Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ha Youn Shin | -- | 2635 | 2022-09-21 09:19:52 | | | |
| 2 | Sirius Huang | Meta information modification | 2635 | 2022-09-22 02:46:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kim, S.; Shin, H.Y. Tissue Specificity of Zika Virus Infection. Encyclopedia. Available online: https://encyclopedia.pub/entry/27419 (accessed on 11 January 2026).
Kim S, Shin HY. Tissue Specificity of Zika Virus Infection. Encyclopedia. Available at: https://encyclopedia.pub/entry/27419. Accessed January 11, 2026.
Kim, Suyeon, Ha Youn Shin. "Tissue Specificity of Zika Virus Infection" Encyclopedia, https://encyclopedia.pub/entry/27419 (accessed January 11, 2026).
Kim, S., & Shin, H.Y. (2022, September 21). Tissue Specificity of Zika Virus Infection. In Encyclopedia. https://encyclopedia.pub/entry/27419
Kim, Suyeon and Ha Youn Shin. "Tissue Specificity of Zika Virus Infection." Encyclopedia. Web. 21 September, 2022.
Copy Citation
Zika virus (ZIKV) is an arthropod-borne virus that belongs to the Flavivirus genus and is principally transmitted by Aedes aegypti mosquitoes. ZIKV infection often causes no or only mild symptoms, but it can also trigger severe consequences, including microcephaly in infants and Guillain-Barré syndrome, uveitis, and neurologic manifestations in adults. Since numerous studies have indicated that there are slightly different tissue-specific pathologies in each animal model of ZIKV, the distinct ZIKV tropism of a given animal model must be understood to enable effective vaccine development.
ZIKV
vaccine
tissue tropism
animal model
1. Introduction
Zika virus (ZIKV) is a mosquito-transmitted RNA virus; it belongs to genus Flavivirus in the Flaviviridae family and is closely related to the dengue, yellow fever, Japanese encephalitis, and West Nile viruses [1]. Like other flaviviruses, ZIKV is enveloped and icosahedral and has an 11-kb positive single-stranded RNA genome. ZIKV was first isolated in 1947 from a rhesus monkey from the Zika forest of Uganda [2]. Sporadic ZIKV epidemic outbreaks were reported in French Polynesia (2013–2014), Brazil (2015), and South and Central America (2016) [3]. After human-to-human transmission was reported in 2016, the World Health Organization (WHO) declared ZIKV to be a major threat to public health. So far, two major strains of ZIKV have been identified: the African and Asian strains. Both are transmitted primarily via mosquitoes and can also be transmitted through mother-to-fetus transmission, breastfeeding, sexual contact, or blood transfusion [4]. The primary target tissues of ZIKV infection have been recognized as the maternal placenta and fetal brain. However, ZIKV can also be transmitted to other adult tissues, including the reproductive system, brain, eyes, and lymph nodes (Figure 1). ZIKV infection causes several clinical complications, including congenital microcephaly, autoimmune Guillain-Barré syndrome, and ocular diseases, such as uveitis and unilateral acute maculopathy [5][6][7][8].
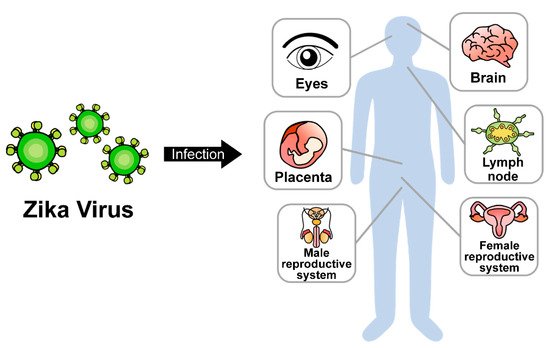
Figure 1. Dissemination of ZIKV in multiple organs.
2. ZIKV Transmission from Maternal Placenta to Fetal Brain
A key feature of congenital ZIKV syndrome is microcephaly, wherein an infant displays a smaller-than-normal head due to immature brain development during pregnancy. Severe microcephaly is correlated with mental retardation, learning disability, and poor motor function [9]. ZIKV infection in pregnant women has received considerable attention due to the vertical transmission of ZIKV from the maternal placenta to fetuses, resulting in high rates of microcephaly [10][11]. Several studies have demonstrated that ZIKV can replicate in placental cells [12][13][14] and in fetal endothelial cells upon the infection of the placental barrier [15]. Accordingly, many researchers have focused primarily on understanding the mechanisms of ZIKV infection in the placenta and fetus when seeking to develop vaccines and therapeutics.
