
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Radu Claudiu Fierascu | -- | 2892 | 2022-09-20 10:52:50 | | | |
| 2 | Catherine Yang | + 38 word(s) | 2930 | 2022-09-21 03:39:58 | | | | |
| 3 | Catherine Yang | Meta information modification | 2930 | 2022-09-21 03:40:45 | | | | |
| 4 | Catherine Yang | Meta information modification | 2930 | 2022-09-21 03:41:48 | | | | |
| 5 | Catherine Yang | Meta information modification | 2930 | 2022-09-21 03:43:46 | | | | |
| 6 | Catherine Yang | Meta information modification | 2930 | 2022-09-21 04:17:49 | | |
Video Upload Options
The species belonging to the Polypodiopsida class have been part of human culture since the beginning of civilization, often being utilized due to the presence of antimicrobial substances (such as alkaloids, terpenes as tannins, saponins, anthraquinones, cardiac glycosides, etc.). Application of the ferns in nanotechnology is presented with practical examples.
1. Introduction
2. Polypodiopsida and the Nanomaterials
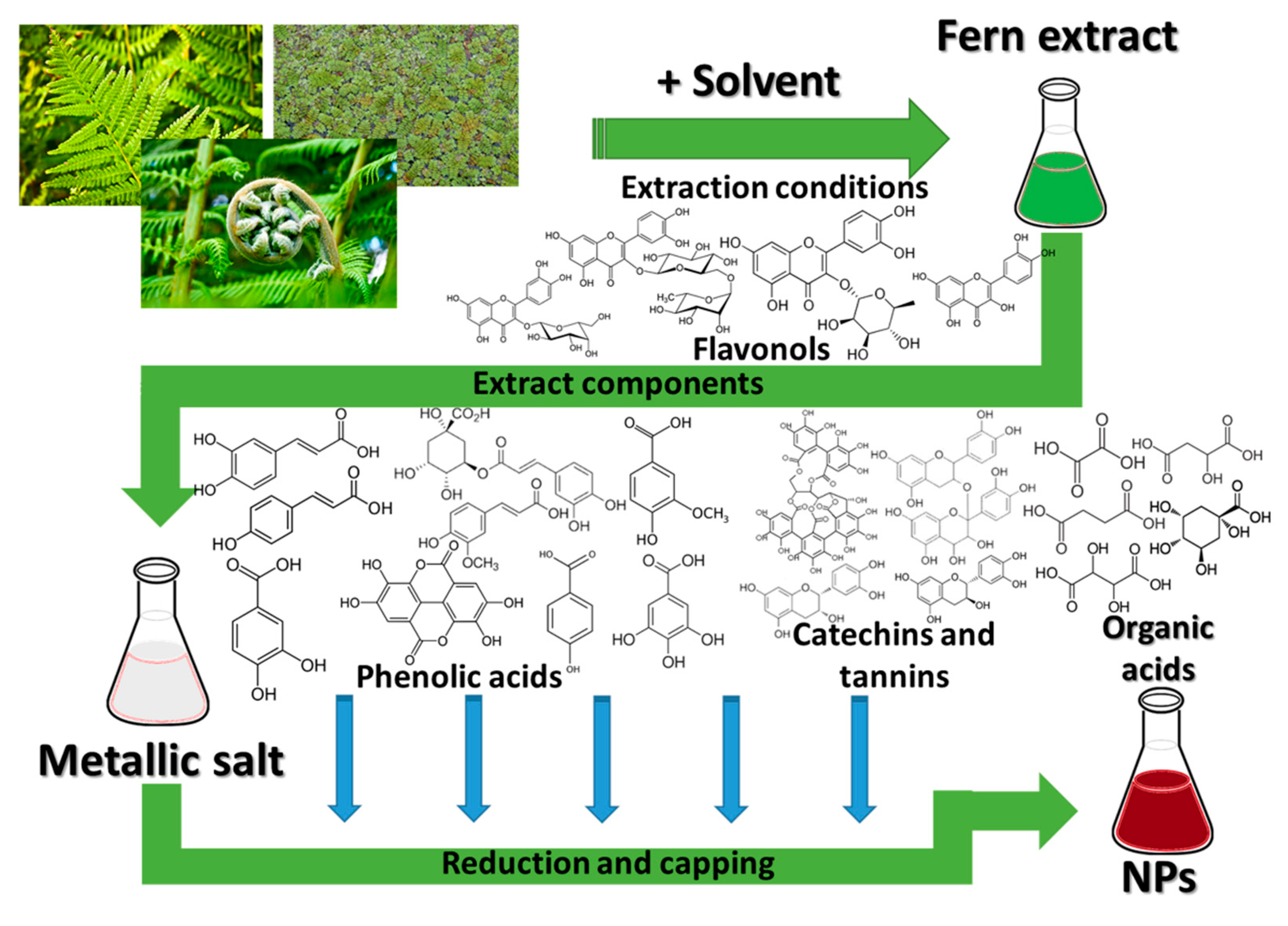
- -
-
factors related to the vegetal extract used: the intrinsic properties of the plants, related to their phytocomponents, the part of the plant used, extraction procedure, used solvents, the vegetal material to solvent ratio, plant pre-treatment, etc.;
- -
-
factors related to the phytosynthesis process: concentration of the metallic salt precursors, reaction conditions (temperature, pH, reaction time), extract to metallic salt ratio, etc.
