 1993 | 2022-10-14 02:54:49 | | |
Video Upload Options
Renal artery stenosis (RAS) is one of the major causes of secondary hypertension and renal impairment. Ultrasound (US) is a noninvasive, real-time examination method for detecting RAS. The available US scanners enable the depiction of small vessels or organs. Gray-scale US can assess the morphology of the renal artery and kidney. Hemodynamic changes in the renal artery and kidney are evaluated with color and spectral Doppler US. Contrast-enhanced US may directly show the diameter change in the renal artery with intravascular contrast material that is not harmful to patients with poor renal function. Therefore, US is a useful examination method for detecting RAS, regardless of patient renal function.
1. Introduction
There are many imaging studies on detecting RAS with ultrasound (US) [1][2][3], computed tomography (CT) [4][5], magnetic resonance imaging (MRI) [4][5][6], digital subtraction angiography (DSA) [7][8][9], and angiotensin-converting enzyme inhibitor scintigraphy [10][11]. CT or MRI is preferred because radiologists are familiar with CT and MR angiography. However, these examinations require the use of intravascular contrast material for evaluating the diameter of the renal artery. Given that these patients frequently have decreased renal function, serious complications can be induced by the intravascular administration of iodine [12][13][14] or gadolinium contrast material [6][15][16].
| US Techniques and Accuracy | Renal Artery US | Renal US |
|---|---|---|
| Imaging techniques | More difficult | Less difficult |
| Scan time | Longer | Shorter |
| Breath hold | Unnecessary | Necessary |
| Bowel artifact | Frequent | Infrequent |
| Diagnostic performance | Higher | Lower |
2. Renal Artery US: Imaging Techniques
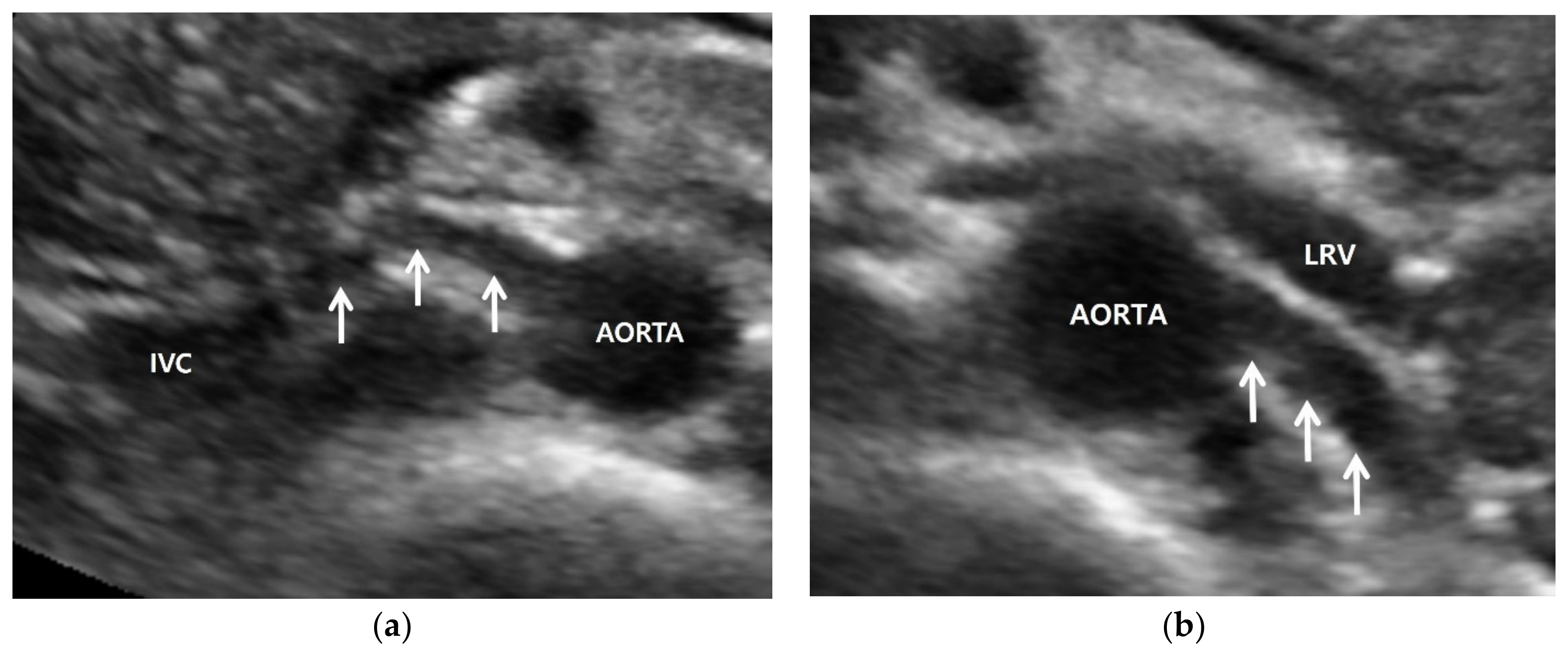
