Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anandkumar Naorem | -- | 3540 | 2022-09-21 08:24:46 | | | |
| 2 | Jessie Wu | + 5 word(s) | 3545 | 2022-09-22 07:29:06 | | | | |
| 3 | Jessie Wu | Meta information modification | 3545 | 2022-09-22 07:31:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Naorem, A.; Jayaraman, S.; Dalal, R.C.; Patra, A.; Rao, C.S.; Lal, R. Factors Affecting SIC Formation in Arid Soils. Encyclopedia. Available online: https://encyclopedia.pub/entry/27412 (accessed on 07 February 2026).
Naorem A, Jayaraman S, Dalal RC, Patra A, Rao CS, Lal R. Factors Affecting SIC Formation in Arid Soils. Encyclopedia. Available at: https://encyclopedia.pub/entry/27412. Accessed February 07, 2026.
Naorem, Anandkumar, Somasundaram Jayaraman, Ram C. Dalal, Ashok Patra, Cherukumalli Srinivasa Rao, Rattan Lal. "Factors Affecting SIC Formation in Arid Soils" Encyclopedia, https://encyclopedia.pub/entry/27412 (accessed February 07, 2026).
Naorem, A., Jayaraman, S., Dalal, R.C., Patra, A., Rao, C.S., & Lal, R. (2022, September 21). Factors Affecting SIC Formation in Arid Soils. In Encyclopedia. https://encyclopedia.pub/entry/27412
Naorem, Anandkumar, et al. "Factors Affecting SIC Formation in Arid Soils." Encyclopedia. Web. 21 September, 2022.
Copy Citation
Soil inorganic carbon (SIC) has received increasing attention due to the high accumulation of SIC in arid soils contributed by its high temperature, low soil moisture, less vegetation, high salinity, and poor microbial activities. SIC storage in dryland soils is a complex process comprising multiple interactions of several factors such as climate, land use types, farm management practices, irrigation, inherent soil properties, soil biotic factors, etc. In addition, soil C studies in deeper layers of drylands have opened-up several study aspects on SIC storage.
arid
carbonate
soil inorganic carbon
dryland
carbon
1. Climatic Factors
A growing body of research suggests that soil inorganic carbon (SIC) may be just as dynamic as SOC [1][2][3]. Kim et al. [4] pointed out a potentially dynamic SIC pool that is sensitive to hydrological changes. SIC storage can be affected by a wide number of factors, including climate, land use, and soil characteristics (Figure 1). Precipitation, temperature, and other climate factors significantly affect the processes of evaporation and leaching, which in turn influence the dissolution and re-precipitation of carbonates (Figure 1). Raheb et al. [5] investigated the influence of climates on soil C pools under arid, semi-arid, and dry sub-humid conditions along a soil climosequence. With the increase in mean annual precipitation, total SOC and SIC storage increased from 3.75 and 6.28 kg m−2 under arid and semi-arid conditions, respectively, to 11.32 kg m−2 under dry sub-humid conditions. Although SOC was found to be low in arid soils, the ratio of SIC/SOC was the highest in arid regions. The high value of this ratio depicts the crucial role of climate on SIC storage as compared to SOC [5]. However, the time required for SIC storage was found to be higher in drier conditions as Raheb et al. [5] calculated the average time (in years) needed to store SIC in arid (26,000 years), semi-arid (23,100 years), and sub-humid conditions (15,400 years).
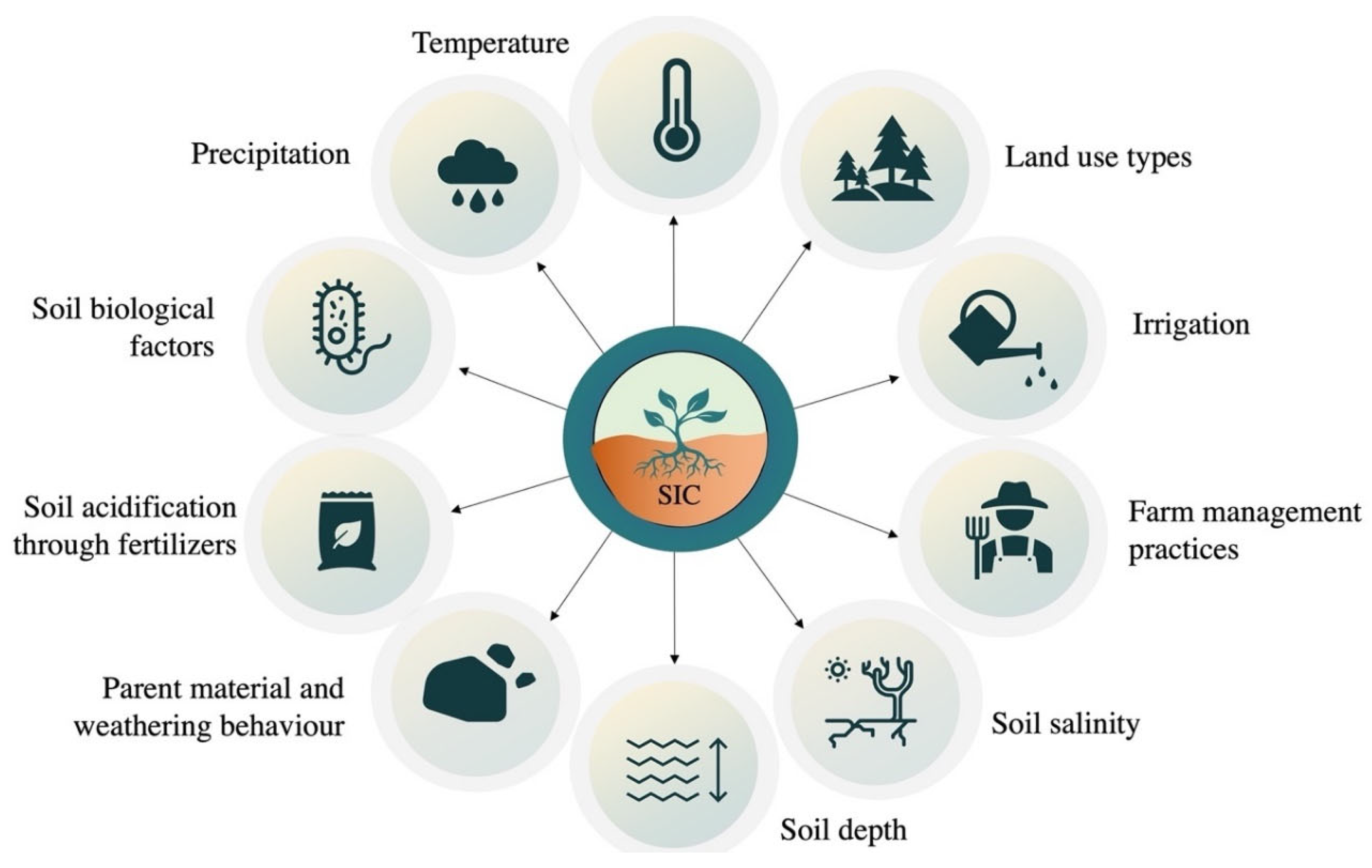
Figure 1. Natural and anthropogenic factors influencing the formation of soil inorganic carbon in dryland soils.
