
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Agenor Santos | + 7839 word(s) | 7839 | 2020-10-05 15:23:18 | | | |
| 2 | Vivi Li | -6091 word(s) | 1748 | 2020-10-23 07:00:11 | | |
Video Upload Options
Conventional petrochemical plastics have become a serious environmental problem. Its unbridled use, especially in non-durable goods, has generated an accumulation of waste that is difficult to measure, threatening aquatic and terrestrial ecosystems. The replacement of these plastics with cleaner alternatives, such as polyhydroxyalkanoates (PHA), can only be achieved by cost reductions in the production of microbial bioplastics, in order to compete with the very low costs of fossil fuel plastics. The biggest costs are carbon sources and nutrients, which can be appeased with the use of photosynthetic organisms, such as cyanobacteria, that have a minimum requirement for nutrients, and also using agro-industrial waste, such as the livestock industry, which in turn benefits from the by-products of PHA biotechnological production, for example pigments and nutrients. Circular economy can help solve the current problems in the search for a sustainable production of bioplastic: reducing production costs, reusing waste, mitigating CO2, promoting bioremediation and making better use of cyanobacteria metabolites in different industries.
1. Introduction
An urgent demand for biotechnology is to find alternatives to conventional plastics, derived from hydrocarbons, which are harmful to the environment not only in its exploration and refining, but also in its disposal. In 2018, more than 359 million tons of plastic was produced worldwide [1]. Since traditional plastic is not biodegradable, it depends on human action for its degradation, however a very small portion of fossil plastic is actually recycled. About 35.4 million tons of plastic is discarded annually by the United States alone, and only an estimated 8.4% is sent for recycling [2]. In the last 50 years, we have primarily and almost exclusively depended on petrochemical plastics, due to its wide range of applications and cheap manufacture; for example, in 1995, a kilo of polypropylene cost less than US $1.00 to produce, which justifies the predilection for this type of polymer [3].
The consequences of its unbridled use are already visible and have been studied for a long time. Since the 1970s, researchers have been warning about the high prevalence of microplastics, the result of the wear of fossil fuel plastics with a size of less than 1 mm, in marine environments and its damage to this ecosystem [4]. Alternatives to conventional plastics are being studied and some are already on the market, these polymers can be classified as polynucleotides, polyamides, polysaccharides, polyoxoesters, polythioesters, polyphosphates, polyisoprenoides and polyphenols [5]. We focus here on polyhydroxyalkanoates (PHA), especially polyhydroxybutyrates (PHB), examples of polyesters, which have similar applications as polypropylene with physical-chemical characteristics comparable to this petrochemical plastic [6]. In addition to its favorable structural properties, this thermoplastic of natural origin is biodegradable, water resistant and liable to be manipulated by techniques that are already widespread in the industry, such as injection, being better absorbed by current industrial equipment [7].
An alternative has to be found, one that does not produce non-biodegradable waste such as petrochemical residues with its high molecular masses accumulating in the soil and water for a long period of time [8][9]. Despite the environmental advantages of PHA over conventional plastics, for its replacement to be a reality, it is necessary to reduce the costs associated with the microbiological production of these biopolymers. The main obstacle in the process is the carbon source used to maintain fermentation costs, the yield of the chosen entries, the productivity and the downstream processing, including purification [10][11].
The use of cyanobacteria as industrial PHA producers makes it possible to reduce the cost of nutritional inputs, since these photosynthetic organisms have fewer nutritional needs than heterotrophic bacteria [12][13]. The potential application of cyanobacteria by-products in industries with high added value [14][15] is interesting from an economic and environmental point of view, even more so if this system is implemented in light of the circular economy (Figure 1).