2.1. Mice
Mouse models have shown slight differences in viral susceptibility to and clinical manifestations of ZIKV infection in the maternal placenta and fetus depending on the genetic background and/or route of administration. For example, the subcutaneous injection of ZIKV in immunocompetent mice, such as C57BL/6, CD-1, or SJL mice, yielded insignificant clinical signs and no viral replication due to the intact innate immunity [16][17][18]. However, several studies found that intravenous or intrauterine ZIKV infection in these mice induced viral replication in the placenta and the resorption of fetuses [17] (Table 1). When C57BL/6 mice were inoculated with a high dose of ZIKV via an intravenous route at embryonic day 9.5 (E9.5), fetuses were resorbed at day E17.5 even though the ZIKV RNA was only sparsely detected in the fetal head [19]. When ZIKV was inoculated directly into the uterus of pregnant CD-1 mice, viral RNA was observed in the uterine horn, placenta, and fetal head [20]. This inoculation also increased fetal abortion, placental inflammation, and IFN-β levels. In ICR mice, the injection of ZIKV into the embryonic brains at E15.5 led to fetal death and microcephaly [21][22]. The intravenous injection of ZIKV into SJL mice also induced the congenital malformation of the brain [23]. Human STAT2 KI mice and immunodeficient mice showed more enhanced viral susceptibility and evident clinical symptoms. Even the subcutaneous injection of ZIKV into human STAT2 KI mice resulted in higher levels of ZIKV RNA in the placenta and fetal heads compared with the immunocompetent wild-type mice [24]. The subcutaneous injection of ZIKV into IFN-deficient A129 and AG129 mice resulted in a small fetal head and the detection of viral RNA in the placenta and fetal head [25][26]. Taken together, these results indicate that mice are naturally resistant to ZIKV infection but that mouse models may exhibit human-like symptoms such as microcephaly depending on their genetic background and/or the route of ZIKV administration.
2.2. Non-Human Primates
Compared with the mouse models, the pathological symptoms of ZIKV infection during pregnancy in nonhuman primates are more analogous to those of humans [27]. Several studies have shown that the subcutaneous injection of ZIKV in rhesus monkeys induced robust placental and fetal inflammation, placental dysfunction, and fetal neuropathology, such as neural progenitor cell dysfunction and neuronal apoptosis [28][29]. The direct inoculation of ZIKV into the amniotic fluid at early pregnancy resulted in more severe symptoms, including loss of neural precursor cells and early fetal death [30]. The subcutaneous inoculation of ZIKV into pregnant pigtail monkeys induced fetal brain damage, such as fetal brain growth arrest and a significant loss of fetal neural progenitor cells [31][32]. The intramuscular injection of ZIKV into a rat-sized marmoset induced human-like fetal neurodevelopmental abnormalities and spontaneous fetal loss accompanied by extensive viral replication in the placenta [33]. Although the gestational duration is shorter in these nonhuman primates than in humans, the above-described results indicate that nonhuman primates may recapitulate the human situation for clinical symptoms of ZIKV infection during pregnancy.
Table 1. Pathological features of ZIKV infection in maternal placentas and fetuses of animal models.
| Animal Species | ZIKV Strain | Route of Administration |
Pathologic Features | Reference |
|---|---|---|---|---|
| Mouse | ||||
| C57BL/6 | PRVABC59 | Intravenous | Fetal resorption, little or no viral RNA in placenta and fetal heads | [19] |
| CD-1 | IBH 30656, PRVABC59, FS13025 |
Intrauterine | Placental dysfunction and inflammation, increased aborted fetus numbers, reduced neonatal brain and cortical thickness | [20] |
| SJL | ZIKV-BR | Intravenous | Malformations of congenital brain, intrauterine growth restriction, upregulation of apoptosis-related genes | [23] |
| ICR | GZ01 | Intra-ventricular | Fetal death, microcephaly | [22] |
| Human STAT2 KI | ZIKV-Dak-MA | Subcutaneous | Viral RNA in placenta and fetal heads | [24] |
| Ifnar1−/− | FSS13025 | Subcutaneous | Fetal resorption, reduced fetal weight and size | [34] |
| A129 | PRVABC59 | Subcutaneous | Viral RNA in placenta, maternal brain, and fetal heads, reduced fetal weight | [26] |
| AG129 | P6-740 | Subcutaneous | Viral RNA in placenta and fetal heads, decreased weight and head length | [25] |
| Monkey | ||||
| Rhesus macaque | PRVABC59 | Subcutaneous | Viral RNA in fetus, increased maternal-placental-fetal inflammatory response, placenta damage, reduced oxygen permeability of the placental villi | [28] |
| Rhesus macaque | ZIKV-BR | Subcutaneous | Fetal neuropathology, neuroprogenitor apoptosis and gliosis, placental pathology | [29] |
| Rhesus macaque | SPH 2015 | Intra-amniotic | Viral RNA in fetal and placental tissues, early death of fetus, fetal ZIKV neurotropism, neuropathology at the end of gestation | [30] |
| Pigtail macaque | FSS13025 | Subcutaneous | Reduced growth of the fetal biparietal diameter and fetal brain lesions, viral RNA in fetal brain and placenta | [31] |
| Pigtail macaque | FSS13025, ZIKV-BR |
Subcutaneous | Decreased fetal non-cortical brain volume, injury in ependymal epithelium with underlying gliosis, reduced late fetal neuronal progenitor cells in the subventricular zone | [32] |
| Marmoset | SPH 2015 | Intramuscular | Fetal demise and abortion, increase of proinflammatory cytokines, fetal neurocellular disorganization, viral RNA in placenta and fetus | [33] |
3. ZIKV Infection in Reproductive Organs
3.1. Female Animal Models
In addition to vertical transmission from mother to fetus, ZIKV can be transmitted through sexual contact and can cause genital infection. Several research groups have demonstrated that ZIKV can infect the female reproductive organs and detected ZIKV RNA in the female genital tract in animal models, including cervical mucus, vaginal secretions, and ovaries [35][36] (Table 2). Even in immunocompetent C57BL/6 mice, vaginal infection with ZIKV resulted in local viral replication [35]. In immunodeficient Ifnar1−/− and Irf3−/−Irf7−/− mice, vaginal ZIKV infection induced higher levels of viral replication. In C57BL/6 mice treated with anti-Ifnar1 monoclonal Ab (mAb) to block the IFN pathway, viral RNA was detected in the vaginal lumen, cervix, ovaries, and uterine horns after intravaginal inoculation of ZIKV [37]. Subcutaneous inoculation of ZIKV in anti-Ifnar1 mAb-treated T-cell-deficient CD8−/− and TCRβδ−/− mice resulted in higher ovarian cell death compared to that in C57BL/6 mice [36]. Transmission of ZIKV from female reproductive organs has also been found in nonhuman primates. In rhesus monkeys, direct injection of ZIKV into the vagina led to preferential viral replication in the female reproductive tract [38]. Even after subcutaneous injection, ZIKV RNA was detected in the vaginal secretions and reproductive tissues of female rhesus and cynomolgus monkeys [39].
