Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tae-Don Kim | -- | 2589 | 2022-09-21 10:08:11 | | | |
| 2 | Rita Xu | Meta information modification | 2589 | 2022-09-21 10:25:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, C.; Kong, L.; Kim, S.; Lee, S.; Oh, S.; Jo, S.; Jang, I.; Kim, T. IL-7 and IL-7R in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/27423 (accessed on 08 February 2026).
Wang C, Kong L, Kim S, Lee S, Oh S, Jo S, et al. IL-7 and IL-7R in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/27423. Accessed February 08, 2026.
Wang, Chunli, Lingzu Kong, Seokmin Kim, Sunyoung Lee, Sechan Oh, Seona Jo, Inhwan Jang, Tae-Don Kim. "IL-7 and IL-7R in Cancer" Encyclopedia, https://encyclopedia.pub/entry/27423 (accessed February 08, 2026).
Wang, C., Kong, L., Kim, S., Lee, S., Oh, S., Jo, S., Jang, I., & Kim, T. (2022, September 21). IL-7 and IL-7R in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/27423
Wang, Chunli, et al. "IL-7 and IL-7R in Cancer." Encyclopedia. Web. 21 September, 2022.
Copy Citation
Interleukin-7 (IL-7) is a multipotent cytokine that maintains the homeostasis of the immune system. IL-7 plays a vital role in T-cell development, proliferation, and differentiation, as well as in B cell maturation through the activation of the IL-7 receptor (IL-7R). IL-7 is closely associated with tumor development and has been used in cancer clinical research and therapy.
IL-7
IL-7R signal
cancer
immunotherapy
1. Introduction
Interleukin 7 (IL-7) is a cytokine necessary for the adaptive immune system, which is critical for B cell development [1][2][3] as well as proliferation and survival of memory and naive T cells, and T cell development in the thymus [4]. IL-7 performs its biological functions mainly through the activation of the IL-7 receptor (IL-7R) [5]. IL-7R is a heterodimer that is composed of the IL-7R α chain (CD127) and the common γ chain (CD132, IL-2Rγ) shared by multiple cytokines such as IL-2, IL-7, IL-4, IL-9, IL-15, and IL-21 [1]. IL-7 and IL-7Rα promote cell survival and inhibit cell apoptosis mainly by the activation of Janus kinase (JAK), signal transduction factor and transcription activator 5 (STAT5), and the phosphatidylinositol 3-kinase (PI3K)—protein kinase B (AKT)-mediated signal pathway [6][7][8]. IL-7 has strong immunomodulatory effects, which can directly or indirectly act on tumor cells and exert anti-tumor effects by enhancing tumor eradication or adoptive immunity [9]. Conversely, IL-7 also has potential pro-tumor effects via the activation of downstream JAK/STAT5 and PI3K–AKT pathways [10][11][12]. IL-7 is closely associated with tumor development and has been used in clinical research and treatment [5][13].
2. Biology and Functions of IL-7 and IL-7R
2.1. Biology and Functions of IL-7
IL-7 is widely expressed in many tissues, including lymphoid organs such as the bone marrow, thymus, lymph nodes, and spleen, as well as in non-lymphoid sites such as the skin, lung, intestine, and liver [14][15]. However, IL-7 is predominantly secreted by the bone marrow, thymus, and lymph nodes to maintain the body’s immune self-stability [5]. The human IL-7 gene, located on chromosome 8, has 534 bp, contains 6 exons and 5 introns, and encodes a protein of 177 amino acids with a molecular weight of approximately 20 kDa. The active form of IL-7 encodes a 25 kDa, single-chain glycoprotein that is predicted to form a structure containing four α-helices with a hydrophobic core [2].
IL-7 can promote early B cell proliferation in mice, in vitro, and can also promote the growth of precursor B cells [16][17]. IL-7 knockout mice showed developmental retardation of bone marrow, inability to convert from pro-B cells to pre-B cells, lack of mature T cells and B cells, and a 20-fold reduction in thymic cells, indicating that IL-7 plays a crucial role in the development and maturation of the bone marrow, and the central T and B cells of the thymus [18]. Moreover, IL-7 could increase the viability of naive T cells in the absence of antigenic stimulation, suggesting that it plays an essential role in protecting the naive T cell repertoire [19][20][21]. It also plays a vital role in increasing memory T cell survival and expansion [22][23]. The deficiency of IL-7 and its receptor affects the development of B cells, T cells, natural killer (NK) cells, monocytes, macrophages, dendritic cells, and innate lymphoid cells, indicating that IL-7 plays crucial regulatory roles in the entire immune system [24].
2.2. Biology and Functions of IL-7Rα
IL-7 function is mediated by the IL-7R, a heterodimer consisting of the IL-7R α chain (CD127) and a common γ chain (CD132, IL-2Rγ). The human IL-7Rα gene, located on chromosome 5, contains 1380 bp, includes 8 exons and 7 introns, and encodes for a protein of 459 amino acids with a molecular weight of approximately 49.5 kDa [2]. IL-7Rα is expressed in hematopoietic cells, particularly the lymphoid lineage, including fetal NK/dendritic precursors, mature T cells and bone marrow macrophages, and developing T cells and B cells. Human marrow stromal cells [25], endothelial cells [26], normal human intestinal epithelial cells, and several malignant tumor cell lines containing breast cancer, melanoma, leukemia, lung cancer and cutaneous T cell lymphoma [27][28][29][30] were all found to express IL-7Rα. IL-7Rα has two forms, membrane-bound IL-7Rα and soluble IL-7Rα (sIL-7Rα), with different biological functions [31][32]. sIL-7Rα competes with membrane IL-7R to reduce excessive IL-7 consumption and antagonizes IL-7 signaling, hence enhancing the biological activity of IL-7 when cytokines are restricted [33]. In addition, sIL-7R directly bind to IL-2Rγ on membrane surface and inhibit IL-7 signaling in IL-2Rγ-positive cells [34]. Previous studies found that sIL-7Rα aggravate autoimmune diseases [33][35][36]. However, sIL-7Rα concentrations were demonstrated to be significantly enhanced in the serum of HIV-positive patients, and high concentrations of sIL-7Rα inhibit IL-7-mediated CD8+ T cell proliferation, indicating that sIL-7Rα may play dual regulatory roles in vivo [33][37][38]. Membrane-bound IL-7Rα promotes cell growth and proliferation, and it inhibits apoptosis by regulating the IL-7 signaling pathway [37]. During this process, IL-7 first binds to IL-7Rα and then recruits IL-2Rγ to form a ternary signaling complex [39], which activates two main downstream signaling pathways, the JAK/STAT5 and the PI3K–AKT signal pathways [6][7][8]. Furthermore, IL-7 also induces the activation of mitogen-activated protein kinases (MAPK) pathway [40] (Figure 1).