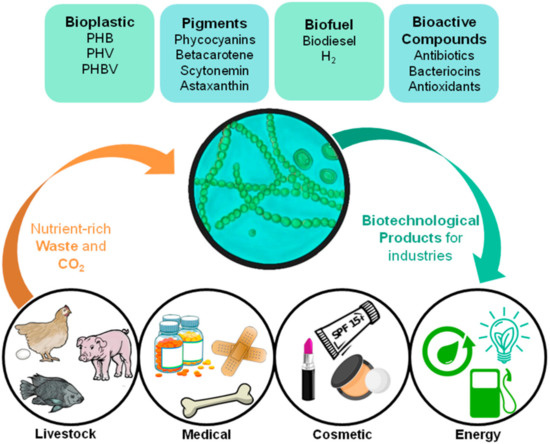
Figure 1. Diagrammatic representation showing cyanobacteria’s role in a circular economy-based system for various industries, and its possible products and waste assimilation.
2. Cyanobacteria Potential Application in Circular Economy
Despite the advantages PHA has over conventional plastics in terms of sustainability, for fossil plastic to be viably replaced, it is necessary to reduce the costs associated with the microbiological plastic production. Research and investments in the area have been making production cheaper. In 2002, the cost for manufacturing conventional petroleum-based plastic was €1.00/kg, a fraction of the PHA cost of €9.00/kg [16]. Two decades later, microbiological production of PHA can be obtained at €2.49/kg, which is still expensive, even compared to other sustainable polymers, such as PLA, costing €1.72/kg [9].
The main obstacles in the process concerns the carbon source used [17], the costs of maintaining the fermentation, the yield of the chosen inputs, the productivity and the downstream processing, including the extraction and purification of the polymer [10][11]. There are different strategies to face these obstacles; here, we will address only a few that are related to circular economy and industrial ecosystems, an approach that has already been applied with microalgae and heterotrophic bacteria [18][19][20][21][22]. The use of cyanobacteria is interesting because of the possibility of integrated production of different metabolites—with more than one type of compound as a salable product—and application of a “cradle-to-cradle” system [23], using by-products or production residues as a substrate for another product. Like the use of carbon monoxide (CO) in synthesis gas (syngas) for the production of PHB by the proteobacterium Rhodospirillum rubrumde [24], this author evens refers to this process as “grave-to-cradle”, turning a waste into a new product, bioplastic. Another example of waste being reapplied to the production process, now using microalgae, is the reuse of effluents from the refining of olive oil in the cultivation of microalgae for biodiesel and biopolymers [25]. This approach can benefit from the implementation and maintenance of an “inter-system ecology”, associating different industries [15][26].
From an environmental point of view, cyanobacteria are well-used as bioremediators, feeding on nutrients from domestic and agro-industrial waste, promoting nutrient removal and detoxification, removing heavy metals [27][28][29]. The assimilation of atmospheric carbon dioxide for conversion into biotechnological products [30][31] is another positive environmental impact, making the implementation of a circular bioeconomy more tangible.
An alternative to make microbial PHB cheaper is to integrate the production of bioplastic with other desirable products, reusing by-products and residues of the microbiological production [14][32][33]. The production of acids for the cosmetic and pharmaceutical industry, such as eicosapentaenoic acid, by cyanobacteria of the genus Nannochloropsis sp., and γ-linoleic acid by cyanobacteria Spirulina platensis, is a viable alternative [34]. This species is also relevant for its expressive biomass production, with high protein content, suitable for application in nutraceuticals or animal feed [35][36]. The implementation of a biorefinery, integrating the PHB production of Synechocystis salina, with pigments of commercial interest, specifically phycocyanin and chlorophyll, commonly abundant in this phylum, and carotenoids, presented promising results [37]. The extraction of pigments without their degradation is not only possible, but essential, as the quality of the obtained polymer is directly affected by purification, which includes the removal of pigments, that can be used in production chains of higher value.
In addition to pigments, S. salina biomass has carbohydrates, lipids and proteins [37], which can be used for animal feed [38], provided that the necessary nutritional requirements and laws regarding the presence of contaminants such as heavy metals or mycotoxins are observed [39], and in this case, cyanotoxins [40], giving priority to non-toxin-producing cyanobacteria. The residual biomass of cyanobacteria would therefore be well-used in the nutrition of livestock and aquaculture, but it is possible to go further in the optimization of this production chain. Residues from these same livestock farming can be re-applied as supplementary nutrients to the growth of cyanobacteria in an integrated bio-factory [41]. The return of cyanobacterial by-products such as pigments and biomass to animal feed completes the proposed circular economy. Still, using Spirulina sp. as an example, its supplementation to animal feed has already been studied in shrimp, fish and chicken farming [38][42], valuing the production of this associated industry, improving the growth and coloring of tilapia [43] and egg yolks of chickens fed with S. platensis astaxanthin [44]. Animal health is also benefited by nutritional supplementation with cyanobacteria, with Spirulina sp. biomass improving the humoral and immunological response of chickens [45][46].