2.1. Nanoparticle Phytosynthesis Using Ferns
- -
-
silver ions can bind to form different insoluble precipitates, which can negatively affect their properties [9];
- -
-
particularly for the case of phytosynthesized nanoparticles, the use of different phytocomponents as capping agents can not only contribute to an increase in their antimicrobial or antioxidant potential (for example) [7], but can decrease their toxic potential against non-target organisms [7], which is actually lower for NPs, compared with silver ions [11];
- -
-
the large surface area to volume ratio of nanoparticles (an element common for all types of NPs) provides better contact with microorganisms, thus increasing their antimicrobial potential, as well as contributing to their successful application in other areas [12].
2.2. Potential Applications of Phytosynthesized Nanoparticles
The phytosynthesis of the NPs leads to the attainment of nanoparticles with characteristics depending on the extract used. Being closely correlated with the natural extract, the phytosynthesized NPs finds applications in areas in which the extracts have a historical use, such as antioxidant or antimicrobial fields, in which various phytoconstituents (such as phenolic acids, flavonoids, terpenes, carotenoids and proanthocyanidins) have proven applicability.
| Fern Used | Applied NPs | Application Results | Ref. |
|---|---|---|---|
| Antimicrobial potential | |||
| Asplenium scolopendrium L. | AgNPs, 10–12 nm | Evaluated against Staphylococcus aureus, Pseudomonas aeruginosa; MIC-1/32 (against S. aureus); MCBE-1/16 (P. aeruginosa); | [14] |
| Dryopteris crassirhizoma Nakai (1920) | AgNPs, spherical, 5–60 nm | Evaluated against Bacillus cereus and P. aeruginosa; best inhibition zones (IZ): 10 mm/250 μg under green LED (B. cereus); 6 mm/250 μg under green LED (P. aeruginosa). | [15] |
| Equisetum arvense L. | AgNPs, nearly spherical, 10–60 nm | Evaluated against Escherichia coli, S. aureus, Candida albicans, commercial probiotic Saccharomyces boulardii; Selective activity against E. coli (effective at low concentrations-0.72 mg/L); |
[13] |
| Equisetum arvense L. | AgNPs, spherical, 170.5 nm | evaluated against Salmonella enterica, B. cereus, Listeria monocytogenes, Enterococcus faecium, S. aureus, Aeromonas hydrophila) IZ (mm) = 11.64/10.75/12.46/9.68/12.53/10.80 |
[16] |
| Equisetum giganteum L. | AgNPs, spherical, 20 nm | Evaluated against E. coli, S. aureus, Alternaria alternata, Chaetomium globosum; Fungal resistance test and antibacterial biofilm tests after incorporation in waterborne paints; active against all strains; MIC-3.3/13.3/3.3/67.5 μg/mL; paint films inhibited fungal and bacterial biofilm development |
[17] |
| Dicranopteris linearis (Burm.f.) Underw | AgNPs, spherical, 40–60 nm | Evaluated against Bacillus subtilis, Klebsiella pneumoniae and Salmonella typhi; IZ (mm) = 21.01/20.1/19 at 75 ppm | [18] |
| Gleichenella pectinata (Willd.) Ching | AgNPs, spherical, 7.51 nm. | Evaluated against P. aeruginosa, E. coli, K. pneumoniae and C. albicans; IZ (mm) = 15/11/10/13 at 5 mM | [19] |
| Nephrolepis biserrata (Sw.) Schott |
RuNPs, ~26 nm | Evaluated against Aspergillus flavus; 50% inhibition at 0.6 mL; | [20] |
| Nephrolepis cordifolia (L.) K. Presl | SiO2@Au–Ag composites (200–246 nm SiO2 decorated with 3-nm AuNPs/AgNPs) | Evaluated against E. coli, S. aureus; IZ (mm) = 21/14 | [21] |