3.2. Male Animal Models
ZIKV can also infect the male reproductive system. Cohort studies have revealed that the ZIKV RNA can be detected in semen and can persist in the male reproductive tract [40][41][42][43][44]. Persistent ZIKV replication in the male genital tract can cause genitourinary symptoms such as inflammation of the prostate gland, bloody semen, and infertility. The subcutaneous inoculation of ZIKV into C57BL/6 and Ifnar1−/− mice resulted in decreased testis size and testosterone levels and degradation of the seminiferous tubule due to persistent viral replication in semen [45][46] (Table 2). On the other hand, infection of ZIKV via an intraperitoneal route into immunodeficient Ifnar1−/− and AG129 mice caused inflammation in the testis and epididymis, leading to male infertility [47][48][49]. Similarly, ZIKV RNA was detected in monkey testicles after subcutaneous injection [50].
Table 2. Effects of ZIKV on reproductive systems in animal models.
| Animal Species | ZIKV Strain | Route of Administration |
Pathologic Features | Reference |
|---|---|---|---|---|
| Female | ||||
| C57BL/6 | FSS13025 | Intravaginal | Viral RNA in vagina until 4 dpi (local replication) | [35] |
| Ifnar1−/−, Irf3−/−Irf7−/− | FSS13025 | Intravaginal | High viral RNA levels in vagina | [35] |
| C57BL/6J (+ anti-Ifnar1 mAb) |
ZIKV-BR | Intravaginal | ZIKV in the vaginal lumen, cervix, ovaries, uterine horns | [37] |
| C57BL/6, CD8−/−, TCRβδ−/− (+ anti-Ifnar1 mAb) |
Dakar 41525, PRVABC59, H/PF/2013 |
Subcutaneous | Viral replicates in ovary, increased inflammation in the ovary, acute oophoritis | [36] |
| Rhesus macaque | SPH 2015 | Intravaginal | Viral RNA in female reproductive tract, increased cytokine levels in cervicovaginal lavage | [38] |
| Rhesus macaque, Cynomolgus macaque |
Thai, Puerto Rican |
Subcutaneous | Viral RNA in female reproductive tissues | [39] |
| Male | ||||
| C57BL/6 (+ anti-Ifnar1 mAb) |
Dakar 41519, H/PF/2013 |
Subcutaneous | Viral RNA in the testis and epididymis, decreased testis size, low testosterone level, infected spermatogonia, degradation of seminiferous tubule | [45] |
| Ifnar−/− | MEX2-81 | Subcutaneous | Viral RNA and antigen in the epididymal lumen, testicular atrophy, low testosterone level | [46] |
| Ifnar−/− | SMGC-1 | Intraperitoneal | Atrophy of the reproductive tract, virus in testicles, increased inflammation in the testis and epididymis | [48] |
| AG129 | PRVABC59 | Intraperitoneal | Viral RNA and virus in semen, increased inflammation in testis and epididymis | [49] |
| AG129 | SL1602 | Intraperitoneal | Viral RNA in testis and epididymis, increased inflammation in testis | [47] |
| Cynomolgus macaque | IBH30656, PRVABC59, FSS13025 |
Subcutaneous | Virus in the testis | [50] |
4. ZIKV Tropism in Other Tissues
4.1. Brain
One unique feature of ZIKV infection is that it preferentially infects the fetal brain, causing congenital or postnatal microcephaly [51]. ZIKV can also affect the adult brain to cause Guillain-Barré syndrome, an autoimmune disease in which the immune system attacks the peripheral nervous system [4][52]. Guillain-Barré syndrome further leads to muscle weakness, paralysis, and ultimately death [53][54][55]. Several animal models can recapitulate the brain or neurological damage seen in humans (Table 3). In C57BL/6 mice treated with anti-Ifnar1 mAb, either the subcutaneous or the intraperitoneal injection of ZIKV induced inflammatory responses and neuronal cell death in the brain and spinal cord [56]. The subcutaneous injection of ZIKV into human STAT2 KI mice resulted in high levels of viral RNA in the brain [24]. However, intraperitoneal infection yielded higher viral susceptibility (100% mortality) than that achieved with subcutaneous infection (40% mortality). In immunodeficient A129 mice, ZIKV RNA was also detected in adult brain tissue after subcutaneous injection, and nuclear fragments were found with necrosis in a portion of the hippocampus [57]. Adult AG129 mice showed high viral susceptibility with severe brain pathology after subcutaneous, intraperitoneal, or foot-pad ZIKV inoculation [58][59][60]. Histopathological examination revealed neutrophil infiltration in the hippocampus, neuronal degeneration, and necrotic cell debris. High viral RNA loads were also observed in the brain and spinal cords. Mice with triple knockout of Irf3, Ifr5, and Irf7 were highly vulnerable to subcutaneous, intraperitoneal, or intravenous ZIKV infection and developed neurological diseases such as hindlimb weakness and paralysis [18]. Taken together, these animal studies clearly demonstrated that ZIKV can damage the adult brain and nervous tissue, although the detailed mechanism underlying Guillain-Barré syndrome remains to be fully elucidated.