3. Renal Artery US: Imaging Features
3.1. Gray-Scale US
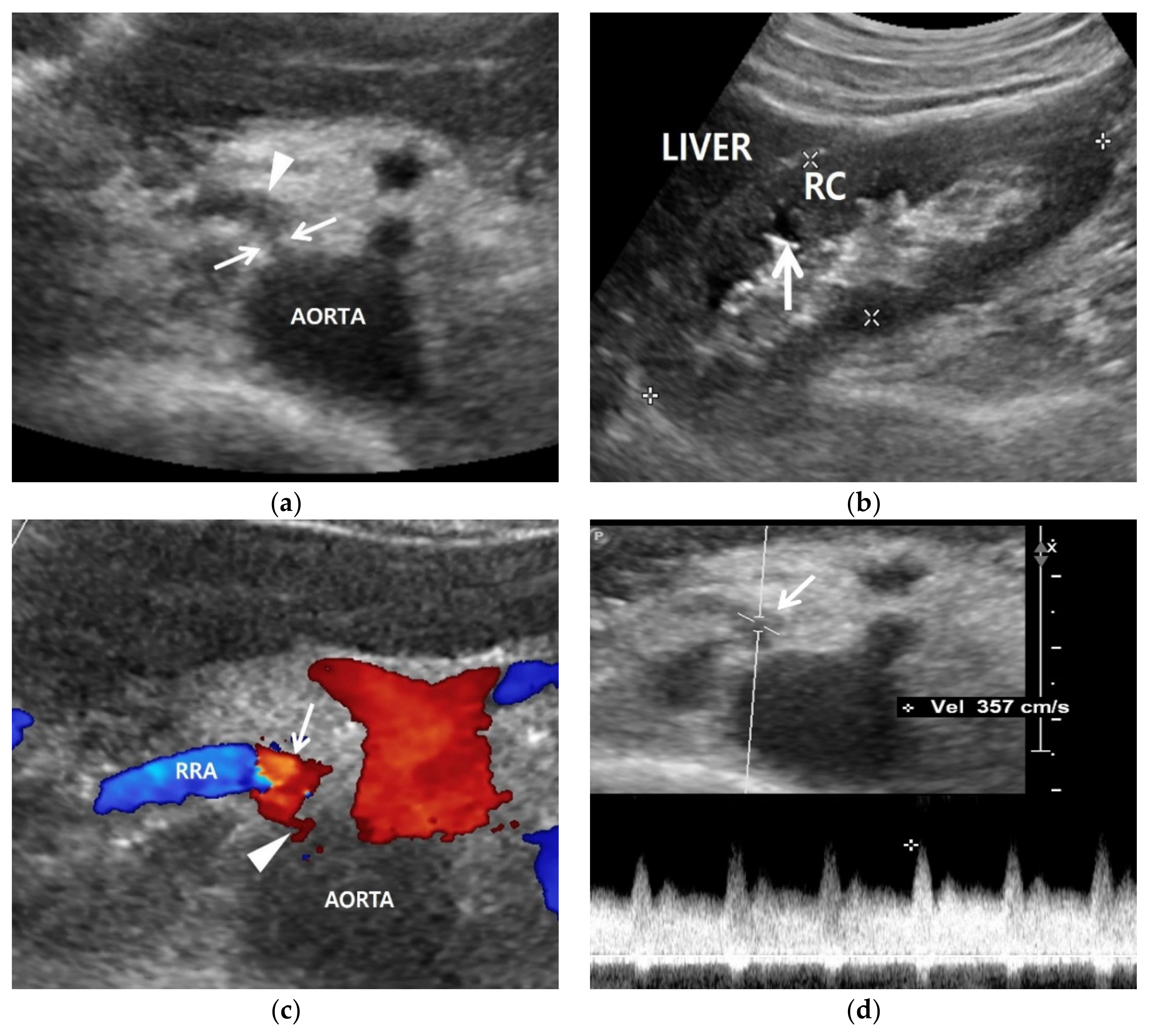
3.2. Color Doppler US
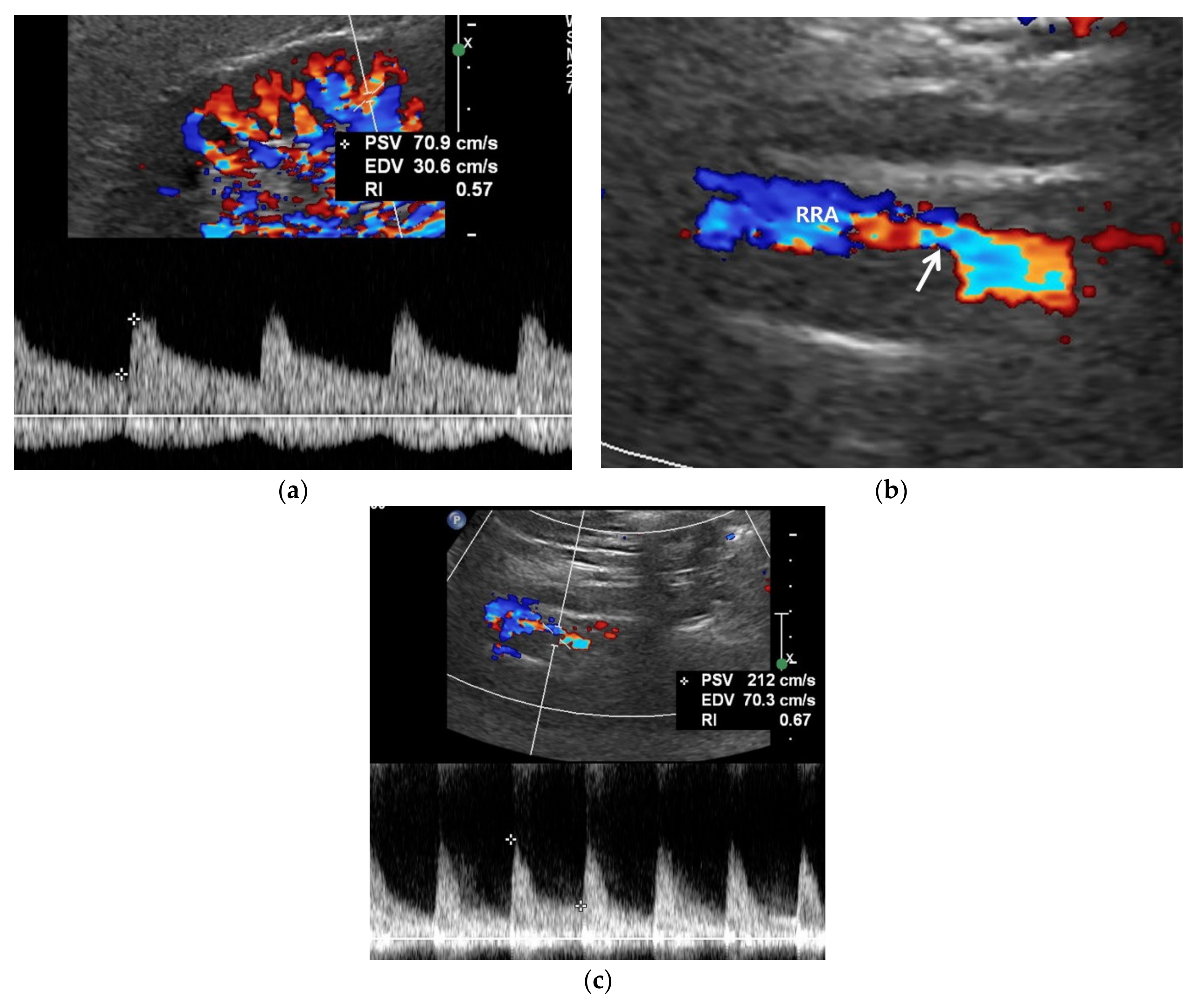
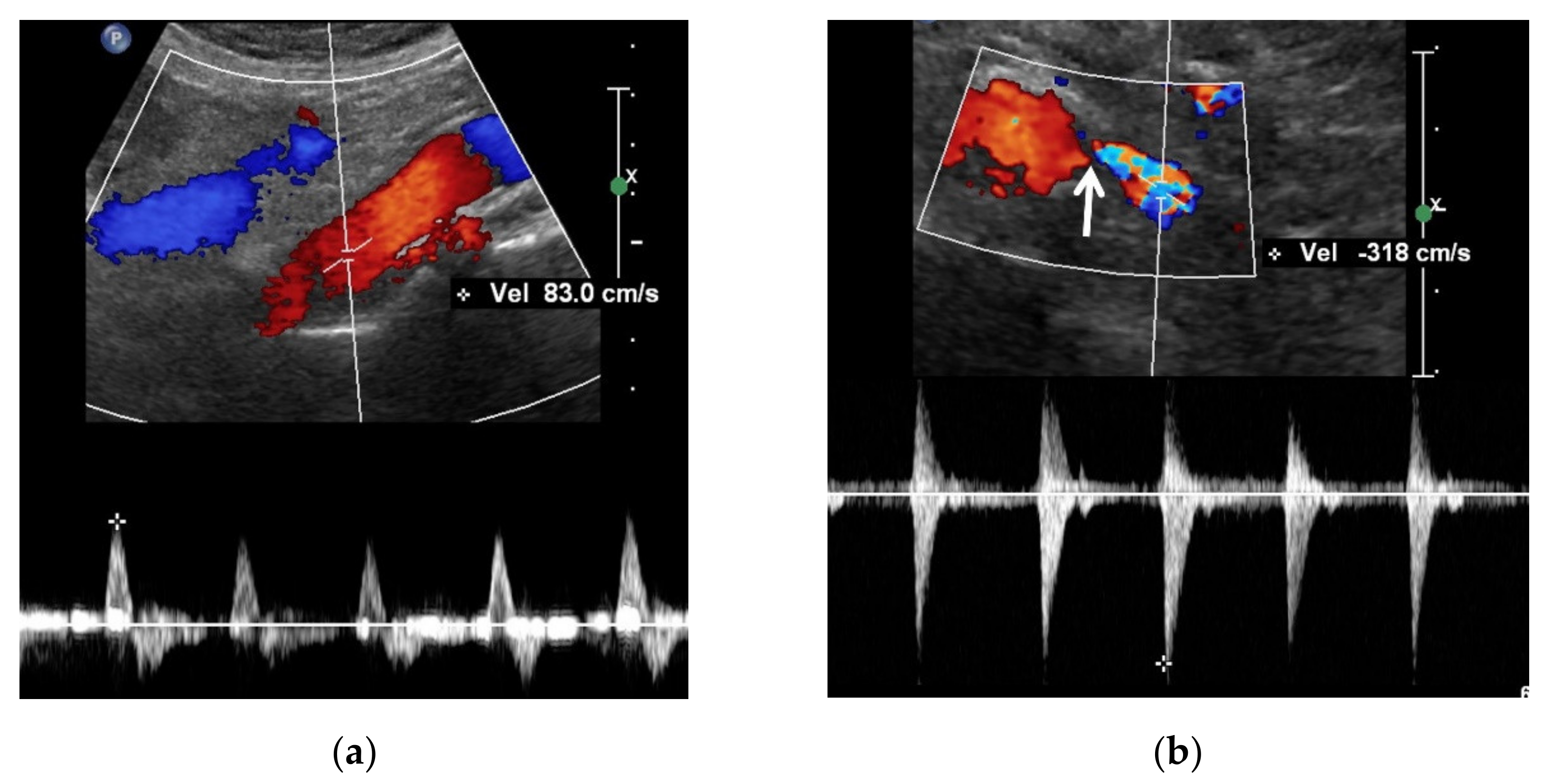
3.3. Spectral Doppler US
3.4. Contrast-Enhanced US
4. Diagnostic Steps for RAS
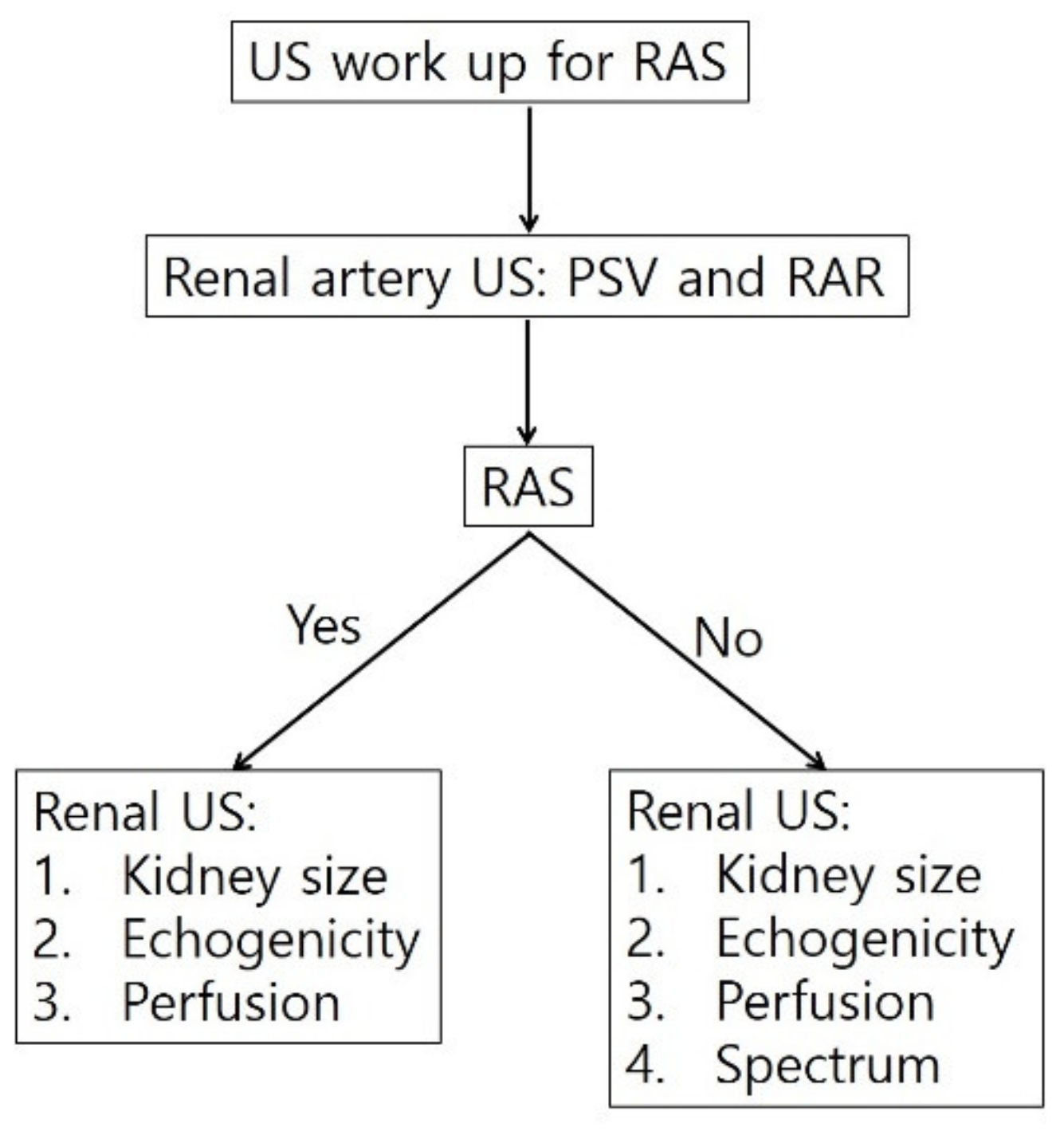
5. Conclusions
References
- Zierler, R.E. Is duplex scanning the best screening test for renal artery stenosis? Semin. Vasc. Surg. 2001, 14, 177–185.