| Adiantum philippense L. |
AuNPs—spherical, triangular, 33.9 nm in AuNPs–amoxicillin composites | Evaluated against E. coli, S. aureus, Staphylococcus epidermis, B. subtilis, B. cereus, MRSA1, MRSA2, MRSA3, MRSA4; in vivo treatment of systemic MRSA infection : IZ (mm) = 31/30/19/35/38/16/15/12/12 MIC/MBC (mg/L) = 2/4; ½; ½; 16/32; 8/16; 16/32; 16/32; 16/32; 32/32. Survival rate at day 7 post-inoculation 96% |
[22] |
| Adiantum philippense L. | AgNPs, quasi-spherical, 10–60 nm | Evaluated against B. subtilis, Listeria monocytogenes, S. aureus, E. coli, K. pneumoniae, Salmonella typhimurium; MIC = 105.41/17.55/17.85/12.36/17.84/28.77 | [23] |
| Pteris ripartite Sw. | AgNPs, different morphologies, 32 nm | Evaluated against B. subtilis, B. cereus, Bacillus megaterium, E. coli, Proteus vulgaris, Serratia marcescens, S. typhi, K. pneumoniae, Vibrio cholerae, Shigella sonnei, Enterobacter aerogenes, P. aeruginosa, A. niger, Aspergillus flavus, Fusarium oxysporum, Penicillium chrysogenum, Rhizopus oryzae; IZ (mm) = 8.33 (B. cereus) − 24.33 (P. aeruginosa) at 10 mg/mL; MIC (at 10 mg/mL, 24 h) = 0.29 (P. aeruginosa) − 1.40 (E. coli); | [24] |
| Adiantum capillus-veneris L. | AgNPs, spherical, 18.4 nm | Evaluated against E. coli and S. aureus; IZ (mm) = 30/19 applied as “thick nanoparticle suspension” |
[25] |
| Adiantum capillus-veneris L. | AuNPs | Evaluated against E. coli, P. aeruginosa, Salmonella enterica, S. aureus, B. subtilis, Trichophyton rubrum, Scedosporium apiospermum, Aspergillus fumigates, A. niger, A. flavus; IZ (mm) = 16 (A. fumigates, S. apiospermum, S. enterica) − 21 (E. coli) | [26] |
| Pteris quadriaurita Retz. | AuNPs | Evaluated against E. coli, P. aeruginosa, S. enterica, S. aureus, B. subtilis, T. rubrum, S. apiospermum, A. fumigates, A. niger, A. flavus; IZ (mm) = 14 (T. rubrum) − 18 (S. aureus) | [26] |
| Marsilea quadrifolia L. | AgNPs, spherical, 9–42 nm | Evaluated against E. coli; MIC = 0.5 nM; | [27] |
| Salvinia molesta D. Mitch. | AgNPs, spherical, 12.46 nm | Evaluated against E. coli, S. aureus; IZ (mm) = 21/16 (at 50 ppm); MIC = 10.5/13 mg/L Cell viability loss = 95.8/92.6% after 8 h. at MIC |
[28] |
| Cibotium barometz (L.) J. Sm. | AgNPs, spherical, 5–40 nm; | Evaluated against E. coli, S. aureus, S. enterica, P. aeruginosa; IZ (mm, AgNPs) = 16/17.5/12.5/12.5 at 45 μg/disc; | [29] |
| Antioxidant potential | |||
| Asplenium scolopendrium L. | AgNPs, < 50 nm | DPPH inhibition 81.34%/80.93% (rhizomes/leaves mediated NPs) | [30] |
| Equisetum arvense L. | AgNPs, spherical, 170.5 nm | IC0.50 (reducing power activity) = 641.24 μg/mL; IC50 (ABTS/DPPH/NOx) = 210.16/92.90/62.52 μg/mL; | [16] |
| Nephrolepis biserrata (Sw.) Schott | RuNPs, ~26 nm | IC50 (mg/mL, DPPH, ABTS, SORS, HSA assays) = 0.986/0.852/1.265/1.389 | [20] |
| Pteris tripartite Sw. | AgNPs, different morphologies, 32 nm | DPPH, chelating activity, Phosphomolybdenum, ABTS, HPSA assays: 47.90 (mg/L)/61.51 ± 0.61 (mg EDTA/g)/41.94 ± 2.29 (mg AAE/g)/8592.70 ± 614.2 (μmol Trolox/g)/16.20 ± 3.86 (%); |
[24] |
| Adiantum capillus-veneris L. | AuNPs | Inhibition: ~90% (DPPH)/~70% (SORS)/~85% (HPSA)/~82% (HSA); |
[26] |
| Pteris quadriaurita Retz. | AuNPs | Inhibition: ~81% (DPPH)/~60% (SORS)/~77% (HPSA)/~75% (HSA); |
[26] |
| Marsilea quadrifolia L. | AuNPs, spherical, 10–40 nm | IC50 (DPPH) = 50 mg/L; | [31] |