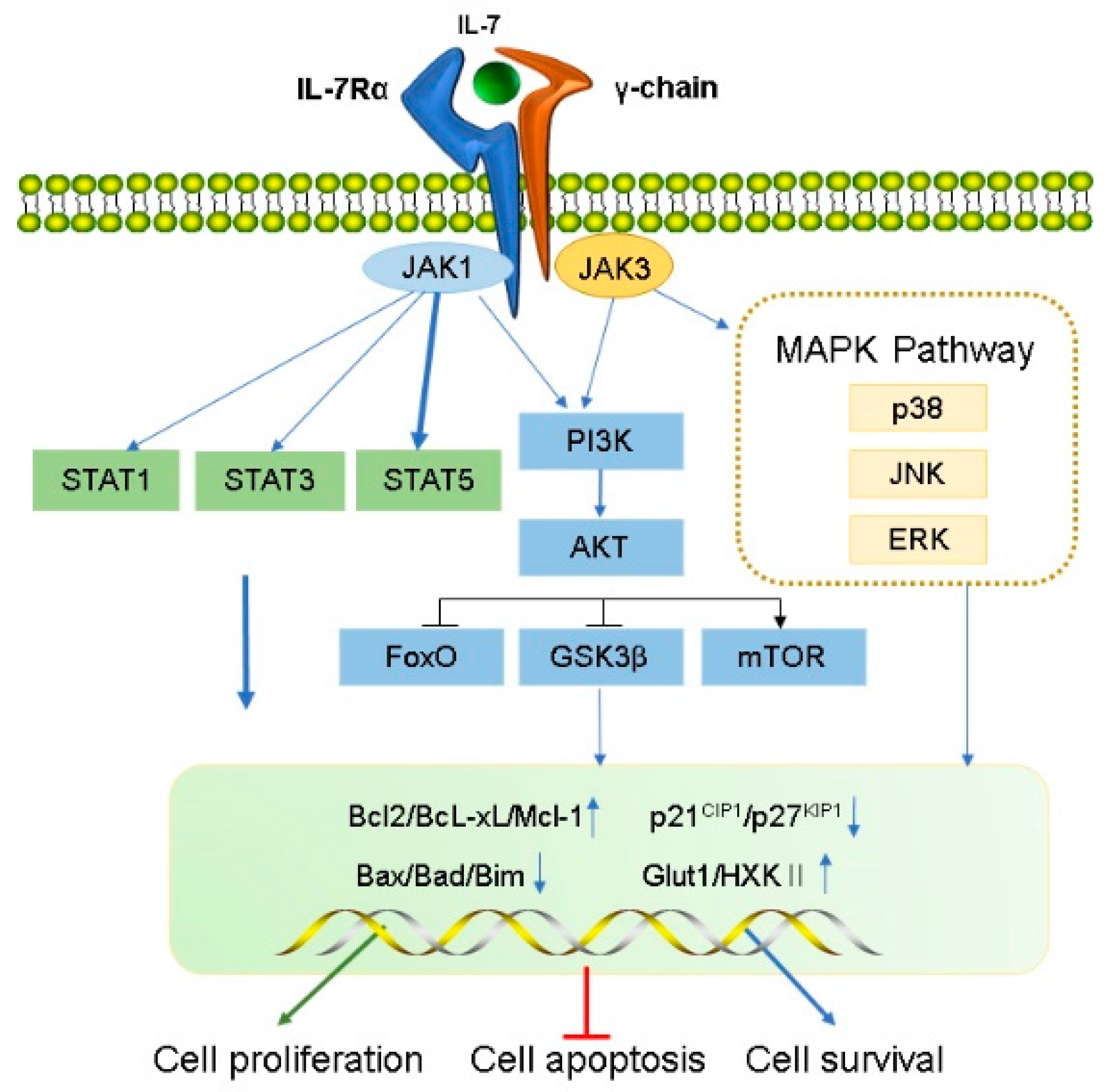
Figure 1. Transduction of IL-7 signaling pathway. IL-7 induces the activation of IL-7R downstream signaling pathway kinases, including JAK1 (linked to IL-7Rα) and JAK3 (linked to common γC), STAT1, STAT3, STAT5, PI3K, AKT, and MAPK. IL-7 signal transduction promotes cell proliferation and survival and inhibits apoptosis by regulating gene expression levels in the nucleus, including a decrease in pro-apoptotic factors (such as Bad and Bax) and cell cycle inhibitors (p21CIP1 and p27KIP1) and an increase in anti-apoptotic factors (such as Bcl-XL, Bcl-2, and McL-1) and glucose metabolism regulators (Glut1, HXKⅡ).
2.2.1. JAK/STAT5 Signaling Pathway
IL-7 binds to IL-7Rα, triggering activation of the IL-7Rα-associated tyrosine kinase, JAK 1 (linked to IL-7Rα), and JAK3 (linked to common γC). The activated JAK protein phosphorylates a specific motif on the IL-7Rα chain to form a binding site for STAT5 (a signaling molecule containing Src homologous 2 (SH2) domains), and then binds and phosphorylates STAT5, which forms a dimer and enters the nucleus.
During this process, a series of genes that modulate cell growth and survival in the nucleus is affected, as well as other pathways such as PI3K–AKT and MEK/extracellular signal-regulated kinase (ERK) are activated. For example, anti-apoptotic proteins Bcl-XL, Bcl-2, McL-1 belonging to the bcl-2 protein family are up-regulated, and pro-apoptotic proteins (BAX, BAD) are down-regulated, which improve the survival of T cells in vivo [41]. IL-7 signaling can maintain survival of memory CD8 T cells by mediating STAT5 and STAT3 activation [42]. However, overexpression of Bcl-2 and Bcl-XL did not prevent effector cell death during lymphocytic choriomeningitis virus infection [43], suggesting that activation of other signaling pathways downstream of IL-7R are crucial for maintaining the survival of memory cell precursors. Correspondingly, basal levels of IL-7 can also regulate the number of memory CD8 T cells formed [44]. Furthermore, IL-7 mediates activation of STAT5 and is necessary for T cell proliferation [45], differentiation [46] and survival [47][48]. It also regulates T cell cytotoxicity [47] and drug resistance [49][50]. Additionally, IL-7 not only leads to IL-7-dependent activation of STAT1 and STAT5 in the presence of lymphopenia, but also enhances T cell response to type-I IFN by regulating STAT1 protein expression level [51][52]. In addition, STAT1 overexpression was related to reduce survival in CD4+ T cells undergoing lymphocytopenia-induced proliferation [52]. These results suggest that STAT1 is involved in the process by which IL-7 regulates T cell survival. IL-7 also activates STAT1 and STAT3 which promote B cell precursor acute lymphoblastic leukemia proliferation [53] and survival of B cell progenitors [54], respectively. Furthermore, The JAK/STAT pathway not only activates the family of cytokine signaling inhibitor proteins (SOCS) but can also be inhibited by them to form a negative feedback loop [55][56]. SOCS proteins inhibit cytokine signaling either by competing with STAT5 to inhibit JAK [57] or by proteasomal degradation of targeted signaling proteins [58][59][60].