- Olin, J.W.; Piedmonte, M.R.; Young, J.R.; DeAnna, S.; Grubb, M.; Childs, M.B. The utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosis. Ann. Intern. Med. 1995, 122, 833–838.
- Riehl, J.; Schmitt, H.; Bongartz, D.; Bergmann, D.; Sieberth, H.G. Renal artery stenosis: Evaluation with colour duplex ultrasonography. Nephrol. Dial. Transpl. 1997, 12, 1608–1614.
- Vasbinder, G.B.; Nelemans, P.J.; Kessels, A.G.; Kroon, A.A.; de Leeuw, P.W.; van Engelshoven, J.M. Diagnostic tests for renal artery stenosis in patients suspected of having renovascular hypertension: A meta-analysis. Ann. Intern. Med. 2001, 135, 401–411.
- Vasbinder, G.B.; Nelemans, P.J.; Kessels, A.G.; Kroon, A.A.; Maki, J.H.; Leiner, T.; Beek, F.J.; Korst, M.B.; Flobbe, K.; de Haan, M.W.; et al. Accuracy of computed tomographic angiography and magnetic resonance angiography for diagnosing renal artery stenosis. Ann. Intern. Med. 2004, 141, 674–682.
- Roditi, G.; Maki, J.H.; Oliveira, G.; Michaely, H.J. Renovascular imaging in the NSF Era. J. Magn. Reson. Imaging 2009, 30, 1323–1334.
- Kim, D.; Porter, D.H.; Brown, R.; Crivello, M.S.; Silva, P.; Leeming, B.W. Renal artery imaging: A prospective comparison of intra-arterial digital subtraction angiography with conventional angiography. Angiology 1991, 42, 345–357.
- Hawkins, I.F., Jr.; Wilcox, C.S.; Kerns, S.R.; Sabatelli, F.W. CO2 digital angiography: A safer contrast agent for renal vascular imaging? Am. J. Kidney Dis. 1994, 24, 685–694.
- Liss, P.; Eklof, H.; Hellberg, O.; Hagg, A.; Bostrom-Ardin, A.; Lofberg, A.M.; Olsson, U.; Orndahl, P.; Nilsson, H.; Hansell, P.; et al. Renal effects of CO2 and iodinated contrast media in patients undergoing renovascular intervention: A prospective, randomized study. J. Vasc. Interv. Radiol. 2005, 16, 57–65.
- Stratigis, S.; Stylianou, K.; Kyriazis, P.P.; Dermitzaki, E.K.; Lygerou, D.; Syngelaki, P.; Stratakis, S.; Koukouraki, S.; Parthenakis, F.; Tsetis, D.; et al. Renal artery stenting for atherosclerotic renal artery stenosis identified in patients with coronary artery disease: Does captopril renal scintigraphy predict outcomes? J. Clin. Hypertens. 2018, 20, 373–381.