4.2. Eyes
Several case reports have shown that ZIKV infection can cause ocular diseases, such as conjunctivitis and optic nerve abnormalities [61][62]. These ocular pathologies have also been observed in animal models (Table 3). The subcutaneous injection of ZIKV into C57BL/6 mice preferentially infected the cornea and retina, resulting in inflammation of the optic nerves and the distortion of eye structures [63]. The direct inoculation of ZIKV into the eyes of C57BL/6 and ISG15 knockout mice yielded ocular abnormalities, including pigment clumping and retinal pigment epithelium (RPE) atrophy [64]. ISG15 knockout mice showed more severe retinitis and retinal cell death with higher levels of ZIKV RNA in the retina. ZIKV inoculation to the anterior chamber of the eye or by intraperitoneal route in C57BL/6 and Ifnar1 knockout mice induced innate immune responses and increased intraocular pressure (IOP), which is a hallmark of glaucoma (a major cause of blindness) [65]. Posterior retinal cell death was observed even following ZIKV infection in the anterior part of the eye, suggesting that ZIKV may spread from the anterior to posterior part of the eye [66]. Together, these results indicate that ZIKV can affect the optic nerve, which is part of the central nervous system.
4.3. Lymph Nodes
ZIKV infection in lymph nodes was commonly found in nonhuman primates (Table 3). Following the subcutaneous injection of ZIKV into rhesus monkeys, ZIKV could persist up to 72 days in the lymph nodes even after the virus had been cleared from the peripheral blood [67]. Among the various cell types within lymphoid tissues, ZIKV RNA was mainly found in the macrophage and B cell subsets, whereas little or no ZIKV RNA was found in the subsets of dendritic cells and T cells [68]. Intravenous inoculation of ZIKV into rhesus monkeys clearly induced hyperplasia of the lymph nodes, but the activation of lymphocytic cells was limited to the monocyte and B cell subsets [69]. Subcutaneous ZIKV injection in pigtail monkeys also resulted in an innate immune response, with increased proinflammatory cytokine levels and long-term ZIKV persistence in the lymph nodes [70]. Vaginal inoculation of ZIKV-infected semen into baboons yielded the persistence of ZIKV RNA in lymph nodes [71]. This persistent ZIKV infection in lymph nodes and lymphocytic inflammatory responses found in nonhuman primates recapitulate the pathogenic features of ZIKV infection in humans.
Table 3. Pathologic features of ZIKV dissemination in brain, eyes, and lymph nodes of animal models.
| Animal Species | ZIKV Strain | Route of Administration |
Pathologic Features | Reference |
|---|---|---|---|---|
| Brain | ||||
| C57BL/6 (+ anti-Ifnar1 Ab) |
Dakar 41525 | Subcutaneous, intraperitoneal |
Lesions in CNS, high viremia, decreased weight | [56] |
| Human STAT2 KI | ZIKV-Dak-MA | Subcutaneous | Viral RNA in brain | [24] |
| A129 | MP1751 | Subcutaneous | Lesions in brain, viral RNA in the brain | [57] |
| AG129 | H/PF/2013 | Footpad, intraperitoneal |
Virus in brain, neurodegeneration, myofiber necrosis, inflammatory cell infiltration, pathology in brain | [58] |
| AG129 | P6–740 | Subcutaneous | Viral load in the brain, limb weakness, paralysis, increased neurological signs including locomotor hyperactivity, tremor, and seizure | [59] |
| AG129 | MR 766 | Intraperitoneal | Paralysis, neurodegeneration, acute encephalitis, viral antigen in brain and spinal cord | [60] |
| Ifnar−/−, Irf3−/−Irf5−/−Ifr7−/− TKO |
H/PF/2013, MR766 |
Subcutaneous, intravenous, intraperitoneal |
Viral RNA in brain and spinal cord, increased neurological disease signs including hindlimb weakness and paralysis | [18] |
| Eye | ||||
| C57BL/6 | PRVABC59 | Subcutaneous | Chorioretinal lesions, induced local inflammation and cellular infiltration | [63] |
| C57BL/6, ISG15−/− |
PRVABC59 | Intravitreal | Chorioretinal atrophy with RPE mottling, increased multiple inflammatory and antiviral response genes in the retina, severe chorioretinitis coinciding with retinal cell death and higher virus replication in ISG15−/− mice | [64] |
| C57BL/6, Ifnar1−/− |
PRVABC59 | Anterior segment of eyes, intraperitoneal |