2.2.2. PI3K/AKT/mTOR Signaling Pathway
Activated IL-7Rα stimulates JAK1/3, and then phosphorylates the P85 subunit of PI3K to activate PI3K and produces the second messenger phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) on the plasma membrane. PIP3 binds to the signaling proteins AKT and (3-phosphoinositide-dependent protein kinase 1) PDK1 (containing Pleckstrin homology domain) and then promotes PDK1 to phosphorylate Ser308 of the AKT protein, thereby activating AKT. IL-7/IL-7R pathways mediate the main downstream targets of AKT such as glycogen synthase kinase (GSK, inhibited), forkhead box O (FoxO, inhibited), and mammalian target of rapamycin (mTOR, activated) [61]. AKT phosphorylates tuberous sclerosis complex 1/2 and prevents negative regulation of small GTP-binding proteins Rheb, resulting in enrichment of Rheb and activation of the mTOR complex (mTORC1) which promotes cell survival and proliferation by inhibiting Bad, Bim, Bax, p21CIP1 and p27KIP1 and activating Cdk2 [62][63]. Additionally, IL-7 increases the expression of glucose transporter 1 (Glut1) and glycolytic enzyme hexokinase II (HXKII), thereby increasing glucose uptake [64][65] and regulating glucose utilization depending on the PI3K/AKT signaling pathway [66]. IL-7 mediates the proliferation and activation of T cells in mice and is attenuated by PI3K inhibitors [67]. Furthermore, PI3K/AKT pathway is inhibited by PTEN; inhibitors of this pathway are critical for pro-B cell development [68]. IL-7 may promote adipose-derived stem cell differentiation by increasing AKT phosphorylation [69]. Therefore, the PI3K/AKT pathway is essential for powerful IL-7 signal transduction in the cell cycle.
2.2.3. MAPK Pathway
Early studies have shown that IL-7 activates MAPK, containing p38 kinase, c-Jun N-terminal kinase (JNK), and ERK [40]. IL-7-induced cell proliferation could be mediated by the inhibition of the downstream effector MAPK-activated proteinase 2, further verifying that IL-7 activates this pathway [70]. Specific P38 inhibitors inhibit IL-7-induced T cell proliferation, suggesting that the P38 MAPK pathway plays a vital role in IL-7 signal transduction [70]. Additionally, IL-7 withdrawal blocks the activation of P38 and JNK kinases, leading to IL-7-dependent thymocyte death [71]. IL-7 rescues rapamycin-induced apoptosis of B-cell precursor acute lymphoblastic leukemia-acute lymphoblastic leukemia (ALL) cells by upregulating MEK/ERK [72]. Hence, the MAPK pathway may play a vital role in regulating cell development via IL-7-mediated signal transduction.
3. Effects of IL-7 and IL-7Rα in Cancer
3.1. Anti-Tumor Effects of IL-7 and IL-7Rα
IL-7 has a powerful immunomodulatory effect, which can directly or indirectly act on tumor cells and exert anti-tumor effects by enhancing tumor eradication or adaptive immunity. The expression levels of IL-7 and IL-7Rα are important for normal T cell development and sustaining the homeostasis of the immune system [18][73][74]. IL-7 enhances the cytotoxicity of NK, NKT, lymphokine-activated killer (LAK) cells, monocytes, and cancer-specific cytotoxic T lymphocytes (CTLs). It induces CTL to secrete perforin in a STAT5-dependent manner [47] and stimulates the expression of interferon-gamma (IFN-γ), mitogen-inducible gene (MIG), IL-12, and IFN-γ-induced protein 10 (IP-10) [75][76]. IL-7 can also increase the cytolytic functions of NK cells [77] and CTL [78] by increasing FasL mRNA and protein expression in the membrane. Furthermore, IL-7 increases the amount of CD4+, CD8+ T, cells and CD19+ B cells to promote antibody-dependent cell-mediated cytotoxicity; moreover, it also enhances the response of antigen-specific CD8+ T cells [79] and improve the recovery of CD4+ T cells after chemotherapy in solid tumors [80]. IL-7 inhibits melanoma growth by promoting the secretion of the cytokines IL-1β, IL-1α, and tumor necrosis factor-α (TNF-α) from monocytes [81]. IL-7 enhances the antitumor effect of IFN-γ in rat gliomas [9]. IL-7 restores the activity of CD8+ T cells by decreasing the expression of exhaustion marker PD- 1 [82][83]. Some tumors secrete TGF-β, which inhibits the proliferation of CD8+ T cells via SMAD proteins. IL-7 can reverse this inhibition by inducing the expression of SMAD ubiquitination regulatory factor 2 (SMURRF2) [82][83] (Figure 2).