- Qanadli, S.D.; Soulez, G.; Therasse, E.; Nicolet, V.; Turpin, S.; Froment, D.; Courteau, M.; Guertin, M.C.; Oliva, V.L. Detection of renal artery stenosis: Prospective comparison of captopril-enhanced Doppler sonography, captopril-enhanced scintigraphy, and MR angiography. Am. J. Roentgenol. 2001, 177, 1123–1129.
- Mehran, R.; Nikolsky, E. Contrast-induced nephropathy: Definition, epidemiology, and patients at risk. Kidney Int. 2006, 69, S11–S15.
- Morcos, S.K.; Thomsen, H.S.; Webb, J.A.; Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Contrast-media-induced nephrotoxicity: A consensus report. Eur. Radiol. 1999, 9, 1602–1613.
- Gleeson, T.G.; Bulugahapitiya, S. Contrast-Induced Nephropathy. Am. J. Roentgenol. 2004, 183, 1673–1689.
- Grobner, T.; Prischl, F.C. Gadolinium and nephrogenic systemic fibrosis. Kidney Int. 2007, 72, 260–264.
- Grobner, T. Gadolinium—A specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transpl. 2006, 21, 1104–1108.
- Granata, A.; Fiorini, F.; Andrulli, S.; Logias, F.; Gallieni, M.; Romano, G.; Sicurezza, E.; Fiore, C.E. Doppler ultrasound and renal artery stenosis: An overview. J. Ultrasound 2009, 12, 133–143.
- Schäberle, W.; Leyerer, L.; Schierling, W.; Pfister, K. Ultrasound diagnostics of renal artery stenosis: Stenosis criteria, CEUS and recurrent in-stent stenosis. Gefasschirurgie 2016, 21, 4–13.
- Park, B.K.; Kim, S.H.; Moon, M.H.; Jung, S.I. Imaging features of gray-scale and contrast-enhanced color Doppler US for the differentiation of transient renal arterial ischemia and arterial infarction. Korean J. Radiol. 2005, 6, 179–184.
- Bokhari, S.W.; Faxon, D.P. Current advances in the diagnosis and treatment of renal artery stenosis. Rev. Cardiovasc. Med. 2004, 5, 204–215.
- Radermacher, J.; Chavan, A.; Schäffer, J.; Stoess, B.; Vitzthum, A.; Kliem, V.; Rademaker, J.; Bleck, J.; Gebel, M.J.; Galanski, M.; et al. Detection of significant renal artery stenosis with color Doppler sonography: Combining extrarenal and intrarenal approaches to minimize technical failure. Clin. Nephrol. 2000, 53, 333–343.
- Hua, H.T.; Hood, D.B.; Jensen, C.C.; Hanks, S.E.; Weaver, F.A. The use of colorflow duplex scanning to detect significant renal artery stenosis. Ann. Vasc. Surg. 2000, 14, 118–124.
- Revzin, M.V.; Imanzadeh, A.; Menias, C.; Pourjabbar, S.; Mustafa, A.; Nezami, N.; Spektor, M.; Pellerito, J.S. Optimizing Image Quality When Evaluating Blood Flow at Doppler US: A Tutorial. RadioGraphics 2019, 39, 1501–1523.
- Hoffmann, U.; Edwards, J.M.; Carter, S.; Goldman, M.L.; Harley, J.D.; Zaccardi, M.J.; Strandness, D.E., Jr. Role of duplex scanning for the detection of atherosclerotic renal artery disease. Kidney Int. 1991, 39, 1232–1239.
- Williams, G.J.; Macaskill, P.; Chan, S.F.; Karplus, T.E.; Yung, W.; Hodson, E.M.; Craig, J.C. Comparative accuracy of renal duplex sonographic parameters in the diagnosis of renal artery stenosis: Paired and unpaired analysis. Am. J. Roentgenol. 2007, 188, 798–811.
