
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Radu Claudiu Fierascu | -- | 3967 | 2022-09-20 10:54:51 | | | |
| 2 | Peter Tang | Meta information modification | 3967 | 2022-09-20 11:34:45 | | |
Video Upload Options
Agro-industrial side streams covers a wide range of products available as raw materials. These can serve as a source of other added value products, under the nomenclature of biowaste. Food waste are generated from different sectors of food industry, such as vegetables, fruits, milk, meat, fish, and wine production. Recovered compounds can be re-utilized as food additives, functional foods, nutra-/pharmaceuticals, cosmeceuticals, beauty products, and bio-packaging.
1. Introduction
|
Compound Group |
Source |
Extracted Compounds |
Ref. |
|---|---|---|---|
|
Phenolic compounds |
Apple seeds |
Phloridzin, ellagic acid, caffeic acid, ferrulic acid, protocatechuic acid, gallic acid |
[11] |
|
Phenolic compounds |
Avocado seeds |
Procyanidin B2, epicatechin, rans-5-O-caffeoyl-d-quinic acid, procyanidin B1, catechin |
[12] |
|
Phenolic compounds |
Rapeseed cake |
Sinapine, sinapic acid and canolol |
[13] |
|
Phenolic compounds |
Citrus peel |
Total phenolic content |
[14] |
|
Phenolic compounds |
Coconut shell |
Total phenolic content |
[15] |
|
Phenolic compounds |
Grape marc, Orange peel, Strawberry, Citrus pulp Camelina cake |
Total phenolic content |
[16] |
|
Phenolic compounds |
Black currant Sea buckthorn |
Delphinidin 3-O-rutinoside, delphinidin 3-O-glucoside, cyanidin 3-O-rutinoside, cyaniding-3-O-glucoside, ellagitannins, proanthocyanidins, p-coumaric acid, caffeic acid-hexosides, coumaroylquinic acid-hexosides, vanillic acid-hexoside, (+)-Catechin, (−)-epicatechin, Quercetin 3-O-rutinoside, 3-O-glucoside, and 3-O-(6′′-malonyl)-glucoside |
[17] |
|
Phenolic acids, flavonoids |
Grape skin |
Gallic acid, caffeic acid, epicatechin, p-coumaric acid, rutin, catechin gallate |
[18] |
|
Flavonols |
Pistachio hulls |
Gallic acid, penta-O-galloyl-β-d-glucose, anacardic acid |
[19] |
|
Flavonoids, carotenoids |
Passion fruit peel |
β-carotene, provitamin A, quercetin, lycopene |
[20] |
|
Carotene |
Carrot pomace |
α- and β-carotene |
[21] |
|
Lycopene |
Tomato peel |
Lycopene |
[22] |
|
Non phenolic compounds |
Lettuce |
Ascorbic acid |
[23] |
|
Non phenolic compounds |
Sugarcane molasses |
Pullulan |
[24] |
|
Non phenolic compounds |
Rice bran oil |
Tocopherol |
[25] |
2. Recovery of Antioxidants from Agro-Industrial Side Streams
2.1. Recent Advances in Recovery of Antioxidants from Agro-Industrial Side Streams
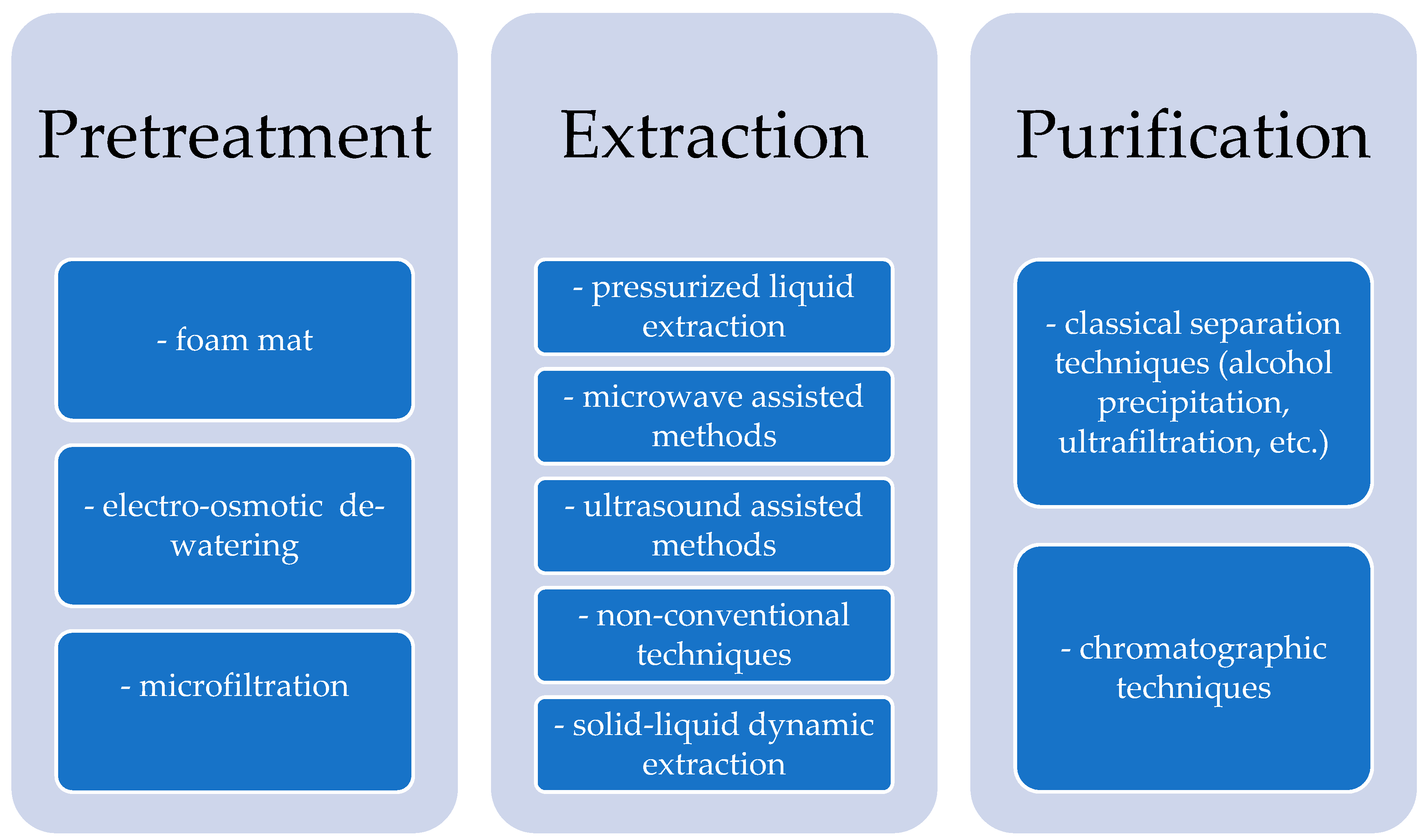
2.2. Recovery of Antioxidant Compounds from Edible Oil Industry Wastes
|
Waste |
Extraction Method |
Optimized Extraction Conditions |
Obtained Compounds |
Antioxidant Assay |
Ref. |
|---|---|---|---|---|---|
|
Flaxseed hulls |
PEF |
Electrode area (cm2)—95 pulse length (µs)—10 Temperature (°C)—20 Electric field (kV/cm)—20 |
Tocopherols, polyphenols, phytosterols |
- |
[61] |
|
Palm pressed fibers |
PLE, Sx, Pc |
Temperature (°C)—35, 35, 78.4 Flow rate (g/min) 2.4 Pressure (Mpa)—0.1; 0.1; 4 |
Carotenoids |
- |
[64] |
|
Palm pressed fiber |
PLE |
Solvents: CO2 and compressed liquefied petroleum gas Temperature (°C)—60 Pressure (MPa)—25.0 |
β-sitosterol, α-tocopherol, squalene |
HPX/XOD |
[65] |
|
Palm pressed fiber |
UAE |
Ultrasound intensity (W.cm−2)—120 Pulse cycle 0.4 Temperature (°C)—20 |
β-sitosterol, α-tocopherol, squalene, |
DPPH ABTS |