Increased intraocular pressure, chorioretinal atrophy, increased expression of inflammatory mediators, retinal ganglion cell death | [65] |
| Lymph node | ||||
| Rhesus macaque | PRVABC59, Brazil/ZKV/2015 |
Subcutaneous | Virus in lymph nodes in both paracortical regions, germinal centers, and cerebrospinal fluid, upregulation of proinflammatory and anti-apoptotic signaling pathways | [67] |
| Rhesus monkeys | ZIKV-BR | Intravenous | Activation of lymphoid tissues | [69] |
| Rhesus monkeys | PRVABC59 | Subcutaneous | Virus in multiple lymphoid tissues, viral RNA in lymphoid and joint/muscle tissues | [68] |
| Pigtail macaque | Brazil_2015_MG | Subcutaneous | Increase of innate immune cells in blood, lymph nodes, and mucosal tissues, activation of monocytes in peripheral lymph nodes | [70] |
| Olive baboon | H/PF/2013, PRVABC59 |
Vaginal deposition of ZIKV-infected semen | Viral RNA in lymph nodes | [71] |
References
- Sharma, V.; Sharma, M.; Dhull, D.; Sharma, Y.; Kaushik, S.; Kaushik, S. Zika virus: An emerging challenge to public health worldwide. Can. J. Microbiol. 2020, 66, 87–98.
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520.
- Shaily, S.; Upadhya, A. Zika virus: Molecular responses and tissue tropism in the mammalian host. Rev. Med. Virol. 2019, 29, e2050.
- Song, B.H.; Yun, S.I.; Woolley, M.; Lee, Y.M. Zika virus: History, epidemiology, transmission, and clinical presentation. J. Neuroimmunol. 2017, 308, 50–64.
- Miner, J.J.; Diamond, M.S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 2017, 21, 134–142.
- Kodati, S.; Palmore, T.N.; Spellman, F.A.; Cunningham, D.; Weistrop, B.; Sen, H.N. Bilateral posterior uveitis associated with Zika virus infection. Lancet 2017, 389, 125–126.
- de Paula Freitas, B.; Ventura, C.V.; Maia, M.; Belfort, R., Jr. Zika virus and the eye. Curr. Opin. Ophthalmol. 2017, 28, 595–599.
- Duca, L.M.; Beckham, J.D.; Tyler, K.L.; Pastula, D.M. Zika Virus Disease and Associated Neurologic Complications. Curr. Infect. Dis. Rep. 2017, 19, 4.
- Baud, D.; Gubler, D.J.; Schaub, B.; Lanteri, M.C.; Musso, D. An update on Zika virus infection. Lancet 2017, 390, 2099–2109.
- Arora, N.; Sadovsky, Y.; Dermody, T.S.; Coyne, C.B. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe 2017, 21, 561–567.
- Pierson, T.C.; Diamond, M.S. The emergence of Zika virus and its new clinical syndromes. Nature 2018, 560, 573–581.
- Quicke, K.M.; Bowen, J.R.; Johnson, E.L.; McDonald, C.E.; Ma, H.; O’Neal, J.T.; Rajakumar, A.; Wrammert, J.; Rimawi, B.H.; Pulendran, B.; et al. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe 2016, 20, 83–90.
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091.
- Sheridan, M.A.; Yunusov, D.; Balaraman, V.; Alexenko, A.P.; Yabe, S.; Verjovski-Almeida, S.; Schust, D.J.; Franz, A.W.; Sadovsky, Y.; Ezashi, T.; et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc. Natl. Acad. Sci. USA 2017, 114, E1587–E1596.
- Richard, A.S.; Shim, B.S.; Kwon, Y.C.; Zhang, R.; Otsuka, Y.; Schmitt, K.; Berri, F.; Diamond, M.S.; Choe, H. AXL-dependent infection of human fetal endothelial cells distinguishes Zika virus from other pathogenic flaviviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 2024–2029.
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a Novel Murine Model to Study Zika Virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369.
- Caine, E.A.; Jagger, B.W.; Diamond, M.S. Animal Models of Zika Virus Infection during Pregnancy. Viruses 2018, 10, 598.
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730.
- Szaba, F.M.; Tighe, M.; Kummer, L.W.; Lanzer, K.G.; Ward, J.M.; Lanthier, P.; Kim, I.J.; Kuki, A.; Blackman, M.A.; Thomas, S.J.; et al. Zika virus infection in immunocompetent pregnant mice causes fetal damage and placental pathology in the absence of fetal infection. PLoS Pathog. 2018, 14, e1006994.
- Vermillion, M.S.; Lei, J.; Shabi, Y.; Baxter, V.K.; Crilly, N.P.; McLane, M.; Griffin, D.E.; Pekosz, A.; Klein, S.L.; Burd, I. Intrauterine Zika virus infection of pregnant immunocompetent mice models transplacental transmission and adverse perinatal outcomes. Nat. Commun. 2017, 8, 14575.