| Leptochilus pteropus (Blume) Fraser-Jenk | AgNPs | IC₅₀ = 47.0 μg/mL (DPPH)/35.8 μg/mL (HPSA) | [32] |
| Cibotium barometz (L.) J. Sm. | AgNPs, spherical, 5–40 nm; AuNPs, spherical, 5–20 nm, |
IC50 (DPPH) = 1.4/1.22 mg/mL (AuNPs/AgNPs) | [29] |
| Cytotoxic potential | |||
| Asplenium scolopendrium L. | AgNPs, < 50 nm | Rhizomes extract mediated NPs-progressive time-related mitoinhibitory effect; for both NPs—increased frequency and variability of chromosomal aberrations in the Allium cepa assay | [30] |
| Asplenium scolopendrium L. | AgNPs, 10–12 nm | Significantly higher frequency of the total aberrant cells compared with the negative control sample in the Allium cepa assay | [14] |
| Equisetum arvense L. | AgNPs, nearly spherical, 10–60 nm | MTT assay (MC3T3-E1): Cytotoxic threshold: >2.25/> 4.5 mg L−1, lower for smaller NPs | [13] |
| Equisetum arvense L. | AgNPs spherical, 170.5 nm | Trypan blue exclusion test (HepG2): 20% viability (at 1 mg/mL) | [16] |
| Nephrolepis cordifolia (L.) K. Presl | SiO2@Au–Ag composites (200–246 nm SiO2 decorated with 3 nm AuNPs/AgNPs) | MTT assay (human keratinocyte cells): 95% cell viability at 500 μg/mL | [21] |
| Adiantum philippense L. | AuNPs-spherical, triangular, 33.9 nm. | MTT assay (L929): 81% viability (AuNPs), 79% viability (AuNPs–amoxicillin composites) | [22] |
| Adiantum sp. | AgNPs, AuNPs | MTT assay: Cytotoxicity against MCF-7 cells at different concentrations (2.5 to 100 μg/mL); non-cytotoxic to HEK293 cells | [33] |
| Marsilea quadrifolia L. | AgNPs, spherical, 9–42 nm | MTT assay: Cell death: 40.04% (MCF-7)/55.88% (HeLa), with NP sonication | [27] |
| Marsilea quadrifolia L. | AuNPs, spherical, 17–40 nm | MTT assay (3T3-L1): Cell viability = 71.23% (100 μM) − 84.02% (30μM); glucose uptake = 60.86% | [34] |
| Marsilea quadrifolia L. | AuNPs, spherical, 10–40 nm | MTT assay: IC50 = 45.88/52.01 mg/L (PA-1/A549) | [31] |
| Cibotium barometz (L.) J. Sm. | AgNPs, spherical, 5–40 nm; AuNPs, spherical, 5–20 nm, |
MTT assay (RAW264.7 and MCF-7): AuNPs—no cell death at 0.1–10 mg/L; AgNPs—cytotoxicity at ≥ 10 mg/L against RAW264.7 |
[29] |
| Alsophila nilgirensis (Holttum) R.M. Tryon | AgNPs, spherical, 45–74 nm | Hatched shrimps bioassay: LC50 = 869.4 μL/10 mL | [35] |
| Larvicidal potential | |||
| Pteridium aquilinum (L.) Kuhn | AgNPs spherical, 35–65 nm | Against Anopheles stephensi Liston, 1901 in laboratory conditions: LC50 of 7.48 ppm (larva I), 10.68 ppm (II), 13.77 ppm (III), 18.45 ppm (IV), and 31.51 ppm (pupae); Larvicidal assays in the field: complete removal of An. stephensi population after 72 h (at 10 × LC50 in water reservoir) |
[36] |
| Dicranopteris linearis (Burm.f.) Underw | AgNPs spherical, 40–60 nm | Against Aedes aegypti (Linnaeus in Hasselquist, 1762); laboratory conditions: LC50 = 18.905 ppm (I)/ 20.929 ppm (II)/23.187 ppm (III)/26.312 ppm (IV)/29.328 ppm (pupae); LC90 = 32.140 ppm (I)/35.489 ppm (II)/39.696 ppm (III)/44.418 ppm (IV)/48.511 ppm (pupae) Field larvicidal activity (by application in water storage tanks), ovicidal assay, oviposition deterrent activity 100% reduction in A. aegypti larval populations at 10 × LC50 (after 72 h); No hatching observed at 25 ppm; ER = 94.29% at 30 ppm; |
[18] |
| Adiantum raddianum C. Presl | AgNPs, 9.69–13.9 nm | Against mosquito larvae, in laboratory conditions (A. stephensi, A. aegypti, and Culex quinquefasciatus Say, 1823): LC50 = 10.33/11.23/12.19 mg/L Low toxicity against non-target organisms (Diplonychus indicus Venk. et Rao and Gambusia affinis (S. F. Baird and Girard, 1853)), LC50 = 517.86–35.98 mg/L |