[66] |
|
Olive leaves |
ASE |
Temperature (°C)—190 Leaf moisture content (%)—5 Aqueous ethanol concentration (%)—80 |
Oleuropein, Luteolin-7-O-glucoside |
DPPH |
[67] |
|
Olive tree pruning biomass Olive mill leaves |
UAE |
Power (W)—400 Frequency (kHz)—24. Liquid/solid ratio of extraction (v/w)—20 mL/g. |
Phenolic compounds Flavonoids |
DPPH, ABTS, FRAP |
[68] |
|
Olive pomace |
UAE, MAE, Se |
Ethanol concentration (%)—90, Temperature (°C)—50, Time (min)—5 Liquid /solid ratio (mL/g)—30 Ultrasound intensity (W/cm2)—135.6 Ultrasound frequency (kHz)—60 |
Hydroxytyrosol, maslinic acid, oleanolic acid |
- |
[69] |
|
Olive leaves and tree bark |
SCe |
Temperature (°C)—60, Pressure (bar)—300 |
α-tocopherol, squalene |
- |
[70] |
|
Olive waste |
UAEH |
Cellulase, pectinase Frequency (kHz)—40 Power (W)—200 |
Phenolic compounds |
DPPH, ABTS, FRAP |
[71] |
|
Sunflower leaves |
PLE, ESE |
CO2 and mixture of solvents (ethanol in water from 0 to 100%) Pressure (bar)—400 Temperature (°C)—55 |
Diterpenoids, flavonoids |
- |
[72] |
|
Rapeseed press-cake |
HVED |
High voltage pulsed power (kV)—40 Intensity (kA)—10 Needle diameter (mm)—10 |
Protein, polyphenols and isothiocyanates |
TEAC |
[73] |
|
Pumpkin seeds |
UAE MAE |
Frequency (GHz)—2.45 Ethanol concentration (%)—60 Time (min)—20 UAE-EtOH—60% UAE-hex/EtOH/ H2O—30:49:21% |
Phenolic compounds |
DPPH |
[74] |
2.3. Recovery of Antioxidant Compounds from Fruits and Vegetable Wastes
|
Waste |
Extraction Method |
Optimized Extraction Conditions |
Obtained Compounds |
Antioxidant Assay |
Ref. |
|---|---|---|---|---|---|
|
Apple pomace |
Cec |
Methanol, ethanol and ethyl acetate |
Phenolic compounds and triterpenic acids |
DPPH, FRAP, ABTS |
[89] |
|
Apple pomace |
MAE |
Solvent—70% acetone and 60% ethanol, Microwave power (W)—735, Solvent volume to sample ratio (mL/g)—5.65 Time (s)—149 |
Phenolic compounds |
DPPH |
[90] |
|
Mango peels |
ScE |
Pressure (MPa)—25.0 Temperature (°C)—60 Solvent—15% w/w ethanol |
Carotene |
- |
[91] |
|
Orange peel |
LSE |
Solvent: cyclopentyl methyl ether, ethyl lactate, isopropyl alcohol, polyethylene glycol 300, isopropyl acetate, dimethyl carbonate, methyl ethyl ketone, 2-methyl-tetrahydrofuran and ethyl acetate Temperature (°C)—70 Time (min)—150 Solid -liquid ratio—1:10 |
Limonene |
- |
[92] |
|
Cocoa shells |
UAE, HC |
Hexane, hydro-alcoholic solution (70:30 EtOH/H2O) ternary mixture (30:49:21 Hex/EtOH/H2O) cycle number 47.1, cycle time (s)—5 residence time (s)—5 total residence time (min)—3.93 |
Catechins epicatechins theobromine caffeine |
DPPH |
[93] |
|
Tomato seeds |
UAE |
Power (W)—90 hexane-acetone-ethanol 2-1-1 |
Lycopene |
- |
[94] |
|
Tomato seeds |
MAE, OT |
Temperature (°C)—70 Time (min)—15 Solvent—70% ethanol |
Rutin |
- |
[95] |
|
Onion waste |
SbWE(PT) |
Temperature (°C)—145 Time (min)—15 intense pulsed light (V)—1200 Time (s)—60 |
Quercitin |
- |
[96] |
|
Pomegra-nate waste |
UAE |
Temperature (°C)—51.5; Amplitude level—58.8% Solvent—sunflower oil |
Carotenoids |
- |
[97] |
1 Where: ABTS—2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid; Cec—classical extraction with centrifugation; DPPH (assay)—2,2-diphenyl-1-picrylhydrazyl; FRAP (assay)—ferric reducing ability of plasma; HC—hydrodynamic cavitation; LSE—liquid solid extraction; MAE—microwave -ssisted extraction; OT—ohmic technologies; SbWE(PT)—Subcritical water extraction with physical pretreatment; ScE—Supercritical extraction; UAE—ultrasound-assisted extraction.