High evaporation: precipitation ratio inhibits carbonate dissolution and leaching, making arid soils rich in PIC [6]. According to Wu et al. [7], increasing aridity was associated with an increase in SIC content. This encourages the production and buildup of petrocalcic and calcic strata in arid places where there is little effective precipitation [8]. The humid circumstances, on the other hand, encourage a vigorous carbonate leaching process. Areas with annual precipitation of <500 mm have a greater concentration of SIC pool [8]. Mi et al. [9] revealed that 84% of China’s total SIC pool was concentrated in locations with an annual rainfall of <500 mm. A total of 4.19 Pg C is also stored in areas with mean annual precipitation between 500 and 800 mm. In another study conducted by Tan et al. [10] in the Loess Plateau region of China, 84% of SIC stock was concentrated in the regions with an annual rainfall of <500 mm. There are two ways that mean annual precipitation could influence carbonate formation: directly by changing the CaCO3 equilibrium solubility constant and indirectly by distributing precipitation inputs between leaching and evapotranspiration [11]. The seasonal drought period is the conducive time for precipitation of carbonates since both soil moisture and root activity are less during seasonal drought [12]. Additionally, elevation has a positive relationship with SIC due to its indirect effects on mean annual precipitation and mean annual temperature with increasing elevation [9].
2. Land Cover and Land Use
Due to changes in vegetation species and soil management approaches, land cover and land use types have a substantial impact on SIC content. Land-use changes from natural vegetation to cropland can rapidly induce the loss of SIC that has been stable for several years due to increased soil water fluxes [4]. SIC distribution patterns and stocks are also impacted by the vertical distribution of roots and SOC content in various land cover types [13]. Multiple biological parameters affecting the SIC pool can be altered by land use, including plant above- and below-ground biomass productivity, soil characteristics, and microbial processes [14]. Mi et al. [9] highlighted that the desert has the highest SIC, followed by grassland, farmland, marsh, shrubland, meadow, and forest. Through changes in C allocation, plant functional types could influence SIC distribution in deeper soil layers. Chang et al. [15] reported that the conversion of cropland to a forest in the central Loess Plateau led to the redistribution of SIC along the soil profile, but no increase in net SIC was observed. Jin et al. [16] found lower values of δ13CSIC in grassland than in the forest, indicating a greater generation of secondary carbonates in grassland than in the forest. SIC content and stock decreased during the conversion of cropland to grassland. During the tillage practices in grassland restoration, the SIC is vertically mixed, and further dissolution and leaching of carbonates to deeper soil layers might lower SIC during grassland restoration. However, the dissolution and leaching of SIC contribute to a little loss of SIC stock since restoration treatments significantly reduce surface runoff, which is one of the major causes of SIC loss [17]. Additionally, the rapidly growing plant biomass in a restored grassland takes up a considerable amount of Ca2+ in the soil and leads to a decline of Ca2+ in the soil. Moreover, the soil water content and high root biomass promote microbial activity and increase soil CO2 production, leading to higher soluble SIC [9]. SIC formation is found to be more sensitive in sandy soil than the clayey type of soil [18].