[37] |
| Phytotoxic potential | |||
| Asplenium scolopendrium L. | AgNPs, 10–12 nm | NPs led to the reduction of the phytotoxic effect of the extracts in Triticum test | [14] |
| Adiantum philippense L. | CuONPs, quasi-spherical, 1–20 nm | Effect on Lens culinaris Medik: 91.26% seed germination, SVI = 4168.43, RWC = 84.37% at 0.025 mg/mL (optimum dose); optimum dose showed highest activity of defense enzymes and total phenolics; higher concentrations of NPs retard all the parameters |
[38] |
| Alsophila nilgirensis (Holttum) R.M. Tryon | AgNPs, spherical, 45–74 nm | Effect on Vigna radiata (L.) R. Wilczek and Sorghum vulgare (L.). Seeds: Germination reduction: 38.65/100% (V. radiata and S. vulgare) at 50 mg/L | [35] |
| Antidiabetic potential | |||
| Equisetum arvense L. | AgNPs, spherical, 170.5 nm | IC50 (alpha-glucosidase) = 1.73 μg/mL; | [16] |
| Anti-inflammatory potential | |||
| Pteris tripartite Sw. | AgNPs, different morphologies, 32 nm | Anti-inflammatory activity Wistar albino adult female rats using the carrageenan-induced paw oedema method = 56.36%, 24 h., 100 mg/kg b.w. | [24] |
| Hepatoprotective potential | |||
| Azolla filiculoides Lam. | AuNPs, spherical, 17–40 nm | Significant increase in cell viability compared to the acetaminophen group (hepatocytes damage); significant reduction in the levels of LDH and CAT (dose dependent); AuNPs significantly reduced the GOT and GPT levels (50/10%), significantly increased the levels of GSH-Px and SOD (60–70%), drastically reduced the formation of MDA (60%) and ROS generation | [39] |
| Catalytic properties | |||
| Diplazium esculentum (Retz.) Sw. | AgNPs, different morphologies, 10–45 nm | Degradation of MB and RhB dyes under solar light illumination: complete disappearance of the adsorption peaks after 8 min. | [40] |
| Diplazium esculentum (Retz.) Sw. | AgNPs—spherical, 10–25 nm; AuNPs-different morphologies, 35–75 nm |
Degradation of MV 6B, RB, 4-nitro phenol: Ag-98.4/98/96.8%; Au-98.2/98.9/97.3% | [41] |
| Nephrolepis cordifolia (L.) K. Presl | Au–Ag@AgCl, average size 30 nm | Synthesis of quinoline derivatives via three component coupling/hydroarylation/dehydrogenation of arylaldehyde, aniline, and phenyl acetylene derivatives; 96% yield for the composite applied, reaction conditions 9h, at reflux | [42] |
| Nephrolepis cordifolia (L.) K. Presl | SiO2@Au–Ag composites (200–246 nm SiO2 decorated with 3 nm AuNPs/AgNPs) | Solvent-free amidation of carboxylic acid catalyst: 97% yield for the composite applied, reaction conditions—8 h, 100 °C | [21] |
| Other environmental applications | |||
| Nephrolepis cordifolia (L.) K. Presl |
FeNPs, spherical, 40–70 nm, other types of iron oxides | Cr(VI) removal: 90.93% | [43] |
2.3. Development of Biogenic Nanoparticles
References
- Song, X.; Liu, Z.; Sun, D.D. Nano gives the answer: Breaking the bottleneck of internal concentration polarization with a nanofiber composite forward osmosis membrane for a high water production rate. Adv. Mater. 2011, 23, 3256–3260.
- Park, J.S.; Kyhm, J.; Kim, H.H.; Jeong, S.; Kang, J.H.; Lee, S.; Lee, K.T.; Park, K.; Barange, N.; Han, J.Y.; et al. Alternative patterning process for realization of large-area, full-color, active quantum dot display. Nano Lett. 2016, 16, 6946–6953.