2.4. Recovery of Antioxidants Compounds from other Different Industries
|
Waste |
Extraction Method |
Optimized Extraction Conditions |
Obtained Compounds |
Antioxidant Assay |
Ref. |
|---|---|---|---|---|---|
|
Squid muscle |
SbWE |
Temperature (°C)—250 for aminoacids; 160 for peptides |
Amino acids Peptides |
ABTS |
[102] |
|
Poultry wastes |
SbWE |
Temperature (K) 533 Reaction time (min)—28 H2SO4 concentration in reactant system 0.02%. |
Amino acids |
- |
[103] |
|
Waste chicken breast muscle |
OT |
Sets of high voltage short pulses and by low voltage long pulses Energy (J/g)—38.4 ± 1.2 |
Proteins |
DPPH ABTS |
[104] |
|
Fucus vesiculosus |
MAE |
Pressure (psi)—120 Time (min)—1 1g alga/25mL water |
Fucoidan |
- |
[105] |
|
Saccharina japonica Aresch |
MAE |
Solvent: 55% ethanol Irradiation power (W)—400 solid/solvent ratio 1:8; Time (min)—25 |
Phlorotannins |
- |
[106] |
|
Undaria pinnatifida and Sargassum fusiforme |
MAE coupled with HSCCC |
Solvent: ethanolic KOH solution (1.5 mol/L) Irradiation power (W)—500 Liquid/solid ratio 20:1 Time (min)—20 Revolution speed (RPM)—800 |
Fucosterol, 24-methylenecholesterol, phytol |
- |
[107] |
|
Porphyridium cruentum |
UAE |
Solvent: 2mL of ethanol, 10mg ascorbic acid, 3mL of n-hexane, Time (min)—20 |
Tocopherol |
- |
[108] |
|
Nannochlorops sp. |
PEF |
The electric field (kV/cm)—20 Consecutive pulses 1–400 |
Carotenoids |
- |
[109] |
1 Where: ABTS—2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid; DPPH (assay)—2,2-diphenyl-1-picrylhydrazyl; HSCCC—high-speed counter current chromatography; PEF—pulsed electric fields; OT—ohmic technologies; MAE—microwave-assisted extraction; SbWE—subcritical water extraction; UAE—ultrasound-assisted extraction.
3. Potential Applications of Antioxidants Recovered from Food Waste and by-Products
|
Waste |
Active Compounds |
Application |
Ref. |
|---|---|---|---|
|
Applications of antioxidant compounds recovered from edible oil industry wastes |
|||
|
Palm pressed fiber |
β-Sitosterol, α-tocopherol, squalene |
Cosmetic formulation with high sun protection factor |
[66] |
|
Sunflower leaves |
Diterpenoids, flavonoids |
Natural herbicide |
[72] |
|
Sunflower seed |
Phenolic compounds |
Antioxidant additive for sunflower oil |
[145] |
|
Soy bean waste |
Proteins |
Biopackaging |
[146] |
|
Olive waste extract |
Phenolic compounds |
Food industry (increasing shelf life of meat) |
[147] |
|
Olive mill wastes |
Phenolic compounds |
Food antioxidants |
[148] |
|
Applications of antioxidant compounds from fruits wastes |
|||
|
Apple seeds |
Phenolic compounds |
Food industry |
[11] |
|
Berries |
Phenolic compounds |
Pharmaceutical formulations |
[144] |
|
Mango peels |
Carotene |
Antioxidant additive for edible oil |
[91] |
|
Banana peels |
Caffeic acid |
Cosmetic formulations |
[149] |
|
Citrus peels |
Phenolic compounds, essential oils and flavonoids |
Pharmaceutical formulations |
[150] |
|
Citrus wastes |
Phenolics and flavonoids |
Cosmetic formulations |
[151] |
|
Citrus peels |
Terpinene, cymene |
Pharmaceutical formulations |
[152] |
|
Cocoa |
Total extract |
Larvicidal nanoparticles |
[153] |
|
Grape pomace |
Phenolic compounds |
Food industry |
[154] |
|
Applications of antioxidant from vegetable wastes |
|||
|
Tomato wastes |
Lycopene |
Health related applications |
[155] |
|
Beetroot pomace |
Betalains |
Medicinal and food applications |
[156] |
|
Carrot pomace |
Carotenoids |
Pharmaceutical formulations |
[157] |
|
Garlic waste |
Ethanolic extract |
Food additive to increase products shelf life |
[158] |
|
Onion waste |
Phenolic compounds |
Food industry |
[159] |
|
Cauliflower by-products |
Isothiocyanates |
Food industry |
[160] |
|
Applications of antioxidant compounds from other industries |
|||
|
Meat industry wastes |
Gelatin Heparin |
Pharmaceutical formulations (antioxidant and antihypertensive) |
[1] |
|
Algal biomass |
Sulfated polysaccharides |
Pharmaceutical formulations |
[161] |
|
Algal biomass |
α-Carnitine |
Nutraceutical products |
[162] |
|
Squid waste |
Astaxanthin |
Pharmaceutical industry |
[163] |
|
Shrimps shells |
Astaxanthin |
Food packaging material |
[164] |
|
Shrimps shells |
Carotenoprotein |
Supplementary nutritive feed |
[165] |
References
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842.
- Michal, J.; Andrea, Š.; Anna, M.; Jozef, Š. Extraction of value-added components from food industry based and agro-forest biowastes by deep eutectic solvents. J. Biotechnol. 2018, 282, 46–66.
- Sladkova, A.; Benedekova, M.; Stopka, J.; Surina, I.; Haz, A.; Strizincová, P.; Cizova, K.; Skulcova, A.; Burcova, Z.; Kreps, F.; et al. Yield of polyphenolic substances extracted from spruce (Picea abies) bark by microwave-assisted extraction. BioResources 2016, 11, 9912–9921.
- Kinsella, Z.; Stewart, V.E.H.; Trombly, J. The Environmental Paper Listening Study. Chapter Four: Tree Free Paper. Available online: http://www.conservatree.org/paperlisteningstudy/TreeFreePaperLS.pdf (accessed on 2 September 2019).
- Reddy, N.; Yang, Y. Biofibers from agricultural byproducts for industrial applications. Trends Biotechnol. 2005, 23, 22–27.
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.; Alves, R.C.; Oliveira, M.B.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148.
- Ferrentino, G.; Asaduzzaman, M.; Scampicchio, M.M. Current technologies and new insights for the recovery of high valuable compounds from fruits by-products. Crit. Rev. Food Sci. Nutr. 2018, 58, 386–404.
- Sindhu, R.; Gnansounou, E.; Rebello, S.; Binod, P.; Varjani, S.; Thakur, I.S.; Nair, R.B.; Pandey, A. Conversion of food and kitchen waste to value-added products. J. Environ. Manag. 2019, 241, 619–630.