Other land-use studies in dryland soils reported a higher contribution of PIC to SIC in croplands than in grasslands [19]. Several authors have reported a high rate of SIC sequestration of 0.02 Mg C ha−1 year−1 under natural vegetation and up to 0.4 Mg C ha−1 year−1 in managed lands [20]. Murty et al. [21] indicated that in temperate and tropical conditions, conversion of grassland to agricultural land had increased CO2 emission with a decrease in soil C stock. Zhao et al. [22] found relatively higher SIC under the shrub cover than in forests and grassland. The larger quantity of PIC under the shrub cover is due to more Ca produced from litter under shrub cover, higher soil microbial biomass, and slow transfer of PIC owing to low soil water content under shrub cover. This indicates that shrubland is one of the best vegetations for the fixation of inorganic C in the soil.
Intensive cultivation practices such as deep tillage disturb the soil and break down soil aggregates [23]. Moreover, plant-derived C inputs to soil are generally less in farmland, thus affecting the dissolution and precipitation of SIC. Therefore, in general, cultivation leads to a decrease in SIC, and restoration of cropped lands could significantly promote SIC storage [24]. However, it is not necessarily true in all cases since carbonate precipitation or dissolution largely depends on soil pH and Ca2+/Mg2+ source. Soil pH and source of Ca2+ or Mg2+ control SIC and its precipitation, respectively [25]. Other researchers showed greater accumulation of both SOC and SIC stocks in agricultural than in non-agricultural lands under arid and semi-arid conditions, e.g., in the middle of the Hexi Corridor, Gansu, China [26], around the Yunwu Mountain, Ningxia, China and other parts of China [27], and in the Russian Chernozems [28] and Loess soils of Russia [29].
Wu et al. [7] reported that 51% of total cultivated soil in China showed SIC loss at the rate of 0.5–4.0 kg C m−2, from the 1980s to 2008, especially in paddy fields, irrigated farms, and dry farmland. Irrigation practices coupled with the application of acidifying fertilizers increase the loss of SIC from soil profile [30]. The carbonate weathering and erosion are further increased through agricultural practices by exposing the calciferous horizon to the soil surface [8]. On the other hand, an increase in SIC was observed in irrigated silty soils, irrigated desert soils, seirozems, and black soils [7]. Irrigation in arid and semi-arid soils increases plant biomass production, thus increasing plant respiration and microbial decomposition of SOM, releasing CO2 [8]. The elevated CO2 leads to increased weathering [31] and promotes the consumption of atmospheric CO2. Another probable reason is the external addition of Ca2+ and Mg2+ from irrigation water or the addition of fertilizer and manure that increases carbonate formation [23]. However, when such irrigation water is applied to arid non-alkaline soil where groundwater contains a high bicarbonate concentration, CO2 is released during carbonate precipitation [31]. These discrepancies complicate researcher's comprehension of the effect of land use changes on SIC or SOC dynamics in arid and semi-arid environments. Such inconsistencies between SIC stock and land use systems may also highlight the intricate interaction between climate, land management, and soil conditions and the formation of carbonates in arid soils [32].
3. Farm Management Practices
Farming practices such as intensive cropping, irrigation, residue, and fertilizer application/management may also increase SOC stock in agricultural lands, which leads to enhanced CO2 production and ultimately increased SIC stock [7]. Higher SIC density in agricultural land than in other land uses [33] may be due to the increased availability of Ca2+/Mg2+ associated with irrigation and fertilization [34]. To enhance carbonate accumulation in the soil profile, it is also essential to increase soil fertility. Wang et al. [34] demonstrated significant enhancement of carbonate accumulation (especially in subsoil) with the application of organic amendments in the cropland of North China. These results were also supported by Zhang et al. [35], who reported an increased SIC stock in fertile soils than in low fertile loess soils of the Lanzhou area, China. Few long-term experiments reported a varying contribution of PIC to SIC (29%–89%) [20][34][35], which indicates the possible major influence of crop management practices on SIC formation. Although, there is limited literature on pedogenic inorganic carbonates (PIC) contribution to SIC. In fact, intensive cropping may enhance PIC formation, and therefore, proper agricultural management is also essential to increase both the SOC and SIC storage in arid soils [36]. Thus, in order to understand SIC variability, it is important to study PIC dynamics in various soil types. Intensive tillage practices expose the calciferous horizon to the soil surface, thereby increasing carbonate weathering [8]. The possible fate of the SIC can be either it is leached down to deeper soil layers or has converted into bicarbonate that is further transported to groundwater or joined the river surfaces or lakes and ultimately to the ocean [37]. For example, the Yellow River across northern China has been experiencing an increase in dissolved Ca2+ and inorganic C over the past 40 years [38].