- Fuertes, G.; Soto, I.; Carrasco, R.; Vargas, M.; Sabattin, J.; Lagos, C. Intelligent packaging systems: Sensors and nanosensors to monitor food quality and safety. J. Sens. 2016, 2016, 4046061.
- Contado, C. Nanomaterials in consumer products: A challenging analytical problem. Front. Chem. 2015, 3, 48.
- Gupta, S.; Kesarla, R.; Omri, A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013, 2013, 848043.
- Kumar, R.; Lal, S. Synthesis of Organic Nanoparticles and their Applications in Drug Delivery and Food Nanotechnology: A Review. J. Nanomater. Mol. Nanotechnol. 2014, 3, 4.
- Fierascu, I.; Fierascu, I.C.; Brazdis, R.I.; Baroi, A.M.; Fistos, T.; Fierascu, R.C. Phytosynthesized metallic nanoparticles-between nanomedicine and toxicology. A brief review of 2019′s findings. Materials 2020, 13, 574.
- Fierascu, I.; Fierascu, I.C.; Dinu-Pirvu, C.E.; Fierascu, R.C.; Anuta, V.; Velescu, B.S.; Jinga, M.; Jinga, V. A short overview of recent developments on antimicrobial coatings based on phytosynthesized metal nanoparticles. Coatings 2019, 9, 787.
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018, 19, 444.
- Li, W.R.; Sun, T.L.; Zhou, S.L.; Ma, Y.K.; Shi, Q.S.; Xie, X.B.; Huang, X.M. A comparative analysis of antibacterial activity, dynamics and effects of silver ions and silver nanoparticles against four bacterial strains. Int. Biodeterior. Biodegrad. 2017, 123, 304–310.
- Velicogna, J.R.; Ritchie, E.E.; Scroggins, R.P.; Princz, J.I. A comparison of the effects of silver nanoparticles and silver nitrate on a suite of soil dwelling organisms in two field soils. Nanotoxicology 2016, 10, 1144–1151.
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83.
- Miljković, M.; Lazić, V.; Davidović, S.; Milivojević, A.; Papan, J.; Fernandes, M.M.; Lanceros-Mendez, S.; Ahrenkiel, S.P.; Nedeljković, J.M. Selective antimicrobial performance of biosynthesized silver nanoparticles by horsetail extract against E. coli. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2598–2607.
- Fierascu, R.C.; Fierascu, I.; Lungulescu, E.M.; Nicula, N.; Somoghi, R.; Diţu, L.M.; Ungureanu, C.; Sutan, A.N.; Drăghiceanu, O.A.; Paunescu, A.; et al. Phytosynthesis and radiation-assisted methods for obtaining metal nanoparticles. J. Mater. Sci. 2020, 55, 1915–1932.
- Lee, J.H.; Lim, J.M.; Velmurugan, P.; Park, Y.J.; Park, Y.J.; Bang, K.S.; Oh, B.T. Photobiologic-mediated fabrication of silver nanoparticles with antibacterial activity. J. Photochem. Photobiol. B 2016, 162, 93–99.
- Das, G.; Patra, J.K.; Shin, H.S. Biosynthesis, and potential effect of fern mediated biocompatible silver nanoparticles by cytotoxicity, antidiabetic, antioxidant and antibacterial, studies. Mater. Sci. Eng. C 2020, 114, 111011.
- Barberia-Roque, L.; Gámez-Espinosa, E.; Viera, M.; Bellotti, N. Assessment of three plant extracts to obtain silver nanoparticles as alternative additives to control biodeterioration of coatings. Int. Biodeterior. Biodegrad. 2019, 141, 52–61.
- Rajaganesh, R.; Murugan, K.; Panneerselvam, C.; Jayashanthini, S.; Aziz, A.T.; Roni, M.; Suresh, U.; Trivedi, S.; Rehman, H.; Higuchi, A.; et al. Fern-synthesized silver nanocrystals: Towards a new class of mosquito oviposition deterrents? Res. Vet. Sci. 2016, 109, 40–51.
- Femi-Adepoju, A.G.; Dada, A.O.; Otun, K.O.; Adepoju, A.O.; Fatoba, O.P. Green synthesis of silver nanoparticles using terrestrial fern (Gleichenia Pectinata (Willd.) C. Presl.): Characterization and antimicrobial studies. Heliyon 2019, 5, e01543.
- Gupta, P.K.; Ranganath, K.V.S.; Dubey, N.K.; Mishra, L. Green synthesis, characterization and biological activity of synthesized ruthenium nanoparticles using fishtail fern, sago palm, rosy periwinkle and holy basil. Curr. Sci. 2019, 117, 1308–1317.