The dual advantage of production associated with bioremediation has already been described for cyanobacteria and microalgae in general, mainly aimed at the production of biodiesel [19][47][48][49]. The same concept can be applied to the production of biopolymers by cyanobacteria [15], naturally transformable organisms, which opens up possibilities for genetic engineering [50][51].
As a way to take advantage of Amazonian biodiversity in the search for microbial metabolites, in recent years, research with cyanobacteria from the Amazon has been developed with good results, and the sequencing of their genomes is an important tool in the search for compounds of biotechnological interest [52][53]. These organisms proved to be good producers of biodiesel, with yields higher than those in the literature and with parameters following international standards [54][55]. Biopolymer has also been detected in cyanobacteria in the region, with efforts being made to increase its production [56]. Subsequent work in this field would benefit from the approach proposed here of a circular economy, optimizing the resources employed, handling the waste and using its by-products and industrial “waste”, which is, as seen here, a potential feedstock for new biotechnological processes.
3. Conclusions
PHB-producing cyanobacteria, due to its lower productivity, in comparison to heterotrophic bacteria, are commercially viable only if combined with exploration of their various metabolites. Feed costs must be reduced, and using non-conventional feedstock, preferably agro-industrial waste, this opens up the possibility of industrial implementation within a circular economy, with the production of more than one product from this bio-factory, or with the use of by-products in other sectors, returning, for example, as animal nutrition to the industry itself, whose residues supplement cyanobacterial growth. Another great advantage of cyanobacteria in relation to other microorganisms producing PHB is its photosynthetic capacity, acting as a solar cell, reducing the CO2 of the environment, which is an assimilated carbon source and can be used as building blocks for the cyanobacterial production of bioplastic.
The critical reading of the studies already carried out in the area shows promising results for the manufacture and large-scale use of cyanobacterial biopolymers to be a reality in the near future, and more than that, demonstrates the need for cooperation between different knowledge and industries and through a circular economy to optimize the production process and reduce environmental impacts.
Author Contributions
Conceptualization, A.V.S. and D.G.G.; Investigation, D.G.G.; Writing—original draft preparation, D.G.G.; Writing—review and editing, A.V.S. and L.P.X.; Supervision, A.V.S. and L.P.X.; Project administration, A.V.S.; Funding acquisition, A.V.S. All authors have read and agreed to the published version of the manuscript.
References
- Plastics—The Facts 2019. Available online: https://issuu.com/plasticseuropeebook/docs/final_web_version_plastics_the_facts2019_14102019 (accessed on 24 June 2020).
- US EPA Plastics: Material-Specific Data. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data (accessed on 24 June 2020).