4. Irrigation
In croplands, irrigation has more pronounced effects on SIC losses than tillage or fertilization [4]. SIC studies under long-term irrigation have shown mixed results: SIC increases in irrigated treatment [7][39], null effect on SIC [40], and SIC increases only in limited irrigation [41]. Greater water content not only acts as a medium for dissolution and transport of carbonates but also allows SIC to rapidly re-equilibrate with CO2 present in soil pores [42]. The greater loss of SIC in irrigated cropland through carbonate dissolution is due to increased reactive area of dampened finer laminar coating of disseminated carbonates under croplands [6]. However, it is equally important to understand whether this SIC mobilization indicates a net C source or sink. The majority of irrigation water in such arid regions contains up to 1% dissolved CO2 [43]; increasing this concentration can accelerate the rate of carbonate production [31]. Due to high solar radiation in arid regions, irrigation water is typically warmer than groundwater [39]. The solubility of CO2 at 0 °C, 25 °C, and 400 °C is 0.02, 0.03, and 0.08 molL−1, respectively [31], demonstrating the solubility of CO2 in irrigation water is significantly temperature sensitive. When irrigation water reaches a field, its temperature can climb to as high as 2000 °C and is further elevated when it comes into touch with the soil surface on hot days. The higher temperature of irrigation water increases the response time and, under favorable conditions, may increase carbonate precipitation. In addition, as irrigation water runs through canals and agricultural fields, dissolved cations can cause its pH to rise. The high pH of the irrigation water may also promote SIC formation. Entry et al. [39] reported that there is a greater possibility of a higher potential amount of SIC sequestration if irrigated areas are enlarged, and land use patterns are altered.
Irrigation in arid areas is advantageous for SIC accumulation. Irrigating with Ca2+-rich water favor bicarbonate formation and an increase in SIC. It also helps in the redistribution of SIC to deeper soil layers. The increase in SIC in irrigated land as compared to rainfed soils is attributed to the dramatic increase in plant biomass production [7]. High biomass production enhances plant respiration and SOM decomposition, thus increasing soil CO2 levels [8]. Higher soil CO2 favors increased carbonate weathering and consumption of atmospheric CO2 [31]. Irrigated soils showing a decrease in SIC is primarily due to high leaching and maintenance of high water content at the soil surface, which is responsible for elevated CO2 concentration and greater dissolution of soil carbonates [44]. Efficient irrigation with leaching less than 30% of the applied water could favor carbonate accumulation in semi-arid and arid regions.
5. Soil Acidification through Fertilizers
A decrease in SIC in topsoil in croplands might possibly be due to a drop in soil pH linked to soil acidification from the application of chemical fertilizers [45]. The decline in soil pH will aid in the dissolution of soil carbonate and low SIC content. According to recent studies, fertilizer may increase soil acidity and cause soil carbonate to dissolve [6][46]. Soil acidification is one of the major global threats to the sustainable development of ecosystems [47] because a change in soil pH can regulate both the SOC and SIC dynamics. Although soil acidification is a natural process, it is accelerated by anthropogenic activities such as the long-term overuse of nitrogen fertilizers [48].
The change in soil pH in calcareous soil could be explained through three processes:
- (a)
-
Equations (3) and (4) depict the release of proton ions through nitrification and soil organic matter decomposition, respectively [22].
- (b)
-
Equations (5) and (6) depict the consumption of H+ ions during the dissolution of SIC, thus releasing CO2 [49].
- (c)
-
Leaching of dissolved inorganic C to groundwater [50].
The application of nitrogen fertilizer can reduce soil pH and alter the C balance in calcareous soils and promote acidification in soils [51]. In Equation (3), it is shown that with one mole of nitrate produced, two moles of H+ ions will be generated under aerobic conditions [52]. These protons are neutralized by SIC in calcareous soils [45]. Therefore, a lower soil pH in calcareous soils leads to a decline in SIC [51][53]. If the soil is limed, SIC will be released faster than the C from SOC [54]. In addition, although fertilizers are applied in topsoil, they can still cause soil acidification in deeper soil layers through the movement of protons, which is expected to induce SIC loss in the deeper soil layer [47].
SIC lost through degassing depends on the type and amount of fertilizers applied in the soil. Long-term application of nitrogen fertilizers causes agricultural acidification at a rate of 30–240 kmol H+ ha−1 year−1, leading to a maximum loss of 0.36–2.8 Mg C ha−1 year−1 [45][55]. Soil acidification is generated by nitrate leaching, removal of alkalinity during crop harvest, accelerated nitrogen fixation by legumes, and the application of ammonium-based fertilizers [56][57]. Soil acidification rates of 10 kmol H+ ha-1 yr−1 were found in highly exploitative agricultural soils [58]. This can be partially offset by accelerated calcite dissolution in calcareous soils, creating a potential net C sink on the order of 0.03–0.12 t C ha−1 yr−1 if all of the HCO−3 leached down the soil profile and join a long-lived reservoir [59].
6. Temperature
Temperature is another important factor that governs SIC storage in an arid region. An increase in global temperature could alter the dissolution of carbonate directly or indirectly through the products of SOC decomposition [60]. With the increase in temperature, the solubility of CO2 in water decreases, affecting the solubility of carbonates [61]. For example, Buysse et al. [62] reported greater CO2 emissions (due to greater CO2 solubility) from limed farmland soils at 5–15 °C than 15–25 °C. On the other hand, in an incubated study, Ahmad et al. [60] contradicted and reported a 59% increase in CO2 emissions from limed soils when the temperature increased from 20 °C to 40 °C. Surface reactions and mass transfer could be influenced by higher temperature, which further leads to higher carbonate dissolution and C release from soil carbonates. The increase in temperature also accelerates the supply of protons (through nitrification and/or humification) and the rate of lime dissolution. Higher temperature during the plant growing season also increases the release of CO2 from the rhizosphere due to higher soil respiration. The increased rate of proton release in the rhizosphere affects the soil pH and further increases carbonate dissolution. The temperature sensitivity of carbonate dissolution must be further studied to understand the effect of climate change on SIC sequestration.