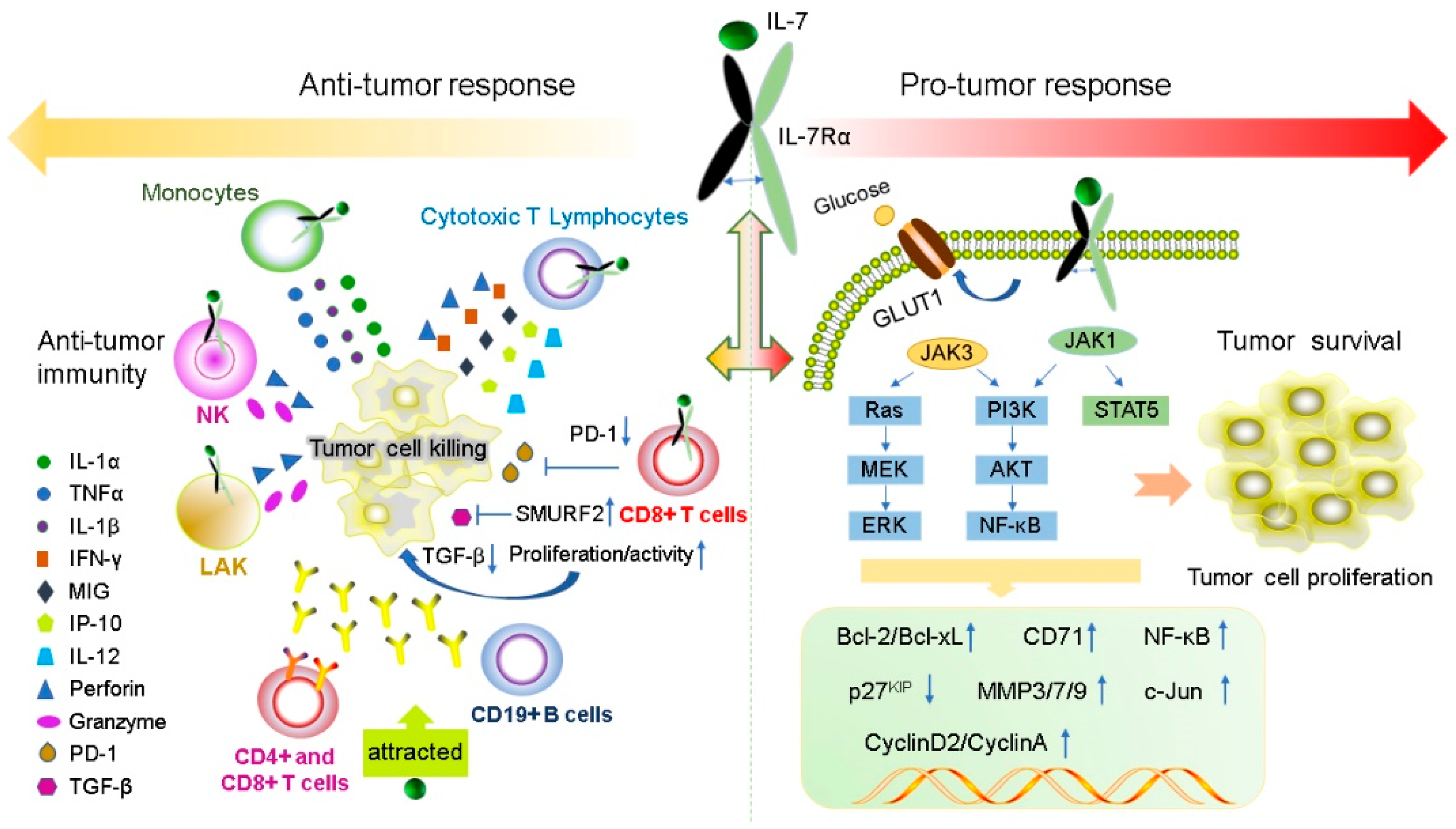
Figure 2. IL-7 and IL-7R have both pro- and anti-tumor functions. IL-7 plays anti-tumor roles by regulating immune cells to release cytokines such as IFN-γ, IL-1β, IL-1α and TNF-α. In contrast, IL-7 can promote the proliferation and survival of tumor cells by binding to the IL-7R to active JAK/STAT5, the PI3K/AKT and Ras/ERK signaling pathways to regulate gene expression levels of Bcl-2, Bcl-XL, CyclinA, CyclinD2, and p27kip.
Recent studies suggest that IL-7Rα may be a beneficial prognostic marker for patients with lung adenocarcinoma (LUAD). Survival analysis showed that IL-7Rα expression is an independent prognostic factor for LUAD. IL-7Rα expression is positively correlated to the overall and progression-free survival in patients with LUAD, and negatively correlated to tumor size. IL-7Rα inhibits the growth of tumor cells by affecting the percentage of infiltrating cells in the tumor immune microenvironment. Thus, IL-7Rα may also be a possible therapeutic target for LUAD [84].
3.2. Pro-Tumor Effects of IL-7 and IL-7Rα
The expression levels of IL-7 and IL-7Rα are important for normal T cell development and preservation of homeostasis in the immune system [18][73][74]. IL-7 and IL-7Rα have bidirectional regulatory effects on tumors. IL-7 transgenic mice induced T cell dysplasia, characterized by decreased CD4+ CD8+ (double-positive) thymocytes and lymphoproliferative diseases such as B and T cell lymphoma [85]. Xenotransplant models of human T-ALL have shown that IL-7 promotes the formation of human T-ALL, providing a new method for the treatment of T-ALL by targeting IL-7/IL-7R signal transduction [86]. Moreover, during normal T-cell development, IL-7 exerts as an anti-apoptotic factor by upregulating the of Bcl-2 expression [24]. A similar situation appears in T-ALL cells, IL-7 not only upregulates the expression of Bcl-2 and down-regulates the cyclin-dependent kinase inhibitor p27kip1 in T-ALL cells to avoid apoptosis, but also leads to continuing reaction of cyclin D2 and cyclin A during cell cycle progression [87][88]. All gamma-cytokines promote the proliferation of primary T-ALL cells, and IL-7 is the most potent cytokine that induces the proliferation of leukemia cells [89]. IL-7 mainly affects the proliferation and apoptosis of T-ALL cells by activating the JAK/STAT5 and PI3K/Akt/mTOR signaling pathways, leading to upregulation of transferrin receptor CD71, glucose transporter Glut1, glucose uptake and mitochondrial integrity [50][55][66][90][91]. In addition to its role in T-ALL formation, IL-7 also affects the invasion and growth of other tumor cells. For instance, expression of IL-7 is closely correlated with poor prognosis in prostate cancer (PCa) [92]. The IL-7/IL-7R pathway promotes the invasion and migration of PCa cells by activating the AKT/NF-κB pathway and regulating the expression of metalloproteinases (MMP 3 and 7), and it promotes the invasion and migration of bladder cancer cells via NF-KB-mediated upregulation of MMP-9 expression [12][93]. Furthermore, IL-7 can also induce the upregulation of cyclin D1 by modulating the c-FOS/c-Jun pathway, thereby promoting the proliferation of lung cancer cells [94]. Therefore, these studies also demonstrated the potential of IL-7 to promote cancer development.
Previous studies have shown that gain-of-function in IL-7R plays a key role in the generation of human T-ALL [95] and specific mutations in IL-7R specifically enhances steroid-resistance in T-ALL. Steroid resistance occurs due to mutations in IL-7R or other signaling molecules in this pathway which activate downstream MEK-ERK and AKT components, thereby upregulating the expression of MC1 and Bcl-XL, leading to a strong anti-apoptotic response. In addition, MEK-ERK and AKT signaling pathways also inhibit BIM, which is an important steroid-induced cell death molecule, and GSK3B, which is an important regulator of pro-apoptotic BIM. However, IL-7R signaling inhibitors can restore steroid resistance [96]. In addition, the abnormal expression of wild-type IL-7R can lead to the occurrence of disease and even carcinogenesis. Insufficient IL-7R expression due to IL-7R gene polymorphism results in decreased T cells numbers; however, B cells numbers remain unaffected [97]. Overexpression of IL-7R also leads to potential thymocyte self-renewal and thymic hyperplasia related to proliferation of T cell precursors, which in turn infiltrates the lymph nodes, spleen, and bone marrow, ultimately resulting in fatal leukemia in a dose-dependent manner [98] (Figure 2).