- Aytac, S.K.; Yigit, H.; Sancak, T.; Ozcan, H. Correlation between the diameter of the main renal artery and the presence of an accessory renal artery: Sonographic and angiographic evaluation. J. Ultrasound Med. 2003, 22, 433–439.
- Bude, R.O.; Forauer, A.R.; Caoili, E.M.; Nghiem, H.V. Is it necessary to study accessory arteries when screening the renal arteries for renovascular hypertension? Radiology 2003, 226, 411–416.
- Labropoulos, N.; Ayuste, B.; Leon, L.R., Jr. Renovascular disease among patients referred for renal duplex ultrasonography. J. Vasc. Surg. 2007, 46, 731–737.
- Zierler, R.E.; Bergelin, R.O.; Davidson, R.C.; Cantwell-Gab, K.; Polissar, N.L.; Strandness, D.E., Jr. A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am. J. Hypertens. 1996, 9, 1055–1061.
- Morel, D.R.; Schwieger, I.; Hohn, L.; Terrettaz, J.; Llull, J.B.; Cornioley, Y.A.; Schneider, M. Human Pharmacokinetics and Safety Evaluation of SonoVue™, a New Contrast Agent for Ultrasound Imaging. Investig. Radiol. 2000, 35, 80.
- Park, B.K.; Kim, S.H.; Choi, H.J. Characterization of renal cell carcinoma using agent detection imaging: Comparison with gray-scale US. Korean J. Radiol. 2005, 6, 173–178.
- Park, B.K.; Kim, B.; Kim, S.H.; Ko, K.; Lee, H.M.; Choi, H.Y. Assessment of cystic renal masses based on Bosniak classification: Comparison of CT and contrast-enhanced US. Eur. J. Radiol. 2007, 61, 310–314.
- Gerst, S.; Hann, L.E.; Li, D.; Gonen, M.; Tickoo, S.; Sohn, M.J.; Russo, P. Evaluation of renal masses with contrast-enhanced ultrasound: Initial experience. Am. J. Roentgenol. 2011, 197, 897–906.
- Ignee, A.; Straub, B.; Schuessler, G.; Dietrich, C.F. Contrast enhanced ultrasound of renal masses. World J. Radiol. 2010, 2, 15–31.
- Barr, R.G. Use of lumason/sonovue in contrast-enhanced ultrasound of the kidney for characterization of renal masses-a meta-analysis. Abdom. Radiol. 2021, 47, 272–287.
- Tenant, S.C.; Gutteridge, C.M. The clinical use of contrast-enhanced ultrasound in the kidney. Ultrasound 2016, 24, 94–103.
- Dong, Y.; Wang, W.P.; Cao, J.; Fan, P.; Lin, X. Early assessment of chronic kidney dysfunction using contrast-enhanced ultrasound: A pilot study. Br. J. Radiol. 2014, 87, 20140350.
- Ma, F.; Cang, Y.; Zhao, B.; Liu, Y.; Wang, C.; Liu, B.; Wu, T.; Song, Y.; Peng, A. Contrast-enhanced ultrasound with SonoVue could accurately assess the renal microvascular perfusion in diabetic kidney damage. Nephrol. Dial. Transplant. 2012, 27, 2891–2898.
- Xu, Y.; Li, H.; Wang, C.; Zhang, M.; Wang, Q.; Xie, Y.; Shao, X.; Tian, L.; Yuan, Y.; Yan, W.; et al. Improving Prognostic and Chronicity Evaluation of Chronic Kidney Disease with Contrast-Enhanced Ultrasound Index-Derived Peak Intensity. Ultrasound Med. Biol. 2020, 46, 2945–2955.
- Ran, X.; Lin, L.; Yang, M.; Niu, G.; Chen, L.; Shao, Y.; Zou, Y.; Wang, B. Contrast-Enhanced Ultrasound Evaluation of Renal Blood Perfusion Changes after Percutaneous Transluminal Renal Angioplasty and Stenting for Severe Atherosclerotic Renal Artery Stenosis. Ultrasound Med. Biol. 2020, 46, 1872–1879.