7. Microbial Soil Factors
While most researchers considered the main mechanism of PIC formation as an abiotic process, some have identified the role of soil (micro) organisms in inducing CaCO3 precipitation [63]. Any biotic factors that affect the deposition rate of SOM also influence soil CaCO3 content [64]. Due to more soil microbial biomass, the unstabilized SOC is mineralized to produce more CO2, which further dissolves in the soil solution and forms carbonates, which later precipitate to CaCO3 in the presence of Ca released from the decomposed litter. This reaction sequesters one mole of CO2 to form one mole of PIC. On the other hand, high soil microbial biomass also means higher production of CO2 since half of the soil respiration is derived from microbial respiration [22]. The higher partial pressure of CO2 could promote the dissolution of PIC in the topsoil layer, which later becomes distributed to deeper soil layers and re-crystallizes under low soil water content. This process neither generates nor consumes CO2 [22].
8. Soil Depth
While assessing SOC and SIC stocks in desert soils, including shrub soils and agricultural soils, SOC showed a decreasing trend with an increase in depth, whereas the opposite trend was observed in the case of SIC. One of the most prevalent errors in C sequestration research under arid conditions is focusing solely on changes in soil total organic C at the surface (e.g., 0–20 cm depth) because sampling and data collection are relatively simple [65]. However, in shrublands, Jobbágy and Jackson [66] found that the relative distribution of SOC was significantly deeper in arid conditions (0–250 mm yr−1) than in semi-arid conditions (250–500 mm yr−1), while no such difference in the vertical distribution of SOC was observed in grasslands regardless of climates. This trend might be attributed to the fact that in arid shrublands, the relatively deep root system of the shrubs may lead to deeper soil C profiles than in arid grasslands [66].
Xie et al. [67] also reported the downward leaching of soil C containing water when dry areas are irrigated sufficiently. The amount of irrigation water used in dry areas influences the depth of water movement and thereby affecting the rate of inorganic C transportation throughout the soil profile. Higher SIC levels are formed in deeper soil layers, even below 2 m in the loess soil [27]. Therefore, the depth of the soil layer is one of the major factors in determining the profile distribution of inorganic C in saline/alkaline areas [32]. Management practices that increase soil erosion remove more weathered surface soils. While SIC is generally found in deeper soil layers, eroded soils show an increase in SIC in surface soils [44]. Approximately 80% of SIC is captured below 1 m, and 50% is stored below 3 m [32].
9. Parent Material
The parent material and its weathering behavior are other aspects that influence SIC formation [68]. For example, high SIC content in the Loess Plateau of China is associated with the primary aeolian deposit rich in CaCO3 [7]. Basalt weathering is crucial in the terrestrial C cycle because it exposes H+ (dissociated from H2CO3) and releases Ca2+ and Mg2+ cations, which combine with bicarbonates to form carbonates in soil [69]. Therefore, pedogenic forms of SIC are found in soil with carbonate-free parent rocks. In arid and semi-arid regions, PIC can also be accumulated in non-carbonate parent materials through the reaction of Ca2+ ions with water (from rainfall) and CO2 (derived from plant root respiration) [70]. Because the weathering rate of parent materials increases with the temperature only in the presence of water, the weathering rate in arid regions is slow.
10. Soil Inorganic Carbon in Salt-Affected Soils
Salt-affected soils tend to dominate in arid and semi-arid regions. Generally, owing to the poor soil’s physical and chemical properties and harsh climate, the plant productivity in salt-affected soils is generally poor. This leads to low plant biomass and lower inputs of organic materials, and low SOC. On the other hand, SIC levels can be high in salt-affected soils and are attributed to the high soil pH and high soil Ca2+ and Mg2+ that can enhance carbonate precipitation [71]. Schlesinger [72] reported poor SIC exchange with the atmosphere, as low as 1.0–5.0 g C m− 2yr− 1 in the desert soils. However, it could also be as high as 62–622 g C m− 2yr− 1 in salt-affected soils [3]. Sodic soil reclamation can both reduce or favor SIC accumulation in the soil [44]. With the application of gypsum to alkaline or sodic soils, the Ca precipitates the soluble bicarbonates and carbonates in the soil, resulting in an increase in SIC. On the other hand, green manuring and the application of sulfur and sulphuric acid tend to elevate CO2 concentration in the soil resulting dissolution of carbonate. Widespread use of acids to prevent emitter clogging in drip irrigation also removes a significant amount of carbonates within 10–20 years and for soils with <3% carbonates [44].
References
- Suarez, D.L. Impact of agriculture on CO2 as affected by changes in inorganic carbon. In Global Climate Change and Pedogenic Carbonates; Lal, R., Kimble, J.M., Eswaran, H., Stewart, B.A., Eds.; NHBS: Totnes, UK, 2000; pp. 257–272.