- Zhu, X.; Li, C.; Afridi, S.K.; Zu, S.; Xu, J.W.; Quanquin, N.; Yang, H.; Cheng, G.; Xu, Z. E90 subunit vaccine protects mice from Zika virus infection and microcephaly. Acta Neuropathol. Commun. 2018, 6, 77.
- Li, C.; Gao, F.; Yu, L.; Wang, R.; Jiang, Y.; Shi, X.; Yin, C.; Tang, X.; Zhang, F.; Xu, Z.; et al. A Single Injection of Human Neutralizing Antibody Protects against Zika Virus Infection and Microcephaly in Developing Mouse Embryos. Cell Rep. 2018, 23, 1424–1434.
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.; Guimaraes, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271.
- Gorman, M.J.; Caine, E.A.; Zaitsev, K.; Begley, M.C.; Weger-Lucarelli, J.; Uccellini, M.B.; Tripathi, S.; Morrison, J.; Yount, B.L.; Dinnon, K.H., 3rd; et al. An Immunocompetent Mouse Model of Zika Virus Infection. Cell Host Microbe 2018, 23, 672–685.e6.
- Julander, J.G.; Siddharthan, V.; Park, A.H.; Preston, E.; Mathur, P.; Bertolio, M.; Wang, H.; Zukor, K.; Van Wettere, A.J.; Sinex, D.G.; et al. Consequences of in utero exposure to Zika virus in offspring of AG129 mice. Sci. Rep. 2018, 8, 9384.
- Xie, X.; Kum, D.B.; Xia, H.; Luo, H.; Shan, C.; Zou, J.; Muruato, A.E.; Medeiros, D.B.A.; Nunes, B.T.D.; Dallmeier, K.; et al. A Single-Dose Live-Attenuated Zika Virus Vaccine with Controlled Infection Rounds that Protects against Vertical Transmission. Cell Host Microbe 2018, 24, 487–499.e5.
- Nguyen, S.M.; Antony, K.M.; Dudley, D.M.; Kohn, S.; Simmons, H.A.; Wolfe, B.; Salamat, M.S.; Teixeira, L.B.C.; Wiepz, G.J.; Thoong, T.H.; et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 2017, 13, e1006378.
- Hirsch, A.J.; Roberts, V.H.J.; Grigsby, P.L.; Haese, N.; Schabel, M.C.; Wang, X.; Lo, J.O.; Liu, Z.; Kroenke, C.D.; Smith, J.L.; et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat. Commun. 2018, 9, 263.
- Martinot, A.J.; Abbink, P.; Afacan, O.; Prohl, A.K.; Bronson, R.; Hecht, J.L.; Borducchi, E.N.; Larocca, R.A.; Peterson, R.L.; Rinaldi, W.; et al. Fetal Neuropathology in Zika Virus-Infected Pregnant Female Rhesus Monkeys. Cell 2018, 173, 1111–1112.e10.
- Coffey, L.L.; Keesler, R.I.; Pesavento, P.A.; Woolard, K.; Singapuri, A.; Watanabe, J.; Cruzen, C.; Christe, K.L.; Usachenko, J.; Yee, J.; et al. Intraamniotic Zika virus inoculation of pregnant rhesus macaques produces fetal neurologic disease. Nat. Commun. 2018, 9, 2414.
- Adams Waldorf, K.M.; Stencel-Baerenwald, J.E.; Kapur, R.P.; Studholme, C.; Boldenow, E.; Vornhagen, J.; Baldessari, A.; Dighe, M.K.; Thiel, J.; Merillat, S.; et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat. Med. 2016, 22, 1256–1259.
- Adams Waldorf, K.M.; Nelson, B.R.; Stencel-Baerenwald, J.E.; Studholme, C.; Kapur, R.P.; Armistead, B.; Walker, C.L.; Merillat, S.; Vornhagen, J.; Tisoncik-Go, J.; et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat. Med. 2018, 24, 368–374.
- Seferovic, M.; Sanchez-San Martin, C.; Tardif, S.D.; Rutherford, J.; Castro, E.C.C.; Li, T.; Hodara, V.L.; Parodi, L.M.; Giavedoni, L.; Layne-Colon, D.; et al. Experimental Zika Virus Infection in the Pregnant Common Marmoset Induces Spontaneous Fetal Loss and Neurodevelopmental Abnormalities. Sci. Rep. 2018, 8, 6851.
- Regla-Nava, J.A.; Elong Ngono, A.; Viramontes, K.M.; Huynh, A.T.; Wang, Y.T.; Nguyen, A.T.; Salgado, R.; Mamidi, A.; Kim, K.; Diamond, M.S.; et al. Cross-reactive Dengue virus-specific CD8(+) T cells protect against Zika virus during pregnancy. Nat. Commun. 2018, 9, 3042.
- Yockey, L.J.; Varela, L.; Rakib, T.; Khoury-Hanold, W.; Fink, S.L.; Stutz, B.; Szigeti-Buck, K.; Van den Pol, A.; Lindenbach, B.D.; Horvath, T.L.; et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell 2016, 166, 1247–1256.e4.