- Sapkota, K.; Chaudhary, P.; Han, S.S. Environmentally sustainable route to SiO2@Au-Ag nanocomposites for biomedical and catalytic applications. RSC Adv. 2018, 8, 31311–31321.
- Kalita, S.; Kandimalla, R.; Sharma, K.K.; Kataki, A.C.; Deka, M.; Kotoky, J. Amoxicillin functionalized gold nanoparticles reverts MRSA resistance. Mater. Sci. Eng. C 2016, 61, 720–727.
- Chatterjee, A.; Khatua, S.; Acharya, K.; Sarkar, J. A green approach for the synthesis of antimicrobial bio-surfactant silver nanoparticles by using a fern. Dig. J. Nanomater. Biostruct. 2019, 14, 479–490.
- Baskaran, X.; Vigila, A.V.G.; Parimelazhagan, T.; Muralidhara-Rao, D.; Zhang, S. Biosynthesis, characterization, and evaluation of bioactivities of leaf extract-mediated biocompatible silver nanoparticles from an early tracheophyte, Pteris tripartita Sw. Int. J. Nanomed. 2016, 11, 5789–5805.
- Omidi, S.; Sedaghat, S.; Tahvildari, K.; Derakhshi, P.; Motiee, F. Biosynthesis of silver nanoparticles with Adiantum capillus-veneris l leaf extract in the batch process and assessment of antibacterial activity. Green Chem. Lett. Rev. 2018, 11, 544–551.
- Rautray, S.; Rajananthini, A.U. Therapeutic potential of green, synthesized gold nanoparticles. BioPharm. Int. 2020, 33, 30–38.
- Maji, A.; Beg, M.; Mandal, A.K.; Das, S.; Jha, P.K.; Kumar, A.; Sarwar, S.; Hossain, M.; Chakrabarti, P. Spectroscopic interaction study of human serum albumin and human hemoglobin with Mersilea quadrifolia leaves extract mediated silver nanoparticles having antibacterial and anticancer activity. J. Mol. Struct. 2017, 1141, 584–592.
- Verma, D.K.; Hasan, S.H.; Banik, R.M. Photo-catalyzed and phyto-mediated rapid green synthesis of silver nanoparticles using herbal extract of Salvinia molesta and its antimicrobial efficacy. J. Photochem. Photobiol. B 2016, 155, 51–59.
- Wang, D.; Markus, J.; Wang, C.; Kim, Y.J.; Mathiyalagan, R.; Aceituno, V.C.; Ahn, S.; Yang, D.C. Green synthesis of gold and silver nanoparticles using aqueous extract of Cibotium barometz root. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1548–1555.
- Sutan, N.A.; Fierascu, I.; Fierascu, R.C.; Manolescu, D.S.; Soare, L.C. Comparative analytical characterization and in vitro citogenotoxic activity evaluation of Asplenium scolopendrium L. leaves and rhizome extracts prior to and after Ag nanoparticles phytosynthesis. Ind. Crops Prod. 2016, 83C, 379–386.
- Balashanmugam, P.; Mosachristas, K.; Kowsalya, E. In vitro cytotoxicity and antioxidant evaluation of biogenic synthesized gold nanoparticles from Marsilea quadrifolia on lung and ovarian cancer cells. Int. J. Appl. Pharmaceut. 2018, 10, 153–158.
- Chick, C.N.; Misawa-Suzuki, T.; Suzuki, Y.; Usuki, T. Preparation and antioxidant study of silver nanoparticles of Microsorum pteropus methanol extract. Bioorg. Med. Chem. Lett. 2020, 30, 127526.
- Zamani, S.; Idrees, D.; Jha, B.; Jha, A.K. Green synthesis of metal nanoparticles from adiantum frond: Comparative analysis on cancer cell lines. Nanosci. Nanotechnol. Asia 2020, 10, 806–816.
- Chowdhury, A.; Kunjiappan, S.; Bhattacharjee, C.; Somasundaram, B.; Panneerselvam, T. Biogenic synthesis of Marsilea quadrifolia gold nanoparticles: A study of improved glucose utilization efficiency on 3T3-L1 adipocytes. In Vitro Cell Dev. Biol. Anim. 2017, 53, 483–493.
- Johnson, M.; Santhanam, A.; Thangaiah, S.; Narayanan, J. Green synthesis of silver nanoparticles using Cyathea nilgirensis Holttum and their cytotoxic and phytotoxic potentials. Part. Sci. Technol. 2018, 36, 578–582.