- Wohlfahrt, G.; Fenstermaker, L.F.; Arnone, J.A. Large annual net ecosystem CO2 uptake of a Mojave desert ecosystem. Global Change Biol. 2008, 14, 1475–1487.
- Guo, Y.; Wang, X.; Li, X.; Wang, J.; Xu, M.; Li, D. Dynamics of soil organic and inorganic carbon in the cropland of Upper Yellow River Delta, China. Sci. Rep. 2016, 6, 36105.
- Kim, J.H.; Jobbágy, E.G.; Richter, D.D.; Trumbore, S.E.; Jackson, R.B. Agricultural acceleration of soil carbonate weathering. Glob. Chang. Biol. 2020, 26, 5988–6002.
- Raheb, A.; Heidari, A.; Mahmoodi, S. Organic and inorganic carbon storage in soils along an arid to dry sub-humid climosequence in Northwest of Iran. Catena 2017, 153, 66–74.
- Zamanian, K.; Pustovoytov, K.; Kuzyakov, Y. Pedogenic Carbonates: Forms and formation processes. Earth Sci. Rev. 2016, 157, 1–17.
- Wu, H.; Guo, Z.; Gao, Q.; Peng, C. Distribution of soil inorganic carbon storage and its changes due to agricultural land use activity in China. Agric. Ecosyst. Environ. 2009, 129, 413–421.
- Lal, R.; Kimble, J.M.; Kimble, H. Pedogenic Carbonates and the Global Carbon Cycle; CRC Press (LewisPublishers): London, UK, 2000.
- Mi, N.A.; Wang, S.; Liu, J.; Yu, G.; Zhang, W.; Jobbágy, E. Soil inorganic carbon storage pattern in China. Glob. Chang. Biol. 2008, 14, 2380–2387.
- Tan, W.F.; Zhang, R.; Cao, H.; Huang, C.Q.; Yang, Q.K.; Wang, M.K.; Koopal, L.K. Soil inorganic carbon stock under different soil types and land uses on the Loess Plateau region of China. Catena 2014, 121, 22–30.
- Feng, Q.; Cheng, G.D.; Kunihiko, E. Carbon storage in desertified lands: A case study from North China. Geo J. 2000, 51, 181–189.
- Schlesinger, W.H. Carbon storage in the caliche of Arid soils: A case study from Arizona. Soil Sci. 1982, 133, 247–255.
- Deng, L.; Liu, G.B.; Shangguan, Z.P. Land-use conversion and changing soil carbon stocks in China’s “Grain-for-Green” program: A synthesis. Glob. Chang. Biol. 2014, 20, 3544–3556.
- Hombegowda, H.C.; van Straaten, O.; Köhler, M.; Hölscher, D. On the rebound: Soil organic carbon stocks can bounce back to near forest levels when agroforests replace agriculture in Southern India. Soil 2016, 2, 13–23.
- Chang, R.; Fu, B.; Liu, G.; Wang, S.; Yao, X. The effects of afforestation on soil organic and inorganic carbon: A case study of the Loess Plateau of China. Catena 2012, 95, 145–152.
- Jin, Z.; Dong, Y.; Wang, Y.; Wei, X.; Wang, Y.; Cui, B.; Zhou, W. Natural vegetation restoration is more beneficial to soil surface organic and inorganic carbon sequestration than tree plantation on the Loess Plateau of China. Sci. Total Environ. 2014, 485–486, 615–623.
- Wang, G.; Zhang, L.; Zhuang, Q.; Yu, D.; Shi, X.; Xing, S.; Xiong, D.; Liu, Y. Quantification of the soil organic carbon balance in the Tai-Lake paddy soils of China. Soil Tillage Res. 2016, 155, 95–106.
- Rasmussen, C. Distribution of soil organic and inorganic carbon pools by biome and soil taxa in Arizona. Soil Sci. Soc. Am. J. 2006, 70, 256–265.
- Gao, Y.; Dang, P.; Zhao, Q.; Liu, J.; Liu, J. Effects of vegetation rehabilitation on soil organic and inorganic carbon stocks in the Mu Us Desert, Northwest China. Land Degrad. Dev. 2018, 29, 1031–1040.
- Bughio, M.A.; Wang, P.; Meng, F.; Qing, C.; Kuzyakov, Y.; Wang, X.; Junejo, S.A. Neoformation of pedogenic carbonates by irrigation and fertilization and their contribution to carbon sequestration in soil. Geoderma 2016, 262, 12–19.
- Murty, D.; Kirschbaum, M.U.F.; Mcmurtrie, R.E.; Mcgilvray, H. Does conversion of forest to agricultural land change soil carbon and nitrogen? A review of the literature. Glob. Chang. Biol. 2002, 8, 105–123.
- Zhao, W.; Zhang, R.; Huang, C.; Wang, B.; Cao, H.; Koopal, L.K.; Tan, W. Effect of different vegetation cover on the vertical distribution of soil organic and inorganic carbon in the Zhifanggou watershed on the Loess Plateau. Catena 2016, 139, 191–198.