- Nasini, L.; De Luca, G.; Ricci, A.; Ortolani, F.; Caselli, A.; Massaccesi, L.; Regni, L.; Gigliotti, G.; Proietti, P. Gas emissions during olive mill waste composting under static pile conditions. Int. Biodeterior. Biodegrad. 2016, 107, 70–76.
- Sharholy, M.; Ahmad, K.; Mahmood, G.; Trivedi, R.C. Municipal solid waste management in Indian cities—A review. Waste Manag. 2008, 28, 459–467.
- Gunes, R.; Palabiyik, I.; Toker, O.S.; Konar, N.; Kurultay, S. Incorporation of defatted apple seeds in chewing gum system and phloridzin dissolution kinetics. J. Food Eng. 2019, 255, 9–14.
- Tremocoldi, M.A.; Rosalen, P.L.; Franchin, M.; Massarioli, A.P.; Denny, C.; Daiuto, E.R.; Paschoal, J.A.R.; Melo, P.S.; de Alencaret, S.M. Exploration of avocado by-products as natural sources of bioactive compounds. PLoS ONE 2018, 13, e0192577.
- Zago, E.; Lecomte, J.; Barouh, N.; Aouf, C.; Carre, P.; Fine, F.; Villeneuve, P. Influence of rapeseed meal treatments on its total phenolic content and composition in sinapine, sinapic acid and canolol. Ind. Crops Prod. 2015, 76, 1061–1070.
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels II: Enzyme-assisted extraction method. Sep. Purif. Technol. 2006, 48, 189–196.
- Rodrigues, S.; Pinto, G.A.S.; Fernandes, F.A.N. Optimization of ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder by response surface methodology. Ultrason. Sonochem. 2008, 15, 95–100.
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Italian J. Anim. Sci. 2018, 18, 336–341.
- Puganen, A.; Kallio, H.P.; Schaich, K.M.; Suomela, J.P.; Yang, B. Red/green currant and sea buckthorn berry press residues as potential sources of antioxidants for food use. J. Agric. Food Chem. 2018, 66, 3426–3434.
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Feng, X.L.; Xu, X.Y.; Cao, S.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, H.B. Comparison of antioxidant activities of different grape varieties. Molecules 2018, 23, 2432.
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem. 2018, 253, 46–54.
- Dos Reis, L.C.R.; Facco, E.M.P.; Flores, S.H.; Rios, A.D.O. Stability of functional compounds and antioxidant activity of fresh and pasteurized orange passion fruit (Passiflora caerulea) during cold storage. Food Res. Int. 2018, 106, 481–486.
- Stoll, T.; Schweiggert, U.; Schieber, A.; Carle, R. Process for the recovery of a carotene-rich functional food ingredient from carrot pomace by enzymatic liquefaction. Innov. Food Sci. Emerg. Technol. 2003, 4, 415–423.
- Choudhari, S.M.; Ananthanarayan, L. Enzyme aided extraction of lycopene from tomato tissues. Food Chem. 2007, 102, 77–81.
- Santos, F.T.; Goufo, P.; Santos, C.; Botelho, D.; Fonseca, J.; Queirós, A.; Costa, M.S.; Trindade, H. Comparison of five agro-industrial waste-based composts as growing media for lettuce: Effect on yield, phenolic compounds and vitamin C. Food Chem. 2016, 209, 293–301.
- Srikanth, S.; Swathi, M.; Tejaswini, M.; Sharmila, G.; Muthukumaran, C.; Jaganathan, M.K.; Tamilarasan, K. Statistical optimization of molasses based exopolysaccharide and biomass production by Aureobasidium pullulans MTCC 2195. Biocatal. Agric. Biotechnol. 2014, 3, 7–12.
- Pestana-Bauer, V.R.; Zambiazi, R.C.; Mendonça, C.R.; Beneito-Cambra, M.; Ramis-Ramos, G. γ-Oryzanol and tocopherol contents in residues of rice bran oil refining. Food Chem. 2012, 134, 1479–1483.
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Recovery and preservation of phenols from olive waste in ethanolic extracts. J. Chem. Technol. Biotechnol. 2010, 85, 1148–1155.
- Jumah, R.; Al-Asheh, S.; Banat, F.; Al-Zoubi, K. Electro-osmotic dewatering of tomato paste suspension under AC electric field. Dry. Technol. 2005, 23, 1465–1475.
- Koubala, B.B.; Mbome, L.I.; Kansci, G.; Mbiapo, F.T.; Crepeau, M.J.; Thibault, J.F.; Ralet, M.C. Physicochemical properties of pectins from ambarella peels (Spondias cytherea) obtained using different extraction conditions. Food Chem. 2008, 106, 1202–1207.
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87.
- Sowbhagya, H.B.; Chitra, V.N. Enzyme-assisted extraction of flavourings and colorants from plant materials. Crit. Rev. Food Sci. Nutr. 2010, 50, 146–161.
- Tsakona, S.; Galanakis, C.M.; Gekas, V. Hydro-ethanolic mixtures for the recovery of phenols from Mediterranean plant materials. Food Bioproc. Technol. 2012, 5, 1384–1393.
- Strati, I.F.; Oreopoulou, V. Effect of extraction parameters on the carotenoid recovery from tomato waste. Int. J. Food Sci. Technol. 2011, 46, 23–29.
- Fierascu, R.C.; Ortan, A.; Avramescu, S.M.; Fierascu, I. Phyto-nanocatalysts: Green synthesis, characterization, and applications. Molecules 2019, 24, 3418.
- Sarkis, J.R.; Boussetta, N.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Application of pulsed electric fields and high voltage electrical discharges for oil extraction from sesame seeds. J. Food Eng. 2015, 153, 20–27.
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High voltage electrical discharges, pulsed electric field, and ultrasound assisted extraction of protein and phenolic compounds from olive kernel. Food Bioproc. Technol. 2015, 8, 885–894.
- Ghafoor, K.; Park, J.; Choi, Y.H. Optimization of supercritical fluid extraction of bioactive compounds from grape (Vitis labrusca B.) peel by using response surface methodology. Innov. Food Sci. Emerg. Technol. 2011, 11, 485–490.
- Périno-Issartier, S.; Huma, Z.; Abert-Vian, M.; Chemat, F. Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food Bioproc. Technol. 2011, 4, 1020–1028.
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A powerful and greener alternative to the latest solid-liquid extraction techniques. Foods 2019, 8, 245.
- Posadino, A.; Biosa, G.; Zayed, H.; Abou-Saleh, H.; Cossu, A.; Nasrallah, G.; Giordo, R.; Pagnozzi, D.; Porcu, M.C.; Pretti, L.; et al. Protective effect of cyclically pressurized solid–liquid extraction polyphenols from Cagnulari grape pomace on oxidative endothelial cell death. Molecules 2018, 23, 2105.