- Koller, M.; Hesse, P.; Kutschera, C.; Bona, R.; Nascimento, J.; Ortega, S.; Agnelli, J.A.; Braunegg, G. Sustainable Embedding of the Bioplastic Poly-(3-Hydroxybutyrate) into the Sugarcane Industry: Principles of a Future-Oriented Technology in Brazil. In Polymers-Opportunities and Risks II; Eyerer, P., Weller, M., Hübner, C., Eds.; Springer: Berlin, Germany, 2009; Volume 12, pp. 81–96. ISBN 9783642027963. [Google Scholar]
- Carpenter, E.J.; Smith, K.L. Plastics on the Sargasso Sea Surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef] [PubMed]
- Dhaman, Y.; Ugwu, C.U. Poly[(R)-3-hydroxybutyrate]: The Green Biodegradable Bioplastics of the Future! Ferment. Technol 2012, 01. [Google Scholar] [CrossRef]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Wu, G.F.; Wu, Q.Y.; Shen, Z.Y. Accumulation of poly-β-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresour. Technol. 2001, 76, 85–90. [Google Scholar] [CrossRef]
- Akaraonye, E.; Keshavarz, T.; Roy, I. Production of polyhydroxyalkanoates: The future green materials of choice. J. Chem. Technol. Biotechnol. 2010, 85, 732–743. [Google Scholar] [CrossRef]
- Karan, H.; Funk, C.; Grabert, M.; Oey, M.; Hankamer, B. Green Bioplastics as Part of a Circular Bioeconomy. Trends Plant. Sci. 2019, 24, 237–249. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.Y. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl. Microbiol. Biotechnol. 1999, 51, 13–21. [Google Scholar] [CrossRef]
- Lee, G.; Na, J. Future of microbial polyesters. Microb. Cell Fact. 2013, 12, 54. [Google Scholar] [CrossRef]
- Balaji, S.; Gopi, K.; Muthuvelan, B. A review on production of poly β hydroxybutyrates from cyanobacteria for the production of bio plastics. Algal Res. 2013, 2, 278–285. [Google Scholar] [CrossRef]
- Singh, A.K.; Mallick, N. Advances in cyanobacterial polyhydroxyalkanoates production. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Drosg, B.; Fritz, I.; Gattermyer, F.; Silvestrini, L. Photo-autotrophic Production of Poly(hydroxyalkanoates) in Cyanobacteria. Chem. Biochem. Eng. Q. 2015, 29, 145–156. [Google Scholar] [CrossRef]
- Arias, D.M.; García, J.; Uggetti, E. Production of polymers by cyanobacteria grown in wastewater: Current status, challenges and future perspectives. New Biotechnol. 2020, 55, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.A.M.; Serafim, L.S.; Lemos, P.C.; Ramos, A.M.; Aguiar, F.R.; Van Loosdrecht, M.C.M. Production of polyhydroxyalkanoates by mixed microbial cultures. Bioprocess. Biosyst. Eng. 2003, 25, 377–385. [Google Scholar] [CrossRef]
- Halami, P.M. Production of polyhydroxyalkanoate from starch by the native isolate Bacillus cereus CFR06. World J. Microbiol. Biotechnol. 2008, 24, 805–812. [Google Scholar] [CrossRef]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Fuentes Grünewald, C.; Lovitt, R.; et al. Using microalgae in the circular economy to valorise anaerobic digestate: Challenges and opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Chang, C.-H.; Chen, C.-Y.; Chang, J.-S.; Ng, I.-S. Towards protein production and application by using Chlorella species as circular economy. Bioresour. Technol. 2019, 289, 121625. [Google Scholar] [CrossRef]
- Blank, L.M.; Narancic, T.; Mampel, J.; Tiso, T.; O’Connor, K. Biotechnological upcycling of plastic waste and other non-conventional feedstocks in a circular economy. Curr. Opin. Biotechnol. 2020, 62, 212–219. [Google Scholar] [CrossRef]
- Kasting, J.F. EARTH HISTORY: The Rise of Atmospheric Oxygen. Science 2001, 293, 819–820. [Google Scholar] [CrossRef] [PubMed]
- Zinn, M.; Amstutz, V.; Hanik, N.; Pott, J.; Utsunomia, C. Grave-to-cradle: The potential of autotrophic bioprocesses in bioplastic production. New Biotechnol. 2018, 44, S64. [Google Scholar] [CrossRef]