- Caine, E.A.; Scheaffer, S.M.; Broughton, D.E.; Salazar, V.; Govero, J.; Poddar, S.; Osula, A.; Halabi, J.; Skaznik-Wikiel, M.E.; Diamond, M.S.; et al. Zika Virus Causes Acute Infection and Inflammation in the Ovary of Mice Without Apparent Defects in Fertility. J. Infect. Dis. 2019, 220, 1904–1914.
- Scott, J.M.; Lebratti, T.J.; Richner, J.M.; Jiang, X.; Fernandez, E.; Zhao, H.; Fremont, D.H.; Diamond, M.S.; Shin, H. Cellular and Humoral Immunity Protect against Vaginal Zika Virus Infection in Mice. J. Virol. 2018, 92, e00038-18.
- Carroll, T.; Lo, M.; Lanteri, M.; Dutra, J.; Zarbock, K.; Silveira, P.; Rourke, T.; Ma, Z.M.; Fritts, L.; O’Connor, S.; et al. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog. 2017, 13, e1006537.
- Osuna, C.E.; Lim, S.Y.; Deleage, C.; Griffin, B.D.; Stein, D.; Schroeder, L.T.; Omange, R.W.; Best, K.; Luo, M.; Hraber, P.T.; et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat. Med. 2016, 22, 1448–1455.
- Foy, B.D.; Kobylinski, K.C.; Chilson Foy, J.L.; Blitvich, B.J.; Travassos da Rosa, A.; Haddow, A.D.; Lanciotti, R.S.; Tesh, R.B. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882.
- Huits, R.; De Smet, B.; Arien, K.K.; Van Esbroeck, M.; Bottieau, E.; Cnops, L. Zika virus in semen: A prospective cohort study of symptomatic travellers returning to Belgium. Bull. World Health Organ. 2017, 95, 802–809.
- Mead, P.S.; Duggal, N.K.; Hook, S.A.; Delorey, M.; Fischer, M.; Olzenak McGuire, D.; Becksted, H.; Max, R.J.; Anishchenko, M.; Schwartz, A.M.; et al. Zika Virus Shedding in Semen of Symptomatic Infected Men. N. Engl. J. Med. 2018, 378, 1377–1385.
- Paz-Bailey, G.; Rosenberg, E.S.; Sharp, T.M. Persistence of Zika Virus in Body Fluids-Final Report. N. Engl. J. Med. 2019, 380, 198–199.
- Kurscheidt, F.A.; Mesquita, C.S.S.; Damke, G.; Damke, E.; Carvalho, A.; Suehiro, T.T.; Teixeira, J.J.V.; da Silva, V.R.S.; Souza, R.P.; Consolaro, M.E.L. Persistence and clinical relevance of Zika virus in the male genital tract. Nat. Rev. Urol. 2019, 16, 211–230.
- Govero, J.; Esakky, P.; Scheaffer, S.M.; Fernandez, E.; Drury, A.; Platt, D.J.; Gorman, M.J.; Richner, J.M.; Caine, E.A.; Salazar, V.; et al. Zika virus infection damages the testes in mice. Nature 2016, 540, 438–442.
- Uraki, R.; Hwang, J.; Jurado, K.A.; Householder, S.; Yockey, L.J.; Hastings, A.K.; Homer, R.J.; Iwasaki, A.; Fikrig, E. Zika virus causes testicular atrophy. Sci. Adv. 2017, 3, e1602899.
- Jacobs, S.; Delang, L.; Verbeken, E.; Neyts, J.; Kaptein, S.J.F. A Viral Polymerase Inhibitor Reduces Zika Virus Replication in the Reproductive Organs of Male Mice. Int. J. Mol. Sci. 2019, 20, 2122.
- Ma, W.; Li, S.; Ma, S.; Jia, L.; Zhang, F.; Zhang, Y.; Zhang, J.; Wong, G.; Zhang, S.; Lu, X.; et al. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell 2016, 167, 1511–1524.e10.
- Duggal, N.K.; Ritter, J.M.; Pestorius, S.E.; Zaki, S.R.; Davis, B.S.; Chang, G.J.; Bowen, R.A.; Brault, A.C. Frequent Zika Virus Sexual Transmission and Prolonged Viral RNA Shedding in an Immunodeficient Mouse Model. Cell Rep. 2017, 18, 1751–1760.
- Koide, F.; Goebel, S.; Snyder, B.; Walters, K.B.; Gast, A.; Hagelin, K.; Kalkeri, R.; Rayner, J. Development of a Zika Virus Infection Model in Cynomolgus Macaques. Front. Microbiol. 2016, 7, 2028.
- Polonio, C.M.; de Freitas, C.L.; Zanluqui, N.G.; Peron, J.P.S. Zika virus congenital syndrome: Experimental models and clinical aspects. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 41.
- Bido-Medina, R.; Wirsich, J.; Rodriguez, M.; Oviedo, J.; Miches, I.; Bido, P.; Tusen, L.; Stoeter, P.; Sadaghiani, S. Impact of Zika Virus on adult human brain structure and functional organization. Ann. Clin. Transl. Neurol. 2018, 5, 752–762.
- Creange, A. Guillain-Barre syndrome: 100 years on. Rev. Neurol. 2016, 172, 770–774.