- Panneerselvam, C.; Murugan, K.; Roni, M.; Aziz, A.T.; Suresh, U.; Rajaganesh, R.; Madhiyazhagan, P.; Subramaniam, J.; Dinesh, D.; Nicoletti, M.; et al. Fern-synthesized nanoparticles in the fight against malaria: LC/MS analysis of Pteridium aquilinum leaf extract and biosynthesis of silver nanoparticles with high mosquitocidal and antiplasmodial activity. Parasitol. Res. 2016, 115, 997–1013.
- Govindarajan, M.; AlQahtani, F.S.; AlShebly, M.M.; Benelli, G. One-pot and eco-friendly synthesis of silver nanocrystals using Adiantum raddianum: Toxicity against mosquito vectors of medical and veterinary importance. J. Appl. Biomed. 2017, 15, 87–95.
- Sarkar, J.; Chakraborty, N.; Chatterjee, A.; Bhattacharjee, A.; Dasgupta, D.; Acharya, K. Green synthesized copper oxide nanoparticles ameliorate defence and antioxidant enzymes in Lens culinaris. Nanomaterials 2020, 10, 312.
- Kunjiappan, S.; Bhattacharjee, C.; Chowdhury, R. In vitro antioxidant and hepatoprotective potential of Azolla microphylla phytochemically synthesized gold nanoparticles on acetaminophen—Induced hepatocyte damage in Cyprinus carpio L. In Vitro Cell Dev. Biol. Anim. 2015, 51, 630–643.
- Paul, B.; Bhuyan, B.; Purkayastha, D.D.; Dhar, S.S. Green synthesis of silver nanoparticles using dried biomass of Diplazium esculentum (retz.) sw. and studies of their photocatalytic and anticoagulative activities. J. Mol. Liq. 2015, 212, 813–817.
- Sinha, T.; Ahmaruzzaman, M. Indigenous north eastern India fern mediated fabrication of spherical silver and anisotropic gold nano structured materials and their efficacy for the abatement of perilous organic compounds from waste water-A green approach. RSC Adv. 2016, 6, 21076–21089.
- Sapkota, K.; Han, S.S. A novel environmentally sustainable synthesis of nanocomposites and their application as an efficient and recyclable catalyst for quinoline synthesis. New J. Chem. 2017, 41, 5395–5402.
- Yi, Y.; Tu, G.; Tsang, P.E.; Xiao, S.; Fang, Z. Green synthesis of iron-based nanoparticles from extracts of Nephrolepis auriculata and applications for Cr(VI) removal. Mater. Lett. 2019, 234, 388–391.
- Mattos, B.D.; Rojas, O.J.; Magalhães, W.L.E. Biogenic SiO2 in colloidal dispersions via ball milling and ultrasonication. Powder Technol. 2016, 301, 58–64.
- Mattos, B.D.; Rojas, O.J.; Magalhaes, W.L.E. Biogenic silica nanoparticles loaded with neem bark extract as green, slow-release biocide. J. Clean. Prod. 2017, 142, 4206–4213.
- Mattos, B.D.; Gomes, G.R.; de Matos, M.; Ramos, L.P.; Magalhães, W.L.E. Consecutive production of hydroalcoholic extracts, carbohydrates derivatives and silica nanoparticles from Equisetum arvense. Waste Biomass Valor. 2018, 9, 1993–2002.
- Hosseini Mohtasham, N.; Gholizadeh, M. Nano silica extracted from horsetail plant as a natural silica support for the synthesis of H3PW12O40 immobilized on aminated magnetic nanoparticles (Fe3O4@SiO2-EP-NH-HPA): A novel and efficient heterogeneous nanocatalyst for the green one-pot synthesis of pyrano pyrazole derivatives. Res. Chem. Intermed. 2020, 46, 3037–3066.
- Adinarayana, T.V.S.; Mishra, A.; Singhal, I.; Reddy, D.V.R.K. Facile green synthesis of silicon nanoparticles from Equisetum arvense for fluorescence based detection of Fe(III) ions. Nanoscale Adv. 2020, 9, 4125–4132.
- Sola-Rabada, A.; Sahare, P.; Hickman, G.J.; Vasquez, M.; Canham, L.T.; Perry, C.C.; Agarwal, V. Biogenic porous silica and silicon sourced from Mexican Giant Horsetail (Equisetum myriochaetum) and their application as supports for enzyme immobilization. Colloid Surf. B 2018, 166, 195–202.
- Bogireddy, N.K.R.; Sahare, P.; Pal, U.; Méndez, S.F.O.; Gomez, L.M.; Agarwal, V. Platinum nanoparticle-assembled porous biogenic silica 3D hybrid structures with outstanding 4-Nitrophenol degradation performance. Chem. Eng. J. 2020, 388, 124237.