- Morillo, J.A.; Antizar-Ladislao, B.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A.; Russell, N.J. Bioremediation and biovalorisation of olive-mill wastes. Appl. Microbiol. Biotechnol. 2009, 82, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular Economy: The Concept and its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Samantaray, S.; Nayak, J.K.; Mallick, N. Wastewater Utilization for Poly-β-Hydroxybutyrate Production by the Cyanobacterium Aulosira fertilissima in a Recirculatory Aquaculture System. Appl. Environ. Microbiol. 2011, 77, 8735–8743. [Google Scholar] [CrossRef]
- Honda, R.; Boonnorat, J.; Chiemchaisri, C.; Chiemchaisri, W.; Yamamoto, K. Carbon dioxide capture and nutrients removal utilizing treated sewage by concentrated microalgae cultivation in a membrane photobioreactor. Bioresour. Technol. 2012, 125, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of Green Algae Chlorella sp. in Different Wastewaters from Municipal Wastewater Treatment Plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, W.J.; MacDougall, K.M.; Melanson, J.E.; O’Leary, S.J.B.; McGinn, P.J. Pilot-scale supercritical carbon dioxide extractions for the recovery of triacylglycerols from microalgae: A practical tool for algal biofuels research. J. Appl. Phycol. 2012, 24, 547–555. [Google Scholar] [CrossRef]
- Liebal, U.W.; Blank, L.M.; Ebert, B.E. CO2 to succinic acid—Estimating the potential of biocatalytic routes. Metab. Eng. Commun. 2018, 7, e00075. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Haas, R.; Jin, B.; Zepf, F.T. Production of Poly(3-hydroxybutyrate) from Waste Potato Starch. Biosci. Biotechnol. Biochem. 2008, 72, 253–256. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Garcia-Perez, J.S.; Rittmann, B.E.; Parra-Saldivar, R. Photosynthetic bioenergy utilizing CO2: An approach on flue gases utilization for third generation biofuels. J. Clean. Prod. 2015, 98, 53–65. [Google Scholar] [CrossRef]
- Shrivastav, A.; Mishra, S.K.; Mishra, S. Polyhydroxyalkanoate (PHA) synthesis by Spirulina subsalsa from Gujarat coast of India. Int. J. Biol. Macromol. 2010, 46, 255–260. [Google Scholar] [CrossRef]
- Belay, A.; Kato, T.; Ota, Y. Spirulina (Arthrospira): Potential application as an animal feed supplement. J. Appl. Phycol. 1996, 8, 303–311. [Google Scholar] [CrossRef]
- Meixner, K.; Kovalcik, A.; Sykacek, E.; Gruber-Brunhumer, M.; Zeilinger, W.; Markl, K.; Haas, C.; Fritz, I.; Mundigler, N.; Stelzer, F.; et al. Cyanobacteria Biorefinery—Production of poly(3-hydroxybutyrate) with Synechocystis salina and utilisation of residual biomass. J. Biotechnol. 2018, 265, 46–53. [Google Scholar] [CrossRef]
- Ansari, S.; Fatma, T. Cyanobacterial Polyhydroxybutyrate (PHB): Screening, Optimization and Characterization. PLoS ONE 2016, 11, e0158168. [Google Scholar] [CrossRef] [PubMed]
- Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed-COUNCIL Statement. Available online: https://eur-lex.europa.eu/eli/dir/2002/32/2015-02-27 (accessed on 25 June 2020).
- Gradíssimo, D.G.; Mourão, M.M.; Santos, A.V. Importância do Monitoramento de Cianobactérias e Suas Toxinas em Águas Para Consumo Humano. J. Crim. 2020, 9, 15–21. [Google Scholar] [CrossRef]
- Price, S.; Kuzhiumparambil, U.; Pernice, M.; Ralph, P.J. Cyanobacterial polyhydroxybutyrate for sustainable bioplastic production: Critical review and perspectives. J. Environ. Chem. Eng. 2020, 8, 104007. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Triratana, P.; Loha, V.; Tia, S.; Bunnag, B. Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. Int. J. Biol. Macromol. 2013, 58, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.G.; Chaves, F.H.; Barros, R.N.; Moreira, R.L.; Teixeira, E.G.; Moreira, A.G.; Farias, W.R. Dietary supplementation with Spirulina platensis increases growth and color of red tilapia. Rev. Colomb. Ciencias Pecu 2012, 25, 462–471. [Google Scholar]