References
- Mazzucchelli, R.; Durum, S.K. Interleukin-7 receptor expression: Intelligent design. Nat. Rev. Immunol. 2007, 7, 144–154.
- Jiang, Q.; Li, W.Q.; Aiello, F.B.; Mazzucchelli, R.; Asefa, B.; Khaled, A.R.; Durum, S.K. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005, 16, 513–533.
- Parrish, Y.K.; Baez, I.; Milford, T.A.; Benitez, A.; Galloway, N.; Rogerio, J.W.; Sahakian, E.; Kagoda, M.; Huang, G.; Hao, Q.L.; et al. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J. Immunol. 2009, 182, 4255–4266.
- Fry, T.J.; Mackall, C.L. The many faces of IL-7: From lymphopoiesis to peripheral T cell maintenance. J. Immunol. 2005, 174, 6571–6576.
- Barata, J.T.; Durum, S.K.; Seddon, B. Flip the coin: IL-7 and IL-7R in health and disease. Nat. Immunol. 2019, 20, 1584–1593.
- Khaled, A.R.; Durum, S.K. Lymphocide: Cytokines and the control of lymphoid homeostasis. Nat. Rev. Immunol. 2002, 2, 817–830.
- Kittipatarin, C.; Khaled, A.R. Interlinking interleukin-7. Cytokine 2007, 39, 75–83.
- Swainson, L.; Kinet, S.; Mongellaz, C.; Sourisseau, M.; Henriques, T.; Taylor, N. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood 2007, 109, 1034–1042.
- Fritzell, S.; Eberstal, S.; Sanden, E.; Visse, E.; Darabi, A.; Siesjo, P. IFNgamma in combination with IL-7 enhances immunotherapy in two rat glioma models. J. Neuroimmunol. 2013, 258, 91–95.
- Zenatti, P.P.; Ribeiro, D.; Li, W.; Zuurbier, L.; Silva, M.C.; Paganin, M.; Tritapoe, J.; Hixon, J.A.; Silveira, A.B.; Cardoso, B.A.; et al. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nat. Genet. 2011, 43, 932–939.
- Liu, Z.H.; Wang, M.H.; Ren, H.J.; Qu, W.; Sun, L.M.; Zhang, Q.F.; Qiu, X.S.; Wang, E.H. Interleukin 7 signaling prevents apoptosis by regulating bcl-2 and bax via the p53 pathway in human non-small cell lung cancer cells. Int. J. Clin. Exp. Pathol. 2014, 7, 870–881.
- Park, S.L.; Lee, E.J.; Kim, W.J.; Moon, S.K. p27KIP1 is involved in ERK1/2-mediated MMP-9 expression via the activation of NF-kappaB binding in the IL-7-induced migration and invasion of 5637 cells. Int. J. Oncol. 2014, 44, 1349–1356.
- Nakao, S.; Arai, Y.; Tasaki, M.; Yamashita, M.; Murakami, R.; Kawase, T.; Amino, N.; Nakatake, M.; Kurosaki, H.; Mori, M.; et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci. Transl. Med. 2020, 12, 526.
- Kim, G.Y.; Hong, C.; Park, J.H. Seeing is believing: Illuminating the source of in vivo interleukin-7. Immune Netw. 2011, 11, 1–10.
- Sawa, Y.; Arima, Y.; Ogura, H.; Kitabayashi, C.; Jiang, J.J.; Fukushima, T.; Kamimura, D.; Hirano, T.; Murakami, M. Hepatic interleukin-7 expression regulates T cell responses. Immunity 2009, 30, 447–457.
- Namen, A.E.; Schmierer, A.E.; March, C.J.; Overell, R.W.; Park, L.S.; Urdal, D.L.; Mochizuki, D.Y. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J. Exp. Med. 1988, 167, 988–1002.
- Namen, A.E.; Lupton, S.; Hjerrild, K.; Wignall, J.; Mochizuki, D.Y.; Schmierer, A.; Mosley, B.; March, C.J.; Urdal, D.; Gillis, S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature 1988, 333, 571–573.
- von Freeden-Jeffry, U.; Vieira, P.; Lucian, L.A.; McNeil, T.; Burdach, S.E.; Murray, R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 1995, 181, 1519–1526.
- Soares, M.V.; Borthwick, N.J.; Maini, M.K.; Janossy, G.; Salmon, M.; Akbar, A.N. IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J. Immunol. 1998, 161, 5909–5917.
- Webb, L.M.; Foxwell, B.M.; Feldmann, M. Putative role for interleukin-7 in the maintenance of the recirculating naive CD4+ T-cell pool. Immunology 1999, 98, 400–405.
- Brenchley, J.M.; Douek, D.C.; Ambrozak, D.R.; Chatterji, M.; Betts, M.R.; Davis, L.S.; Koup, R.A. Expansion of activated human naive T-cells precedes effector function. Clin. Exp. Immunol. 2002, 130, 432–440.
- Bradley, L.M.; Haynes, L.; Swain, S.L. IL-7: Maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005, 26, 172–176.
- Li, J.; Huston, G.; Swain, S.L. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 2003, 198, 1807–1815.
- Chen, D.; Tang, T.X.; Deng, H.; Yang, X.P.; Tang, Z.H. Interleukin-7 Biology and Its Effects on Immune Cells: Mediator of Generation, Differentiation, Survival, and Homeostasis. Front. Immunol. 2021, 12, 747324.
- Iwata, M.; Graf, L.; Awaya, N.; Torok-Storb, B. Functional interleukin-7 receptors (IL-7Rs) are expressed by marrow stromal cells: Binding of IL-7 increases levels of IL-6 mRNA and secreted protein. Blood 2002, 100, 1318–1325.
- Al-Rawi, M.A.; Rmali, K.; Watkins, G.; Mansel, R.E.; Jiang, W.G. Aberrant expression of interleukin-7 (IL-7) and its signalling complex in human breast cancer. Eur. J. Cancer 2004, 40, 494–502.
- Akashi, K.; Kondo, M.; Weissman, I.L. Two distinct pathways of positive selection for thymocytes. Proc. Natl. Acad. Sci. USA 1998, 95, 2486–2491.
- DiSanto, J.P.; Muller, W.; Guy-Grand, D.; Fischer, A.; Rajewsky, K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA 1995, 92, 377–381.
- Hofmeister, R.; Khaled, A.R.; Benbernou, N.; Rajnavolgyi, E.; Muegge, K.; Durum, S.K. Interleukin-7: Physiological roles and mechanisms of action. Cytokine Growth Factor Rev. 1999, 10, 41–60.