- Goodfellow, J.A.; Willison, H.J. Guillain-Barre syndrome: A century of progress. Nat. Rev. Neurol. 2016, 12, 723–731.
- Willison, H.J.; Jacobs, B.C.; van Doorn, P.A. Guillain-Barre syndrome. Lancet 2016, 388, 717–727.
- Smith, D.R.; Hollidge, B.; Daye, S.; Zeng, X.; Blancett, C.; Kuszpit, K.; Bocan, T.; Koehler, J.W.; Coyne, S.; Minogue, T.; et al. Neuropathogenesis of Zika Virus in a Highly Susceptible Immunocompetent Mouse Model after Antibody Blockade of Type I Interferon. PLoS Negl. Trop. Dis. 2017, 11, e0005296.
- Dowall, S.D.; Graham, V.A.; Rayner, E.; Atkinson, B.; Hall, G.; Watson, R.J.; Bosworth, A.; Bonney, L.C.; Kitchen, S.; Hewson, R. A Susceptible Mouse Model for Zika Virus Infection. PLoS Negl. Trop. Dis. 2016, 10, e0004658.
- Aliota, M.T.; Caine, E.A.; Walker, E.C.; Larkin, K.E.; Camacho, E.; Osorio, J.E. Correction: Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004750.
- Julander, J.G.; Siddharthan, V. Small-Animal Models of Zika Virus. J. Infect. Dis. 2017, 216, S919–S927.
- Zmurko, J.; Marques, R.E.; Schols, D.; Verbeken, E.; Kaptein, S.J.; Neyts, J. The Viral Polymerase Inhibitor 7-Deaza-2’-C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model. PLoS Negl. Trop. Dis. 2016, 10, e0004695.
- Parke, D.W., 3rd; Almeida, D.R.; Albini, T.A.; Ventura, C.V.; Berrocal, A.M.; Mittra, R.A. Serologically Confirmed Zika-Related Unilateral Acute Maculopathy in an Adult. Ophthalmology 2016, 123, 2432–2433.
- Furtado, J.M.; Esposito, D.L.; Klein, T.M.; Teixeira-Pinto, T.; da Fonseca, B.A. Uveitis Associated with Zika Virus Infection. N. Engl. J. Med. 2016, 375, 394–396.
- Manangeeswaran, M.; Kielczewski, J.L.; Sen, H.N.; Xu, B.C.; Ireland, D.D.C.; McWilliams, I.L.; Chan, C.C.; Caspi, R.R.; Verthelyi, D. ZIKA virus infection causes persistent chorioretinal lesions. Emerg. Microbes Infect. 2018, 7, 96.
- Singh, P.K.; Guest, J.M.; Kanwar, M.; Boss, J.; Gao, N.; Juzych, M.S.; Abrams, G.W.; Yu, F.S.; Kumar, A. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight 2017, 2, e92340.
- Singh, P.K.; Kasetti, R.B.; Zode, G.S.; Goyal, A.; Juzych, M.S.; Kumar, A. Zika Virus Infects Trabecular Meshwork and Causes Trabeculitis and Glaucomatous Pathology in Mouse Eyes. mSphere 2019, 4, e00173-19.
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53.
- Aid, M.; Abbink, P.; Larocca, R.A.; Boyd, M.; Nityanandam, R.; Nanayakkara, O.; Martinot, A.J.; Moseley, E.T.; Blass, E.; Borducchi, E.N.; et al. Zika Virus Persistence in the Central Nervous System and Lymph Nodes of Rhesus Monkeys. Cell 2017, 169, 610–620.e4.
- Hirsch, A.J.; Smith, J.L.; Haese, N.N.; Broeckel, R.M.; Parkins, C.J.; Kreklywich, C.; DeFilippis, V.R.; Denton, M.; Smith, P.P.; Messer, W.B.; et al. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017, 13, e1006219.
- Silveira, E.L.V.; Rogers, K.A.; Gumber, S.; Amancha, P.; Xiao, P.; Woollard, S.M.; Byrareddy, S.N.; Teixeira, M.M.; Villinger, F. Immune Cell Dynamics in Rhesus Macaques Infected with a Brazilian Strain of Zika Virus. J. Immunol. 2017, 199, 1003–1011.
- O’Connor, M.A.; Tisoncik-Go, J.; Lewis, T.B.; Miller, C.J.; Bratt, D.; Moats, C.R.; Edlefsen, P.T.; Smedley, J.; Klatt, N.R.; Gale, M., Jr.; et al. Early cellular innate immune responses drive Zika viral persistence and tissue tropism in pigtail macaques. Nat. Commun. 2018, 9, 3371.
- Gurung, S.; Nadeau, H.; Maxted, M.; Peregrine, J.; Reuter, D.; Norris, A.; Edwards, R.; Hyatt, K.; Singleton, K.; Papin, J.F.; et al. Maternal Zika Virus (ZIKV) Infection following Vaginal Inoculation with ZIKV-Infected Semen in Timed-Pregnant Olive Baboons. J. Virol. 2020, 94, e00058-20.
More
Information
Subjects:
Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
647
Revisions:
2 times
(View History)
Update Date:
22 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