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae – An update. TrAC Trends Anal. Chem. 2019, 116, 198–213.
- Alonso, E. The role of supercritical fluids in the fractionation pretreatments of a wheat bran-based biorefinery. J. Supercrit. Fluids 2018, 133, 603–614.
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109.
- Nguyen, T.T.; Barber, A.R.; Corbin, K.; Zhang, W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioresour. Bioprocess 2017, 4, 27.
- Kazan, A.; Celiktas, M.S.; Sargin, S.; Yesil-Celiktas, O. Bio-based fractions by hydrothermal treatment of olive pomace: Process optimization and evaluation. Energy Convers. Manag. 2015, 103, 366–373.
- Ko, M.J.; Kwon, H.L.; Chung, M.S. Pilot-scale subcritical water extraction of flavonoids from satsuma Mandarin (Citrus unshiu Markovich) peel. Innov. Food Sci. Emerg. Technol. 2016, 38, 175–181.
- Vergara-Salinas, J.R.; Vergara, M.; Altamirano, C.; Gonzalez, Á.; Pérez-Correa, J.R. Characterization of pressurized hot water extracts of grape pomace: Chemical and biological antioxidant activity. Food Chem. 2015, 171, 62–69.
- Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Huamán-Castilla, N.; Pedreschi, F.; Iglesias-Rebolledo, N.; Pérez-Correa, J.R. Impact of an integrated process of hot pressurised liquid extraction-macroporous resin purification over the polyphenols, hydroxymethylfurfural and reducing sugars content of Vitis vinifera ‘Carménère’ pomace extracts. Int. J. Food Sci. Technol. 2018, 53, 1072–1078.
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from grape marc. J. Food Eng. 2019, 240, 105–113.
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167–176.
- Da Porto, C.; Natolino, A.; Decorti, D. The combined extraction of polyphenols from grape marc: Ultrasound assisted extraction followed by supercritical CO2 extraction of ultrasound-raffinate. LWT Food Sci. Technol. 2015, 61, 98–104.
- Chen, H.; Fu, X.; Luo, Z. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015, 168, 302–310.
- Sumere, B.R.; de Souza, M.C.; dos Santos, M.P.; Bezerra, R.M.N.; da Cunha, D.T.; Martinez, J.; Rostagno, M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.). Ultrason. Sonochem. 2018, 48, 151–162.
- Boukroufa, M.; Boutekedjiret, C.; Petigny, L.; Rakotomanomana, N. Bio-refinery of orange peels waste: A new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrason. Sonochem. 2015, 24, 72–79.
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Rimac Brnčić, S. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100.
- Minjares-Fuentes, R.; Femenia, A.; Garau, M.C.; Meza-Velázquez, J.A.; Simal, S.; Rosselló, C. Ultrasound-assisted extraction of pectins from grape pomace using citric acid: A response surface methodology approach. Carbohydr. Polym. 2014, 106, 179–189.
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioproc. Technol. 2012, 5, 409–424.
- Şahin, S. A novel technology for extraction of phenolic antioxidants from Mandarin (Citrus deliciosa Tenore) leaves: Solvent-free microwave extraction. Korean J. Chem. Eng. 2015, 32, 950–957.
- Llompart, M.; Celeiro, M.; Dagnac, T. Microwave-assisted extraction of pharmaceuticals, personal care products and industrial contaminants in the environment. TrAC Trends Anal. Chem. 2019, 116, 136–150.
- Phongthai, S.; Lim, S.T.; Rawdkuen, S. Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties. J. Cereal Sci. 2016, 70, 146–154.
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372.
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Saha, S.; Sairam, K.V.S.S. Evaluation of enzyme and microwave-assisted conditions on extraction of anthocyanins and total phenolics from black soybean (Glycine max L.) seed coat. Int. J. Biol. Macromol. 2019, 135, 1070–1081.
- Dávila, I.; Remón, J.; Gullón, P.; Labidi, J.; Budarin, V. Production and characterization of lignin and cellulose fractions obtained from pretreated vine shoots by microwave assisted alkali treatment. Biores. Technol. 2019, 289, 121726.
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370.
- Cardenas-Toro, F.P.; Alcazar-Alay, S.C.; Coutinho, J.P.; Godoy, H.T.; Forster-Carneiro, T.; Meireles, M.A.A. Pressurized liquid extraction and low-pressure solvent extraction of carotenoids from pressed palm fiber: Experimental and economical evaluation. Food Bioprod. Process. 2015, 94, 90–100.
- Dal Prá, V.; Soares, J.F.; Monego, D.L.; Vendruscolo, R.G.; Freire, D.M.G.; Alexandri, M.; Koutinas, A.; Wagner, R.; Mazutti, M.A.; da Rosa, M.B. Extraction of bioactive compounds from palm (Elaeis guineensis) pressed fiber using different compressed fluids. J. Supercrit. Fluids 2016, 112, 51–56.
- Dal Prá, V.; Lunelli, F.C.; Vendruscolo, R.G.; Martins, R.; Wagner, R.; Lazzaretti, A.P., Jr.; Freire, D.M.G.; Alexandri, M.; Koutinas, A.; Mazutti, M.A.; et al. Ultrasound-assisted extraction of bioactive compounds from palm pressed fiber with high antioxidant and photoprotective activities. Ultrason. Sonochem. 2017, 36, 362–366.
- Lama-Muñoz, A.; Del Mar Contreras, M.; Espínola, F.; Moya, M.; de Torres, A.; Romero, I.; Castro, E. Extraction of oleuropein and luteolin-7-O-glucoside from olive leaves: Optimization of technique and operating conditions. Food Chem. 2019, 293, 161–168.
- Martínez-Patiño, J.C.; Gullón, B.; Romero, I.; Ruiz, E.; Brnčić, M.; Šic Žlabur, J.; Castro, E. Optimization of ultrasound-assisted extraction of biomass from olive trees using response surface methodology. Ultrason. Sonochem. 2019, 51, 487–495.
- Xie, P.; Huang, L.; Zhang, C.; Xiaoji, Y.D.; Cheng, W.J. Enhanced extraction of hydroxytyrosol, maslinic acid and oleanolic acid from olive pomace: Process parameters, kinetics and thermodynamics, and greenness assessment. Food Chem. 2019, 276, 662–674.
- Issaoui, A.; Ksibib, H.; Ksibi, M. Supercritical fluid extraction of triterpenes and aliphatic hydrocarbons from olive tree derivatives. Arab. J. Chem. 2017, 10, S3967–S3973.