- Cosenza, L.; Gorgun, G.; Urbano, A.; Foss, F. Interleukin-7 receptor expression and activation in nonhaematopoietic neoplastic cell lines. Cell. Signal. 2002, 14, 317–325.
- Fernandez-Botran, R. Soluble cytokine receptors: Basic immunology and clinical applications. Crit. Rev. Clin. Lab. Sci. 1999, 36, 165–224.
- Fernandez-Botran, R.; Crespo, F.A.; Sun, X. Soluble cytokine receptors in biological therapy. Expert Opin. Biol. Ther. 2002, 2, 585–605.
- Lundstrom, W.; Highfill, S.; Walsh, S.T.; Beq, S.; Morse, E.; Kockum, I.; Alfredsson, L.; Olsson, T.; Hillert, J.; Mackall, C.L. Soluble IL7Ralpha potentiates IL-7 bioactivity and promotes autoimmunity. Proc. Natl. Acad. Sci. USA 2013, 110, E1761–E1770.
- Lee, B.; Hong, C. The role of soluble common gamma chain in autoimmune disease. Anat. Cell Biol. 2015, 48, 10–15.
- Lundmark, F.; Duvefelt, K.; Iacobaeus, E.; Kockum, I.; Wallstrom, E.; Khademi, M.; Oturai, A.; Ryder, L.P.; Saarela, J.; Harbo, H.F.; et al. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat. Genet. 2007, 39, 1108–1113.
- Todd, J.A.; Walker, N.M.; Cooper, J.D.; Smyth, D.J.; Downes, K.; Plagnol, V.; Bailey, R.; Nejentsev, S.; Field, S.F.; Payne, F.; et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat. Genet. 2007, 39, 857–864.
- McKay, F.C.; Swain, L.I.; Schibeci, S.D.; Rubio, J.P.; Kilpatrick, T.J.; Heard, R.N.; Stewart, G.J.; Booth, D.R. Haplotypes of the interleukin 7 receptor alpha gene are correlated with altered expression in whole blood cells in multiple sclerosis. Genes Immun. 2008, 9, 1–6.
- Crawley, A.M.; Faucher, S.; Angel, J.B. Soluble IL-7R alpha (sCD127) inhibits IL-7 activity and is increased in HIV infection. J. Immunol. 2010, 184, 4679–4687.
- Goh, T.S.; Jo, Y.; Lee, B.; Kim, G.; Hwang, H.; Ko, E.; Kang, S.W.; Oh, S.O.; Baek, S.Y.; Yoon, S.; et al. IL-7 Induces an Epitope Masking of gammac Protein in IL-7 Receptor Signaling Complex. Mediat. Inflamm. 2017, 2017, 9096829.
- Huang, J.; Long, Z.; Jia, R.; Wang, M.; Zhu, D.; Liu, M.; Chen, S.; Zhao, X.; Yang, Q.; Wu, Y.; et al. The Broad Immunomodulatory Effects of IL-7 and Its Application In Vaccines. Front. Immunol. 2021, 12, 680442.
- Lu, L.; Chaudhury, P.; Osmond, D.G. Regulation of cell survival during B lymphopoiesis: Apoptosis and Bcl-2/Bax content of precursor B cells in bone marrow of mice with altered expression of IL-7 and recombinase-activating gene-2. J. Immunol. 1999, 162, 1931–1940.
- Zhang, Y.; Li, B.; Bai, Q.; Wang, P.; Wei, G.; Li, Z.; Hu, L.; Tian, Q.; Zhou, J.; Huang, Q.; et al. The lncRNA Snhg1-Vps13D vesicle trafficking system promotes memory CD8 T cell establishment via regulating the dual effects of IL-7 signaling. Signal Transduct. Target. Ther. 2021, 6, 126.
- Petschner, F.; Zimmerman, C.; Strasser, A.; Grillot, D.; Nunez, G.; Pircher, H. Constitutive expression of Bcl-xL or Bcl-2 prevents peptide antigen-induced T cell deletion but does not influence T cell homeostasis after a viral infection. Eur. J. Immunol. 1998, 28, 560–569.
- Kaech, S.M.; Tan, J.T.; Wherry, E.J.; Konieczny, B.T.; Surh, C.D.; Ahmed, R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003, 4, 1191–1198.
- Drake, A.; Kaur, M.; Iliopoulou, B.P.; Phennicie, R.; Hanson, A.; Chen, J. Interleukins 7 and 15 Maintain Human T Cell Proliferative Capacity through STAT5 Signaling. PLoS ONE 2016, 11, e0166280.
- Pallard, C.; Stegmann, A.P.; van Kleffens, T.; Smart, F.; Venkitaraman, A.; Spits, H. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity 1999, 10, 525–535.
- Crawley, A.M.; Vranjkovic, A.; Faller, E.; McGuinty, M.; Busca, A.; Burke, S.C.; Cousineau, S.; Kumar, A.; Macpherson, P.A.; Angel, J.B. Jak/STAT and PI3K signaling pathways have both common and distinct roles in IL-7-mediated activities in human CD8+ T cells. J. Leukoc. Biol. 2014, 95, 117–127.
- Kim, H.K.; Waickman, A.T.; Castro, E.; Flomerfelt, F.A.; Hawk, N.V.; Kapoor, V.; Telford, W.G.; Gress, R.E. Distinct IL-7 signaling in recent thymic emigrants versus mature naive T cells controls T-cell homeostasis. Eur. J. Immunol. 2016, 46, 1669–1680.
- Zhang, X.; Tu, H.; Yang, Y.; Jiang, X.; Hu, X.; Luo, Q.; Li, J. Bone marrow-derived mesenchymal stromal cells promote resistance to tyrosine kinase inhibitors in chronic myeloid leukemia via the IL-7/JAK1/STAT5 pathway. J. Biol. Chem. 2019, 294, 12167–12179.
- Delgado-Martin, C.; Meyer, L.K.; Huang, B.J.; Shimano, K.A.; Zinter, M.S.; Nguyen, J.V.; Smith, G.A.; Taunton, J.; Winter, S.S.; Roderick, J.R.; et al. JAK/STAT pathway inhibition overcomes IL7-induced glucocorticoid resistance in a subset of human T-cell acute lymphoblastic leukemias. Leukemia 2017, 31, 2568–2576.
- Le Saout, C.; Hasley, R.B.; Imamichi, H.; Tcheung, L.; Hu, Z.; Luckey, M.A.; Park, J.H.; Durum, S.K.; Smith, M.; Rupert, A.W.; et al. Chronic exposure to type-I IFN under lymphopenic conditions alters CD4 T cell homeostasis. PLoS Pathog. 2014, 10, e1003976.