- West, T.O.; McBride, A.C. The contribution of agricultural lime to carbondioxide emissions in the United States: Dissolution, transport, and net emissions. Agric. Ecosyst. Environ. 2005, 108, 145–154.
- Woodbury, P.B.; Heath, L.S.; Smith, J.E. Effects of land use change on soil carbon cycling in the Conterminous United States from1900 to 2050. Glob. Biogeochem. Cycles 2007, 21, GB3006.
- Monger, H.; Martinez-Rios, J. Inorganic Carbon Sequestration in Grazing Lands. In The Potential of U.S. Grazing Lands to Sequester Carbon and Mitigate the Greenhouse Effect; CRC Press: Boca Raton, FL, USA, 2000.
- Su, Y.Z.; Wang, X.F.; Yang, R.; Lee, J. Effects of sandy decertified land rehabilitation on soil carbon sequestration and aggregation in an arid region in China. J. Environ. 2010, 91, 2109–2116.
- Lu, T.; Wang, X.; Zhang, W. Total and dissolved soil organic and inorganic carbon and their relationships in typical Loess cropland of Fengu Basin. Geosci. Lett. 2020, 7, 17.
- Mikhailova, E.A.; Post, C.J. Effects of land use on soil inorganic carbon stocks in the Russian Chernozem. J. Environ. Qual. 2006, 35, 1384–1388.
- Gocke, M.; Pustovoytov, K.; Kuzyakov, Y. Pedogenic carbonate formation: Recrystallization versus migration-process rates and periods assessed by 14C labeling. Glob. Biogeochem. Cycles 2012, 26, GB1018.
- Sartori, F.; Lal, R.; Ebinger, M.H.; Eaton, J.A. Changes in soil carbon and nutrient pools along a chronosequence of Poplar plantations in the Columbia Plateau, Oregon, USA. Agric. Ecosyst. Environ. 2007, 122, 325–339.
- Doner, H.E.; Lynn, W.C. Carbonate, halide, sulfate, and sulfide minerals. In SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 1989; pp. 279–330. ISBN 9780891188605.
- Wang, Z.P.; Han, X.G.; Chang, S.X.; Wang, B.; Yu, Q.; Hou, L.Y.; Li, L.H. Soil organic and inorganic carbon contents under various land uses across a transect of Continental Steppes in inner Mongolia. Catena 2013, 109, 110–117.
- Wang, X.; Wang, J.; Xu, M.; Zhang, W.; Fan, T.; Zhang, J. Carbon accumulation in arid croplands of Northwest China: Pedogenic carbonate exceeding organic carbon. Sci. Rep. 2015, 5, 11439.
- Wang, X.J.; Xu, M.G.; Wang, J.P.; Zhang, W.J.; Yang, X.Y.; Huang, S.M.; Liu, H. Fertilization enhancing carbon sequestration as carbonate in arid cropland: Assessments of long-term experiments in Northern China. Plant. Soil 2014, 380, 89–100.
- Zhang, F.; Wang, X.; Guo, T.; Zhang, P.; Wang, J. Soil organic and inorganic carbon in the Loess profiles of Lanzhou area: Implications of deep soils. Catena 2015, 126, 68–74.
- Lu, T.; Wang, X.; Xu, M.; Yu, Z.; Luo, Y.; Smith, P. Dynamics of pedogenic carbonate in the cropland of the North China plain: Influences of intensive cropping and salinization. Agric. Ecosyst. Environ. 2020, 292, 106820.
- Raymond, P.A.; Cole, J.J. Increase in the export of alkalinity from North America’s largest river. Science 2003, 301, 88–91.
- Chen, J.; He, D.; Cui, S. The response of river water quality and quantity to the development of irrigated agriculture in the last 4 decades in the Yellow River Basin, China. Water Resour. Res. 2003, 39, 1047.
- Entry, J.A.; Sojka, R.E.; Shewmarker, G.E. Irrigation increase inorganic carbon in agriculture soils. Environ. Manag. 2004, 33, 309–317.
- Denef, K.; Stewart, C.E.; Brenner, J.; Paustian, K. Does long-term center-pivot irrigation increase soil carbon stocks in semi-arid agro-ecosystems? Geoderma 2008, 145, 121–129.
- Halvorson, A.D.; Schlegel, A.J. Crop rotation effect on soil carbon and nitrogen stocks under limited irrigation. Agron. J. 2012, 104, 1265–1273.
- Gocke, M.; Pustovoytov, K.; Kuzyakov, Y. Carbonate recrystallization in root-free soil and rhizosphere of Triticum Aestivum and Lolium Perenne estimated by 14C labeling. Biogeochemistry 2010, 103, 209–222.
- Suarez, D.L. Ion activity products of calcium carbonate in waters below the root zone. Soil Sci. Soc. Am. J. 1977, 41, 310–315.
- Suarez, D.L. Inorganic Carbon: Land Use Impacts. In Encyclopedia of Soil Science; Lal, R., Ed.; Taylor and Francis: Abingdon, UK, 2006; pp. 597–895.
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010.
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob. Chang. Biol. 2020, 26, 3738–3751.