- Wang, Z.; Wang, C.; Zhang, C.; Li, W. Ultrasound-assisted enzyme catalyzed hydrolysis of olive waste and recovery of antioxidant phenolic compounds. Innov. Food Sci. Emerg. Technol. 2017, 44, 224–234.
- Fuentes-Gandara, F.; Torres, A.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Varela, R.; Martínez de la Ossa-Fernández, E.J.; Macías, F.A. Selective fractionation and isolation of allelopathic compounds from Helianthus annuus L. leaves by means of high-pressure techniques. J. Supercrit. Fluids 2019, 143, 32–41.
- Barba, F.J.; Boussetta, N.; Vorobiev, E. Emerging technologies for the recovery of isothiocyanates, protein and phenolic compounds from rapeseed and rapeseed press-cake: Effect of high voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2015, 31, 67–72.
- Ferreira, D.F.; Barin, J.S.; Binello, A.; Veselov, V.V.; Cravotto, G. Highly efficient pumpkin-seed extraction with the simultaneous recovery of lipophilic and hydrophilic compounds. Food Bioprod. Process. 2019, 117, 224–230.
- Chang, J.I.; Tsai, J.J.; Wu, K.H. Composting of vegetable waste. Waste Manag. Res. 2006, 24, 354–362.
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 2017, 63, 51–59.
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data (accessed on 2 September 2019).
- Coman, V.; Teleky, B.E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. Adv. Food Nutr. Res. 2019, in press.
- Food and Agriculture Organization of the United Nations. Global Food Losses and Waste. Extent, Causes and Prevention. Available online: http://www.fao.org/docrep/014/mb060e/mb060e00.pdf (accessed on 2 September 2019).
- Porat, R.; Lichter, A.; Terry, L.A.; Harker, R.; Buzby, J. Postharvest losses of fruit and vegetables during retail and in consumers’ homes: Quantifications, causes, and means of prevention. Postharvest Biol. Technol. 2018, 139, 135–149.
- De Laurentiis, V.; Corrado, S.; Sala, S. Quantifying household waste of fresh fruit and vegetables in the EU. Waste Manag. 2018, 77, 238–251.
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT Food Sci. Technol. 2017, 80, 136–144.
- Tarko, T.; Duda-Chodak, A.; Bebak, A. Aktywność biologiczna wybranych wytłoków owocowych oraz warzywnych. Żywność-Nauka Technologia Jakość 2012, 4, 55–65.
- Majerska, J.; Michalsk, A.; Figiel, A. A review of new directions in managing fruit and vegetable processing by-products. Trends Food Sci. Technol. 2019, 88, 207–219.
- Johnson, M.H.; De Mejia, E.G.; Fan, J.; Lila, M.A.; Yousef, G.G. Anthocyanins and proanthocyanidins from blueberry–blackberry fermented beverages inhibit markers of inflammation in macrophages and carbohydrate-utilizing enzymes in vitro. Mol. Nutr. Food Res. 2013, 57, 1182–1197.
- Juan, C.; Jianquan, K.; Junni, T.; Zijian, C.; Ji, L. The profile in polyphenols and volatile compounds in alcoholic beverages from different cultivars of mulberry. J. Food Sci. 2012, 77, C430–C436.
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897.
- Alhamad, M.N.; Rababah, T.M.; Al-u’datt, M.; Ereifej, K.; Esoh, R.; Feng, H.; Yang, W. The physicochemical properties, total phenolic, antioxidant activities, and phenolic profile of fermented olive cake. Arab. J. Chem. 2017, 10, 136–140.
- Nile, S.H.; Nile, A.; Liu, J.; Kim, D.H.; Kai, G. Exploitation of apple pomace towards extraction of triterpenic acids, antioxidant potential, cytotoxic effects, and inhibition of clinically important enzymes. Food Chem. Toxicol. 2019, 131, 110563.
- Chandrasekar, V.; San Martín-González, M.F.; Hirst, P.; Ballard, T.S. Optimizing microwave-assisted extraction of phenolic antioxidants from Red Delicious and Jonathan apple pomace. J. Food Process. Eng. 2015, 38, 571–582.
- Del Pilar Sánchez-Camargo, A.; Gutiérrez, L.F.; Milena Vargas, S.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574.
- Ozturk, B.; Winterburn, J.; Gonzalez-Miquel, M. Orange peel waste valorisation through limonene extraction using bio-based solvents. Biochem. Eng. J. 2019, 151, 107298.
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa bean shell waste valorisation; extraction from lab to pilot-scale cavitational reactors. Food Res. Int. 2019, 115, 200–208.
- Yilmaz, T.; Kumcuoglu, S.; Tavman, S. Ultrasound-assisted extraction of lycopene and β-carotene from 1242 tomato-processing wastes. Italian J. Food Sci. 2016, 29, 186–194.
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of tomato by-products’ bioactive compounds using ohmic technology. Food Bioprod. Process. 2019, 117, 329–339.
- Kim, S.W.; Ko, M.J.; Chung, M.S. Extraction of the flavonol quercetin from onion waste by combined treatment with intense pulsed light and subcritical water extraction. J. Clean. Prod. 2019, 231, 1192–1199.
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830.
- Food and Agriculture Organization of the United Nations—Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/ (accessed on 5 September 2019).
- Kaspar, A.A.; Reichert, J.M. Future directions for peptide therapeutics development. Drug Discov. Today 2013, 18, 807–817.
- Stenmarck, A.; Jensen, C.; Quested, T.; Moates, G.; Buksti, M.; Cseh, B.; Juul, S.; Parry, A.; Polotano, A.; Redlinghofer, B.; et al. Estimates of European Food Waste Levels; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016.
- Tonini, D.; Albizzati, P.F.; Astrup, T.F. Environmental impacts of food waste: Learnings and challenges from a case study on UK. Waste Manag. 2018, 76, 744–766.
- Asaduzzaman, A.K.M.; Chun, B.S. Recovery of functional materials with thermally stable antioxidative properties in squid muscle hydrolyzates by subcritical water. J. Food Sci. Technol. 2015, 52, 793–802.
- Zhu, G.Y.; Zhu, X.; Wan, X.L.; Fan, Q.; Ma, Y.H.; Qian, J.; Liu, X.L.; Shen, Y.J.; Jiang, J.H. Hydrolysis technology and kinetics of poultry waste to produce amino acids in subcritical water. J. Anal. Appl. Pyrol. 2010, 88, 187–191.
- Ghosh, S.; Gillis, A.; Sheviryov, J.; Levkov, K.; Golberg, A. Towards waste meat biorefinery: Extraction of proteins from waste chicken meat with non-thermal pulsed electric fields and mechanical pressing. J. Clean. Prod. 2019, 208, 220–231.