- Le Saout, C.; Luckey, M.A.; Villarino, A.V.; Smith, M.; Hasley, R.B.; Myers, T.G.; Imamichi, H.; Park, J.H.; O’Shea, J.J.; Lane, H.C.; et al. IL-7-dependent STAT1 activation limits homeostatic CD4+ T cell expansion. JCI Insight 2017, 2, e96228.
- van der Plas, D.C.; Smiers, F.; Pouwels, K.; Hoefsloot, L.H.; Lowenberg, B.; Touw, I.P. Interleukin-7 signaling in human B cell precursor acute lymphoblastic leukemia cells and murine BAF3 cells involves activation of STAT1 and STAT5 mediated via the interleukin-7 receptor alpha chain. Leukemia 1996, 10, 1317–1325.
- Chou, W.C.; Levy, D.E.; Lee, C.K. STAT3 positively regulates an early step in B-cell development. Blood 2006, 108, 3005–3011.
- Ribeiro, D.; Melao, A.; van Boxtel, R.; Santos, C.I.; Silva, A.; Silva, M.C.; Cardoso, B.A.; Coffer, P.J.; Barata, J.T. STAT5 is essential for IL-7-mediated viability, growth, and proliferation of T-cell acute lymphoblastic leukemia cells. Blood Adv. 2018, 2, 2199–2213.
- Patra, A.K.; Avots, A.; Zahedi, R.P.; Schuler, T.; Sickmann, A.; Bommhardt, U.; Serfling, E. An alternative NFAT-activation pathway mediated by IL-7 is critical for early thymocyte development. Nat. Immunol. 2013, 14, 127–135.
- Trengove, M.C.; Ward, A.C. SOCS proteins in development and disease. Am. J. Clin. Exp. Immunol. 2013, 2, 1–29.
- Ilangumaran, S.; Ramanathan, S.; Rottapel, R. Regulation of the immune system by SOCS family adaptor proteins. Semin. Immunol. 2004, 16, 351–365.
- Shuai, K.; Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911.
- Ghazawi, F.M.; Faller, E.M.; Sugden, S.M.; Kakal, J.A.; MacPherson, P.A. IL-7 downregulates IL-7Ralpha expression in human CD8 T cells by two independent mechanisms. Immunol. Cell Biol. 2013, 91, 149–158.
- Michael, P.E.; Jahncke, J.; Hyrenbach, K.D. Placing Local Aggregations in a Larger-Scale Context: Hierarchical Modeling of Black-Footed Albatross Dispersion. PLoS ONE 2016, 11, e0153783.
- Sharfe, N.; Dadi, H.K.; Roifman, C.M. JAK3 protein tyrosine kinase mediates interleukin-7-induced activation of phosphatidylinositol-3′ kinase. Blood 1995, 86, 2077–2085.
- Tal, N.; Shochat, C.; Geron, I.; Bercovich, D.; Izraeli, S. Interleukin 7 and thymic stromal lymphopoietin: From immunity to leukemia. Cell. Mol. Life Sci. CMLS 2014, 71, 365–378.
- Wofford, J.A.; Wieman, H.L.; Jacobs, S.R.; Zhao, Y.; Rathmell, J.C. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 2008, 111, 2101–2111.
- Chehtane, M.; Khaled, A.R. Interleukin-7 mediates glucose utilization in lymphocytes through transcriptional regulation of the hexokinase II gene. Am. J. Physiol. Cell Physiol. 2010, 298, C1560–C1571.
- Barata, J.T.; Silva, A.; Brandao, J.G.; Nadler, L.M.; Cardoso, A.A.; Boussiotis, V.A. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J. Exp. Med. 2004, 200, 659–669.
- Lali, F.V.; Crawley, J.; McCulloch, D.A.; Foxwell, B.M. A late, prolonged activation of the phosphatidylinositol 3-kinase pathway is required for T cell proliferation. J. Immunol. 2004, 172, 3527–3534.
- Zeng, H.; Yu, M.; Tan, H.; Li, Y.; Su, W.; Shi, H.; Dhungana, Y.; Guy, C.; Neale, G.; Cloer, C.; et al. Discrete roles and bifurcation of PTEN signaling and mTORC1-mediated anabolic metabolism underlie IL-7-driven B lymphopoiesis. Sci. Adv. 2018, 4, eaar5701.
- Sun, Y.; Lu, B.; Deng, J.; Jiang, Z.; Cao, W.; Dai, T.; Li, S. IL-7 enhances the differentiation of adipose-derived stem cells toward lymphatic endothelial cells through AKT signaling. Cell Biol. Int. 2019, 43, 394–401.
- Crawley, J.B.; Rawlinson, L.; Lali, F.V.; Page, T.H.; Saklatvala, J.; Foxwell, B.M. T cell proliferation in response to interleukins 2 and 7 requires p38MAP kinase activation. J. Biol. Chem. 1997, 272, 15023–15027.
- Rajnavolgyi, E.; Benbernou, N.; Rethi, B.; Reynolds, D.; Young, H.A.; Magocsi, M.; Muegge, K.; Durum, S.K. IL-7 withdrawal induces a stress pathway activating p38 and Jun N-terminal kinases. Cell. Signal. 2002, 14, 761–769.
- Kariminia, A.; Ivison, S.M.; Leung, V.M.; Sung, S.; Couto, N.; Rozmus, J.; Rolf, N.; Narendran, A.; Dunn, S.E.; Reid, G.S.; et al. Y-box-binding protein 1 contributes to IL-7-mediated survival signaling in B-cell precursor acute lymphoblastic leukemia. Oncol. Lett. 2017, 13, 497–505.
- Fry, T.J.; Mackall, C.L. Interleukin-7: From bench to clinic. Blood 2002, 99, 3892–3904.
- Peschon, J.J.; Morrissey, P.J.; Grabstein, K.H.; Ramsdell, F.J.; Maraskovsky, E.; Gliniak, B.C.; Park, L.S.; Ziegler, S.F.; Williams, D.E.; Ware, C.B.; et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 1994, 180, 1955–1960.
- Andersson, A.; Yang, S.C.; Huang, M.; Zhu, L.; Kar, U.K.; Batra, R.K.; Elashoff, D.; Strieter, R.M.; Dubinett, S.M.; Sharma, S. IL-7 promotes CXCR3 ligand-dependent T cell antitumor reactivity in lung cancer. J. Immunol. 2009, 182, 6951–6958.
- Sharma, S.; Batra, R.K.; Yang, S.C.; Hillinger, S.; Zhu, L.; Atianzar, K.; Strieter, R.M.; Riedl, K.; Huang, M.; Dubinett, S.M. Interleukin-7 gene-modified dendritic cells reduce pulmonary tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Hum. Gene Ther. 2003, 14, 1511–1524.