- Rengel, Z. (Ed.) Handbook of Soil Acidity; Marcel Dekker: New York, NY, USA, 2003; ISBN 9780824747398.
- Meng, H.Q.; Xu, M.G.; Lü, J.L.; He, X.H.; Li, J.W.; Shi, X.J.; Peng, C.; Wang, B.R.; Zhang, H.M. Soil pH dynamics and nitrogen transformations under long-term chemical fertilization in four Typical Chinese croplands. J. Integr. Agric. 2013, 12, 2092–2102.
- Ramnarine, R.; Wagner-Riddle, C.; Dunfield, K.E.; Voroney, R.P. Contributions of carbonates to soil CO2 emissions. Can. J. Soil Sci. 2012, 92, 599–607.
- Li, S.; Li, H.; Yang, C.; Wang, Y.; Xue, H.; Niu, Y. Rates of soil acidification in tea plantations and possible causes. Agric. Ecosyst. Environ. 2016, 233, 60–66.
- Yang, Y.; Ji, C.; Ma, W.; Wang, S.; Wang, S.; Han, W.; Mohammat, A.; Robinson, D.; Smith, P. Significant soil acidification across Northern China’s grasslands during 1980′s-2000′s. Glob. Chang. Biol. 2012, 18, 2292–2300.
- Bolan, N.S.; Adriano, D.C.; Curtin, D. Soil acidification and liming interactions with nutrient and heavy metal transformation and bioavailability. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2003; pp. 215–272. ISBN 9780120007967.
- Shi, Y.; Baumann, F.; Ma, Y.; Song, C.; Kühn, P.; Scholten, T.; He, J.S. Organic and inorganic carbon in the topsoil of the Mongolian and Tibetan grasslands: Pattern, control and implications. Biogeosciences 2012, 9, 2287–2299.
- Adams, T.M.; Adams, S.N. The effects of liming and soil pH on carbon and nitrogen contained in the soil biomass. J. Agric. Sci. 1983, 101, 553–558.
- Jin, S.; Tian, X.; Wang, H. Hierarchical responses of soil organic and inorganic carbon dynamics to soil acidification in a dryland agroecosystem, China. J. Arid Land 2018, 10, 726–736.
- Riley, D.; Barber, S.A. Bicarbonate accumulation and pH changes at Soybean (Glycine max Merr) root-soil interface. Soil Sci. Soc. Am. Proc. 1969, 33, 905–908.
- Mubarak, A.R.; Nortcliff, S. Calcium carbonate solubilization through H-proton release from some legumes grown in calcareous saline-sodic soils. Land Degrad. Dev. 2010, 21, 24–31.
- Helyar, K.R.; Cregan, P.D.; Godyn, D.L. Soil acidity in New South Wale– Current pH values and estimates of acidification rates. Aust. J. Soil Res. 1990, 28, 523–537.
- Sanderman, J. Can management induced changes in the carbonate system drive soil carbon sequestration? A review with particular focus on Australia. Agric. Ecosyst. Environ. 2012, 155, 70–77.
- Ahmad, W.; Singh, B.; Dalal, R.C.; Dijkstra, F.A. Carbon dynamics from carbonate dissolution in Australian agricultural soils. Soil Res. 2015, 53, 144.
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 Bar. Chem. Geol. 2003, 193, 257–271.
- Buysse, P.; Roisin, C.; Aubinet, M. Fifty years of contrasted residue management of an agricultural crop: Impacts on the soil carbon budget and on soil heterotrophic respiration. Agric. Ecosyst. Environ. 2013, 167, 52–59.
- Zhang, N.; He, X.D.; Gao, Y.B.; Li, Y.H.; Wang, H.T.; Ma, D.; Zhang, R.; Yang, S. Pedogenic carbonate and soil dehydrogenase activity in response to soil organic matter in Artemisia ordosica community. Pedosphere 2010, 20, 229–235.
- Zhou, L.K. Soil Enzyme; Science Press: Beijing, China, 1987.
- Harrison, R.B.; Footen, P.W.; Strahm, B. Deep soil horizons: Contribution and importance to soil carbon pools and in assessing whole ecosystem response to management and global change. Forest Sci. 2011, 57, 67–76.
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423.
- Xie, H.L.; Wang, Y.G.; Li, Y. Study on carbon leaching test of irrigation in arid area. Arid Zone 2015, 32, 903–909.
- McLauchlan, K. The nature and longevity of agricultural impacts on soil carbon and nutrients: A review. Ecosystems 2006, 9, 1364–1382.
- Dessert, C.; Dupré, B.; Gaillardet, J.; François, L.M.; Allègre, C.J. Basalt weathering laws and the impact of basalt weathering on the global carbon cycle. Chem. Geol. 2003, 202, 257–273.
- Brock, A.L.; Buck, B.J. Polygenetic development of the Mormon Mesa, NV petrocalcic horizons: Geomorphic and paleoenvironmental interpretations. Catena 2009, 77, 65–75.
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium mediated stabilization of soil organic carbon. Biogeochemistry 2018, 137, 27–49.
- Schlesinger, W.H. Biogeochemistry. Geotimes 1997, 423, 44.
More
Information
Subjects:
Soil Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
3 times
(View History)
Update Date:
27 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