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown edible algae. Carbohydr. Polym. 2011, 86, 1137–1144.
- He, Z.; Chen, Y.; Chen, Y.; Liu, H.; Yuan, G.; Fan, Y.; Chen, K. Optimization of the microwave-assisted extraction of phlorotannins from Saccharina japonica Aresch and evaluation of the inhibitory effects of phlorotannin-containing extracts on HepG2 cancer cells. Chin. J. Ocean. Limnol. 2013, 31, 1045–1054.
- Xiao, X.H.; Yuan, Z.Q.; Li, G.K. Preparation of phytosterols and phytol from edible marine algae by microwave-assisted extraction and high-speed counter-current chromatography. Sep. Purif. Technol. 2013, 104, 284–289.
- Durmaz, Y.; Monteiro, M.; Bandarra, N.; Gökpinar, Ş.; Işik, O. The effect of low temperature on fatty acid composition and tocopherols of the red microalga, Porphyridium cruentum. J. Appl. Phycol. 2007, 19, 223–227.
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.I.; Vorobiev, E. Selective extraction from microalgae Nannochloropsis sp. using different methods of cell disruption. Biores. Technol. 2014, 153, 254–259.
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-food byproducts as a new source of natural food additives. Molecules 2019, 24, 1056.
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316.
- Lavecchia, R.; Zuorro, A. Evaluation of olive pomace as a source of phenolic antioxidants for the production of functional cosmetics. Int. J. Appl. Eng. Res. 2015, 10, 34405–34409.
- Pacifico, S.; Piccolella, S.; Veneziano, R. New sustainable cosmetic products from food waste: A joined-up approach between design and food chemistry. In Designing Sustainability for All, Proceedings of the 3rd LeNS World Distributed Conference (3rd volume), Milano, Mexico City, Beijing, Bangalore, Curitiba, Cape Town, 3–5 April 2019; Ambrosio, M., Vezzoli, C., Eds.; Edizioni POLI.design: Milano, Italy, 2019; pp. 975–979.
- Han, Y.; Yu, M.; Wang, L. Bio-based films prepared with soybean by-products and pine (Pinus densiflora) bark extract. J. Clean. Prod. 2018, 187, 1–8.
- Han, Y.; Yu, M.; Wang, L. Preparation and characterization of antioxidant soy protein isolate films incorporating licorice residue extract. Food Hydrocoll. 2018, 75, 13–21.
- Maryam, Z.A.; Jamilaha, A.B.; Nur Hanania, Z.A. Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. 2018, 74, 207–218.
- Ahn, J.; Grun, I.; Fernando, L. Antioxidant properties of natural plant extracts containing polyphenolic compounds in cooked ground beef. J. Food Sci. 2002, 67, 1364–1369.
- Azman, N.; Skowyra, M.; Muhammad, K.; Gallego, M.; Almajano, M. Evaluation of the antioxidant activity of Betula pendula leaves extract and its effects on model foods. Pharmaceut. Biol. 2017, 55, 912–919.
- Carpenter, R.; O’Grady, M.; O’Callaghan, Y.; O’Brien, N.; Kerry, J. Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci. 2007, 76, 604–610.
- Lee, C.; Reed, J.; Richards, M. Ability of various polyphenolic classes from cranberry to inhibit lipid oxidation in mechanically separated Turkey and cooked ground pork. J. Muscle Foods. 2001, 67, 248–266.
- Karre, L.; Lopez, K.; Getty, K. Natural antioxidants in meat and poultry products. Meat Sci. 2013, 94, 220–227.
- Raghavan, S.; Richards, M. Partitioning and inhibition of lipid oxidation in mechanically separated Turkey by components of cranberry press cake. J. Agricult. Food Chem. 2006, 54, 6403–6408.
- Zhang, H.; Wu, J.; Guo, X. Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality. Food Sci. Human Wellness 2016, 5, 39–48.
- Casarotti, S.; Jorge, N. Antioxidant activity of rosemary extract in soybean oil under thermoxidation. J. Food Process. Preserv. 2014, 38, 136–145.
- Mohdaly, A.; Sarhan, M.; Mahmoud, A.; Ramadan, M.; Smetanska, I. Antioxidant efficacy of potato peels and sugar beet pulp extracts in vegetable oils protection. Food Chem. 2010, 123, 1019–1026.
- Peng, X.; Ma, J.; Cheng, K.W.; Jiang, Y.; Chen, F.; Wang, M. The effects of grape seed extract fortification on the antioxidant activity and quality attributes of bread. Food Chem. 2010, 119, 49–53.
- Reddy, V.; Urooj, A.; Kumar, A. Evaluation of antioxidant activity of some plant extracts and their application in biscuits. Food Chem. 2005, 90, 317–321.
- Vasileva, I.; Denkova, R.; Chochkov, R.; Teneva, D.; Denkova, Z.; Dessev, T.; Denev, P.; Slavov, A. Effect of lavender (Lavandula angustifolia) and melissa (Melissa officinalis) waste on quality and shelf life of bread. Food Chem. 2018, 253, 13–21.
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; NurHanani, Z.A. Utilization of mango peel extracts on the biodegradable films for active packaging. Food Pack. Shelf Life 2018, 16, 1–7.
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259.
- Souza, B.; Larroza Nunes, I.; Vargas Pereira, F.; Druzian, J. Desenvolvimento e avaliação da eficácia de filmes biodegradáveis de amido de mandioca com nanocelulose como reforço e com extrato de erva-mate como aditivo antioxidante. Ciência Rural 2012, 42, 2085–2091.
- Santana, M.; Machado, B.; Silva, T.; Nunes, I.; Druzian, J. Incorporação de urucum como aditivo antioxidante em embalagens biodegradáveis a base de quitosana. Ciencia Rural 2013, 43, 544–550.
- Knapp, M.A.; dos Santos, D.F.; Pilatti-Riccio, D.; Deon, V.G.; dos Santos, G.H.F.; Pinto, V.Z. Yerba mate extract in active starch films: Mechanical and antioxidant properties. J. Food Process. Preserv. 2019, 43, e13897.
- Díaz Vela, J.; Totosaus, A.; Pérez Chabela, M.L. Integration of agroindustrial co-products as functional food ingrdents: Cactus pear (Opuntia ficus indica) flour and pineapple. J. Food Process. Perserv. 2015, 39, 2630–2638.
- Serna-Cock, L.; García-Gonzales, E.; Torres-León, C. Agro-industrial potential of the mango peel based on its nutritional and functional properties. Food Rev. Int. 2016, 32, 346–376.