- Lum, J.J.; Schnepple, D.J.; Nie, Z.; Sanchez-Dardon, J.; Mbisa, G.L.; Mihowich, J.; Hawley, N.; Narayan, S.; Kim, J.E.; Lynch, D.H.; et al. Differential effects of interleukin-7 and interleukin-15 on NK cell anti-human immunodeficiency virus activity. J. Virol. 2004, 78, 6033–6042.
- Kimura, M.Y.; Pobezinsky, L.A.; Guinter, T.I.; Thomas, J.; Adams, A.; Park, J.H.; Tai, X.; Singer, A. IL-7 signaling must be intermittent, not continuous, during CD8(+) T cell homeostasis to promote cell survival instead of cell death. Nat. Immunol. 2013, 14, 143–151.
- Colombetti, S.; Levy, F.; Chapatte, L. IL-7 adjuvant treatment enhances long-term tumor-antigen-specific CD8+ T-cell responses after immunization with recombinant lentivector. Blood 2009, 113, 6629–6637.
- Merchant, M.S.; Bernstein, D.; Amoako, M.; Baird, K.; Fleisher, T.A.; Morre, M.; Steinberg, S.M.; Sabatino, M.; Stroncek, D.F.; Venkatasan, A.M.; et al. Adjuvant Immunotherapy to Improve Outcome in High-Risk Pediatric Sarcomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 3182–3191.
- Alderson, M.R.; Tough, T.W.; Ziegler, S.F.; Grabstein, K.H. Interleukin 7 induces cytokine secretion and tumoricidal activity by human peripheral blood monocytes. J. Exp. Med. 1991, 173, 923–930.
- Pellegrini, M.; Calzascia, T.; Elford, A.R.; Shahinian, A.; Lin, A.E.; Dissanayake, D.; Dhanji, S.; Nguyen, L.T.; Gronski, M.A.; Morre, M.; et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat. Med. 2009, 15, 528–536.
- Gao, J.; Zhao, L.; Wan, Y.Y.; Zhu, B. Mechanism of Action of IL-7 and Its Potential Applications and Limitations in Cancer Immunotherapy. Int. J. Mol. Sci. 2015, 16, 10267–10280.
- Wang, X.; Chang, S.; Wang, T.; Wu, R.; Huang, Z.; Sun, J.; Liu, J.; Yu, Y.; Mao, Y. IL7R Is Correlated With Immune Cell Infiltration in the Tumor Microenvironment of Lung Adenocarcinoma. Front. Pharmacol. 2022, 13, 857289.
- Rich, B.E.; Campos-Torres, J.; Tepper, R.I.; Moreadith, R.W.; Leder, P. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. J. Exp. Med. 1993, 177, 305–316.
- Silva, A.; Laranjeira, A.B.; Martins, L.R.; Cardoso, B.A.; Demengeot, J.; Yunes, J.A.; Seddon, B.; Barata, J.T. IL-7 contributes to the progression of human T-cell acute lymphoblastic leukemias. Cancer Res. 2011, 71, 4780–4789.
- Barata, J.T.; Cardoso, A.A.; Nadler, L.M.; Boussiotis, V.A. Interleukin-7 promotes survival and cell cycle progression of T-cell acute lymphoblastic leukemia cells by down-regulating the cyclin-dependent kinase inhibitor p27(kip1). Blood 2001, 98, 1524–1531.
- Karawajew, L.; Ruppert, V.; Wuchter, C.; Kosser, A.; Schrappe, M.; Dorken, B.; Ludwig, W.D. Inhibition of in vitro spontaneous apoptosis by IL-7 correlates with bcl-2 up-regulation, cortical/mature immunophenotype, and better early cytoreduction of childhood T-cell acute lymphoblastic leukemia. Blood 2000, 96, 297–306.
- Barata, J.T.; Keenan, T.D.; Silva, A.; Nadler, L.M.; Boussiotis, V.A.; Cardoso, A.A. Common gamma chain-signaling cytokines promote proliferation of T-cell acute lymphoblastic leukemia. Haematologica 2004, 89, 1459–1467.
- Silva, A.; Girio, A.; Cebola, I.; Santos, C.I.; Antunes, F.; Barata, J.T. Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells. Leukemia 2011, 25, 960–967.
- Cramer, S.D.; Aplan, P.D.; Durum, S.K. Therapeutic targeting of IL-7Ralpha signaling pathways in ALL treatment. Blood 2016, 128, 473–478.
- Seol, M.A.; Kim, J.H.; Oh, K.; Kim, G.; Seo, M.W.; Shin, Y.K.; Sim, J.H.; Shin, H.M.; Seo, B.Y.; Lee, D.S.; et al. Interleukin-7 Contributes to the Invasiveness of Prostate Cancer Cells by Promoting Epithelial-Mesenchymal Transition. Sci. Rep. 2019, 9, 6917.
- Qu, H.; Zou, Z.; Pan, Z.; Zhang, T.; Deng, N.; Chen, G.; Wang, Z. IL-7/IL-7 receptor axis stimulates prostate cancer cell invasion and migration via AKT/NF-kappaB pathway. Int. Immunopharmacol. 2016, 40, 203–210.
- Ming, J.; Jiang, G.; Zhang, Q.; Qiu, X.; Wang, E. Interleukin-7 up-regulates cyclin D1 via activator protein-1 to promote proliferation of cell in lung cancer. Cancer Immunol. Immunother. CII 2012, 61, 79–88.
- Vicente, C.; Schwab, C.; Broux, M.; Geerdens, E.; Degryse, S.; Demeyer, S.; Lahortiga, I.; Elliott, A.; Chilton, L.; La Starza, R.; et al. Targeted sequencing identifies associations between IL7R-JAK mutations and epigenetic modulators in T-cell acute lymphoblastic leukemia. Haematologica 2015, 100, 1301–1310.
- Li, Y.; Buijs-Gladdines, J.G.; Cante-Barrett, K.; Stubbs, A.P.; Vroegindeweij, E.M.; Smits, W.K.; van Marion, R.; Dinjens, W.N.; Horstmann, M.; Kuiper, R.P.; et al. IL-7 Receptor Mutations and Steroid Resistance in Pediatric T cell Acute Lymphoblastic Leukemia: A Genome Sequencing Study. PLoS Med. 2016, 13, e1002200.
- Puel, A.; Ziegler, S.F.; Buckley, R.H.; Leonard, W.J. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat. Genet. 1998, 20, 394–397.
- Silva, A.; Almeida, A.R.M.; Cachucho, A.; Neto, J.L.; Demeyer, S.; de Matos, M.; Hogan, T.; Li, Y.; Meijerink, J.; Cools, J.; et al. Overexpression of wild-type IL-7Ralpha promotes T-cell acute lymphoblastic leukemia/lymphoma. Blood 2021, 138, 1040–1052.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
21 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