- Zahroojian, N.; Moravej, H.; Shivazad, M. Effects of Dietary Marine Algae (Spirulina platensis) on Egg Quality and Production Performance of Laying Hens. J. Agric. Sci. Technol. 2013, 15, 1353–1360. [Google Scholar]
- Qureshi, M.A.; Garlich, J.D.; Kidd, M.T. Dietary Spirulina Platensis Enhances Humoral and Cell-Mediated Immune Functions in Chickens. Immunopharmacol. Immunotoxicol. 1996, 18, 465–476. [Google Scholar] [CrossRef]
- Rajeev, K.J.; Xu, Z. Biomedical Compounds from Marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar] [CrossRef]
- Briens, C.; Piskorz, J.; Berruti, F. Biomass Valorization for Fuel and Chemicals Production—A Review. Int. J. Chem. React. Eng. 2008, 6. [Google Scholar] [CrossRef]
- De Godos, I.; Blanco, S.; García-Encina, P.A.; Becares, E.; Muñoz, R. Long-term operation of high rate algal ponds for the bioremediation of piggery wastewaters at high loading rates. Bioresour. Technol. 2009, 100, 4332–4339. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, L.; Subramanian, G.; Nazeer, T.T.; Simpson, H.S.; Rahuman, S.T.; Raju, P. Cyanobacteria cultivation in industrial wastewaters and biodiesel production from their biomass: A review. Biotechnol. Appl. Biochem. 2011, 58, 220–225. [Google Scholar] [CrossRef]
- Kamravamanesh, D.; Pflügl, S.; Nischkauer, W.; Limbeck, A.; Lackner, M.; Herwig, C. Photosynthetic poly-β-hydroxybutyrate accumulation in unicellular cyanobacterium Synechocystis sp. PCC 6714. AMB Expr. 2017, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Sarsekeyeva, F.; Zayadan, B.K.; Usserbaeva, A.; Bedbenov, V.S.; Sinetova, M.A.; Los, D.A. Cyanofuels: Biofuels from cyanobacteria. Reality and perspectives. Photosynth. Res. 2015, 125, 329–340. [Google Scholar] [CrossRef]
- Lima, A.R.J.; Siqueira, A.S.; dos Santos, B.G.S.; da Silva, F.D.F.; Lima, C.P.; Cardoso, J.F.; Vianez Junior, J.L.d.S.G.; Dall’Agnol, L.T.; McCulloch, J.A.; Nunes, M.R.T.; et al. Draft Genome Sequence of the Brazilian Cyanobium sp. Strain CACIAM 14. Genome Announc. 2014, 2, e00669-14. [Google Scholar] [CrossRef]
- Lima, A.R.J.; de Castro, W.O.; Moraes, P.H.G.; Siqueira, A.S.; Aguiar, D.C.F.; de Lima, C.P.S.; Vianez-Júnior, J.L.S.G.; Nunes, M.R.T.; Dall’Agnol, L.T.; Gonçalves, E.C. Draft Genome Sequence of Alkalinema sp. Strain CACIAM 70d, a Cyanobacterium Isolated from an Amazonian Freshwater Environment. Genome Announc. 2017, 5, e00635-17. [Google Scholar] [CrossRef]
- Aboim, J.B.; Oliveira, D.; Ferreira, J.E.; Siqueira, A.S.; Dall’Agnol, L.T.; Rocha Filho, G.N.; Gonçalves, E.C.; Nascimento, L.A. Determination of biodiesel properties based on a fatty acid profile of eight Amazon cyanobacterial strains grown in two different culture media. RSC Adv. 2016, 6, 109751–109758. [Google Scholar] [CrossRef]
- de Oliveira, D.T.; Turbay Vasconcelos, C.; Feitosa, A.M.T.; Aboim, J.B.; de Oliveira, A.N.; Xavier, L.P.; Santos, A.S.; Gonçalves, E.C.; da Rocha Filho, G.N.; do Nascimento, L.A.S. Lipid profile analysis of three new Amazonian cyanobacteria as potential sources of biodiesel. Fuel 2018, 234, 785–788. [Google Scholar] [CrossRef]
- Gradíssimo, D.G.; Mourão, M.M.; do Amaral, S.C.; Lima, A.R.J.; Gonçalves, E.C.; Xavier, L.P.; Santos, A.V. Potencial produção de biomaterial pela cianobactéria amazônica Tolypothrix SP. CACIAM 22. In A Produção do Conhecimento nas Ciências da Saúde; Atena Editora: Belo Horizonte, Brazil, 2019; pp. 213–224. ISBN 9788572472982. [Google Scholar]