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional beverages: The emerging side of functional foods commercial trends, research, and health implications. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206.
- Battino, M.; Beekwilder, J.; Denoyes-Rothan, B.; Laimer, M.; McDougall, G.J.; Mezzetti, B. Bioactive compounds in berries relevant to human health. Nutr. Rev. 2009, 67, S145–S150.
- Shahidi, F.; De Camargo, A.C. Tocopherols and tocotrienols in common and emerging dietary sources: Occurrence, applications, and health benefits. Int. J. Mol. Sci. 2016, 17, 1745.
- Lee, E.; Ryu, G.R.; Ko, S.H.; Ahn, Y.B.; Yoon, K.H.; Ha, H.; Song, K.H. Antioxidant treatment may protect pancreatic beta cells through the attenuation of islet fibrosis in an animal model of type 2 diabetes. Biochem. Biophys. Res. Commun. 2011, 414, 397–402.
- Kim, T.; Yan, Q. Peroxisome-proliferator-activated receptors regulate redox signaling in the cardiovascular system. World J. Cardiol. 2013, 5, 164–174.
- Fang, F.; Kang, Z.; Wong, C. Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Mol. Nutr. Food Res. 2010, 54, 345–352.
- Bajaj, S.; Khan, A. Antioxidants and diabetes. Indian J. Endocrinol Metab. 2012, 16, S267–S271.
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and therapeutic effects of edible berries: A focus on colon cancer prevention and treatment. Molecules 2016, 21, 169.
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2017, 58, 2491–2507.
- De Leonardis, A.; Macciola, V.; Di Rocco, A. Oxidative stabilization of cold-pressed sunflower oil using phenolic compound of the same seed. J. Sci. Food Agricult. 2003, 83, 523–528.
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Properties and antioxidant activity of soy protein concentrate films incorporated with red grape extract processed by casting and compression molding. LWT Food Sci. Technol. 2016, 74, 353–362.
- Muíño, I.; Díaz, M.T.; Apeleo, E.; Pérez-Santaescolástica, C.; Rivas-Cañedo, A.; Pérez, C.; Cañeque, V.; Lauzuric, S.; de la Fuente, J. Valorisation of an extract from olive oil waste as a natural antioxidant for reducing meat waste resulting from oxidative processes. J. Clean. Prod. 2017, 140, 924–932.
- Araújo, M.; Pimentel, F.B.; Alvesa, R.C.; Beatriz, M.; Oliveira, P.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211.
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic compounds within banana peel and their potential uses: A review. J. Funct. Foods 2018, 40, 238–248.
- Shetty, S.B.; Mahin-Syed-Ismail, P.; Varghese, S.; Thomas-George, B.; Kandathil Thajuraj, P.; Baby, D. Antimicrobial effects of Citrus sinensis peel extracts against dental caries bacteria: An in vitro study. J. Clin. Exp. Dent. 2016, 8, 70–77.
- Apraj, V.D.; Pandita, N.S. Evaluation of skin antiaging potential of Citrus reticulata blanco peel. Pharmacog. Res. 2016, 8, 160–168.
- Choi, H.S. Lipolytic effects of citrus peel oils and their components. J. Sci. Food Agric. 2006, 54, 3254–3258.
- Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Azeez, L.; Ojo, S.A.; Gueguim-Kana, E.B.; Beukes, L.S. Cocoa pod husk extract-mediated biosynthesis of silver nanoparticles: Its antimicrobial, antioxidant and larvicidal activities. J. Nanostruct. Chem. 2016, 6, 159–169.
- De Souza de Azevedo, P.O.; Aliakbarian, B.; Casazza, A.A.; LeBlanc, J.G.; Perego, P.; de Souza Oliveira, R.P. Production of fermented skim milk supplemented with different grape pomace extracts: Effect on viability and acidification performance of probiotic cultures. PharmaNutrition 2018, 6, 64–68.
- Martins, N.; Ferreira, I.C.F.R. Wastes and by-products: Upcoming sources of carotenoids for biotechnological purposes and health-related applications. Trends Food Sci. Technol. 2017, 62, 33–48.
- Kushwaha, R.; Kumar, V.; Vyas, G.; Kaur, J. Optimization of different variable for eco-friendly extraction of betalains and phytochemicals from beetroot pomace. Waste Biomass Valoriz. 2018, 9, 1485–1494.
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot-A review. J. Food Sci. Technol. 2012, 49, 22–32.
- Kallel, F.; Ellouz Chaabouni, S. Perspective of garlic processing wastes as low-cost substrates for production of high-added value products: A review. Environ. Progr. Sustain. Energy 2017, 36, 1765–1777.
- Larrosa, M.; Llorach, R.; Espın, J.C.; Tomas-Barberan, F.A. Increase of antioxidant activity of tomato juice upon functionalisation with vegetable byproduct extracts. LWT Food Sci. Technol. 2002, 35, 532–542.
- Amofa-Diatuo, T.; Anang, D.M.; Barba, F.J.; Tiwari, B.K. Development of new apple beverages rich in isothiocyanates by using extracts obtained from ultrasound treated cauliflower by-products: Evaluation of physical properties and consumer acceptance. J. Food Compos. Anal. 2017, 61, 73–81.
- Li, N.; Zhang, Q.; Song, J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem. Toxicol. 2005, 43, 421–426.
- Mayer, M.A.; Finlayson, G.; Fischman, D.; de Paz, C.; Telleriarte, M.R.; Ferrero, A.J.; Bobillo, C.; Fernández, B.E. Evaluation of the satiating properties of a nutraceutical product containing Garcinia cambogia and Ascophyllum nodosum extracts in healthy volunteers. Food Funct. 2014, 5, 773–779.
- Veeruraj, A.; Liu, L.; Zheng, J.; Wu, J.; Arumugam, M. Evaluation of astaxanthin incorporated collagen film developed from the outer skin waste of squid Doryteuthis singhalensis for wound healing and tissue regenerative applications. Mat. Sci. Eng. C 2019, 95, 29–42.
- Xu, J.; Wei, R.; Jia, Z.; Song, R. Characteristics and bioactive functions of chitosan/gelatin-based film incorporated with ε-polylysine and astaxanthin extracts derived from by-products of shrimp (Litopenaeus vannamei). Food Hydrocoll. 2019, 100, 105436.
- Pattanaik, S.S.; Sawant, P.B.; Xavier, K.A.M.; Dube, K.; Srivastava, P.P.; Dhanabalan, V.; Chadha, N.K. Characterization of carotenoprotein from different shrimp shell waste for possible use as supplementary nutritive feed ingredient in animal diets. Aquaculture 2019, 515, 734594.




